To date, between 17% and 35% of patients with rheumatoid arthritis (RA) do not respond as expected to the initial biological therapy. The objective of this project is to recognize and weigh the attributes of biologic DMARD (bDMARD) to identify the most appropriate for each case, in the first lines of treatment of RA (after inadequate response to at least one synthetic DMARD or previous bDMARD).
MethodsTo recognize the possible attributes that could define the bDMARD, we performed a systematic search of the literature that recognized the possible attributes involving general aspects, pharmacology, efficacy, safety, management, and cost. Then a Delphi process was conducted with two rounds among a group of selected expert rheumatologists in the management of RA indicating the degree of agreement with the attributes identified in the literature. The project was completed between February and September 2015, indicating the degree of importance that was ascribed to each attribute. Two criteria were applied to determine the consistency of results: (1) based on the median and interquartile range; and (2) on the simultaneous compliance with mean, median, standard deviation, interquartile range and coefficient of variation. The agreement and final ratification of the expert panel were also determined.
ResultsEighty-three Spanish rheumatologists participated and completed both rounds of the Delphi process. In no case was the importance of the 77 attributes identified considered to be low; 75 of 77 (97.4%) were considered highly important and 76 of 77 (98.7%) were ratified. Fifteen attributes had the support of 100% of the working group.
ConclusionsThere was a high degree of agreement concerning the selected attributes. Fifteen of them had the support of 100% of the working group and could be considered the definition of the ideal bDMARD in the first lines of RA treatment.
Existen pacientes con artritis reumatoide (AR) que no responden de la forma deseada a la terapia biológica. Nuestro objetivo fue reconocer los atributos del FAME biológico (FAMEb) que podrían identificar al más adecuado en las primeras líneas de tratamiento de la AR.
MétodosPara reconocer los atributos que podrían definir el FAMEb, se realizó una búsqueda sistemática de la literatura acerca de aspectos generales, farmacología, eficacia, seguridad, administración y coste. A continuación, se realizó un proceso Delphi a 2 rondas entre un grupo de reumatólogos expertos en el manejo de la AR para determinar el grado de acuerdo con los atributos identificados, indicando el grado de importancia que se le daba a cada atributo. Se aplicaron 2 criterios para determinar la consistencia de los resultados: 1) sobre la base de la mediana y el rango intercuartílico, y 2) el cumplimiento simultáneo de media, mediana, desviación estándar, rango intercuartílico y coeficiente de variación. Se determinaron también la concordancia y la ratificación final del panel de expertos.
ResultadosOchenta y tres reumatólogos españoles completaron las 2 circulaciones del proceso Delphi. Ninguno de los 77 atributos identificados se consideró de baja importancia, 75 de los 77 (97,4%) se consideraron de alta importancia y 76 de los 77 (98,7%) fueron ratificados. Quince tuvieron el apoyo del 100% del grupo de trabajo.
ConclusionesQuince atributos tuvieron el apoyo del 100% del grupo de trabajo y podrían considerarse los que definirían el FAMEb ideal en las primeras líneas de tratamiento de la AR.
The main objective of treatment for rheumatoid arthritis (RA) is to control the inflammatory activity and prevent structural damage. It should be begun with a disease-modifying antirheumatic drug (DMARD) as soon as possible.1 Patients in whom treatment with a synthetic DMARD (sDMARD) has failed to achieve the therapeutic objective should be considered candidates to receive biological therapy.1,2 Nevertheless, it has been observed that between 17% and 35% of RA patients do not respond adequately to the first biological therapy.3
The choice of a biological DMARD (bDMARD) is generally based on clinical needs or the preference of the patient, the experience of the prescribing physician and the cost and availability of the drug. It is not usual to pose individualized therapy, although it would be desirable, in order to choose the best agent for each patient. In other diseases, there have been notable advances in individualized therapy through the use of biological markers that predict the response to certain drugs. In RA, this is still not possible.
For these reasons, the purpose of the ACORDAR project is to identify and assess the attributes that would help to decide on the most suitable bDMARD for each case.
Material and MethodsThe project was carried out between the months of February and September 2015.
To respond to the research question “what are the relevant attributes of bDMARD therapy that make it suitable in the first lines of treatment of RA?”, we started with a strategy involving a systematic literature search, following the PICO method, where the population consisted of adults with RA, the intervention was treatment with a bDMARD, comparisons were carried out and the outcome was the general attributes of these agents, their pharmacology, efficacy, safety, administration and cost.
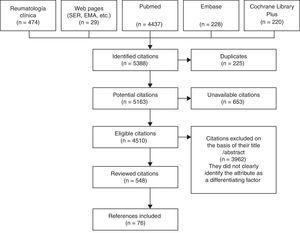
As primary sources, we reviewed the journal Reumatología Clínica, as well as the web pages of the Spanish Society of Rheumatology and of other prestigious entities (the European Medicines Agency, editorials, etc.), PubMed, EMBASE and the Cochrane Library Plus. We employed combinations of MeSH terms: population, “rheumatoid arthritis”; intervention, “biologic” or “biologic + DMARD” in first lines; outcome, “characteristic”, “efficacy”, “safety”, “side effects”, “pharmacology”, “adherence”, “route-administration” and “cost”.
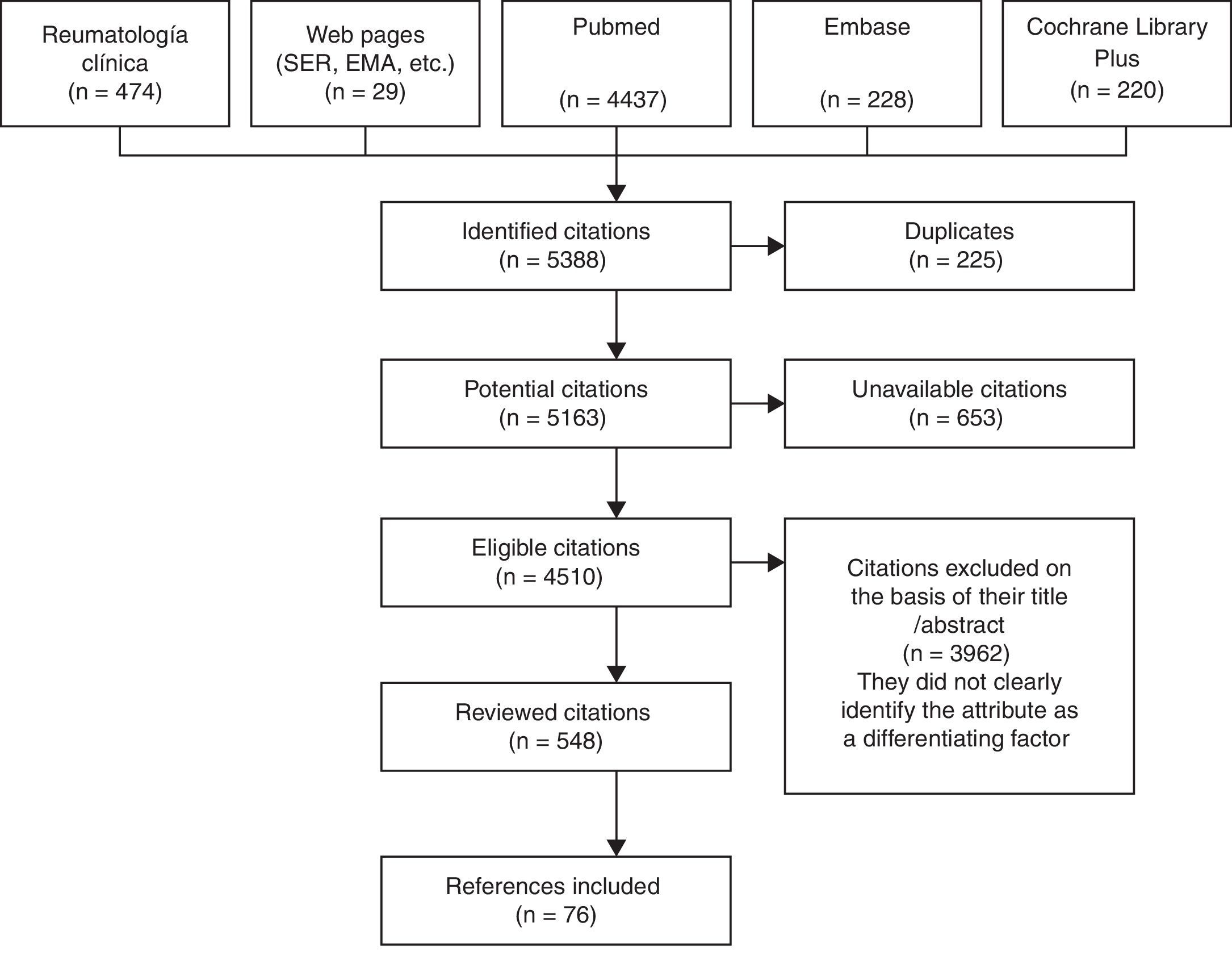
The terms were looked for in both Castilian and English, starting in 1990 to coincide with the development of the bDMARD. We reviewed the abstracts and/or articles of a total of 548 publications of the 4510 found in the different databases investigated. This was carried out by a single person who is an expert in the matter. This collaborator identified attributes of these agents that, aside from their other values, could help to evaluate or compare the use of biological drugs as applied in RA. In all, 109 attributes were proposed to the committee, of which ultimately 77 were selected, grouped and drawn up: 7 on general aspects, 5 related to pharmacological aspects, 18 relative to efficacy, 31 to safety, 6 to their administration and 10 to cost (Fig. 1).1,4–27
In the months of May, June and July, 12 meetings were held in various localities in Spain. To each of them, we invited 7 rheumatologists who were experts in this matter, and explained the project to 83 rheumatologists from all parts of Spain. After each meeting, we distributed the first Delphi findings, in which each attribute was assigned the level of importance it had been designated using a numerical Likert scale with scores ranging from 1 to 9. Each segment had the following correlations: from 1 to 3, minor importance; from 4 to 6, intermediate importance; and from 7 to 9, maximum importance (questionnaire included in the supplementary material). The Delphi process was completed with a second round that was sent in the month of July, when we had received responses from all of the participants.
To determine the consistency of the level of agreement, we simultaneously assessed 2 criteria (the standard deviation [SD], interquartile range [IQR] and coefficient of variation [CV]):
- –
Criterion based on the median and dispersion, assigning the corresponding importance to the median, if the IQR≤1.00.
- –
Criterion based on simultaneously establishing the following values: mean and median in the same segment, in addition to the SD and IQR≤1, and the CV≤0.25.
A result was considered to be in agreement, if less than a third of the responses remained outside the segment of importance attributed by the median.
Finally, we distributed the list of attributes with the resulting level of importance together with the criteria for consistency and agreement, for their approval by the participants. We were thus able to obtain the percentage of those who ultimately supported each attribute.
The analysis of the segmentation of the 77 selected attributes was done using the statistical tools provided by Microsoft Excel 2012: mean and SD; median, quartiles and IQR; mode, minimum and maximum values; as well as the CV. The Spearman correlation coefficient was applied to assess the correlation between the 2 rounds and Cronbach's alpha to quantify the internal consistency of the questionnaire.
ResultsThe 83 participating rheumatologists completed the 2 Delphi rounds, and those who approved had a mean experience in the management of RA of 19.07 years (SD±7.5), and had been prescribing bDMARD therapy for an average of 13.19 years (SD±4.38). In all, 8.43% of the participants were department heads, 10.84% were section heads and the remainder (80.72%) were staff members.
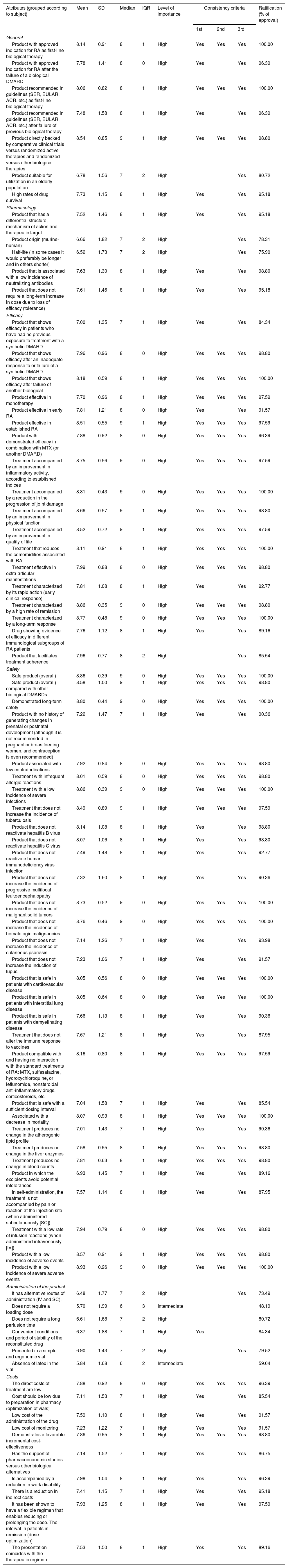
Table 1 summarizes the levels of importance and agreement with each of the attributes included in the study in accordance with the rheumatologists. Only 2 attributes related to the administration of the drug (“a loading dose is not necessary” and “does not contain latex in the vial”) were considered to be of intermediate importance. In the rest, the level of importance was found to be high. The level of agreement with all of the attributes was over 73%, with the exception of those mentioned above, in which the degree of agreement was lower (48.19% and 59.04%, respectively).
Analysis of the Attributes of Biological Disease-modifying Antirheumatic Drugs and Results of the 2 Delphi Rounds.
| Attributes (grouped according to subject) | Mean | SD | Median | IQR | Level of importance | Consistency criteria | Ratification (% of approval) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||||||
| General | |||||||||
| Product with approved indication for RA as first-line biological therapy | 8.14 | 0.91 | 8 | 1 | High | Yes | Yes | Yes | 100.00 |
| Product with approved indication for RA after the failure of a biological DMARD | 7.78 | 1.41 | 8 | 0 | High | Yes | Yes | 96.39 | |
| Product recommended in guidelines (SER, EULAR, ACR, etc.) as first-line biological therapy | 8.06 | 0.82 | 8 | 1 | High | Yes | Yes | Yes | 100.00 |
| Product recommended in guidelines (SER, EULAR, ACR, etc.) after failure of previous biological therapy | 7.48 | 1.58 | 8 | 1 | High | Yes | Yes | 96.39 | |
| Product directly backed by comparative clinical trials versus randomized active therapies and randomized versus other biological therapies | 8.54 | 0.85 | 9 | 1 | High | Yes | Yes | Yes | 98.80 |
| Product suitable for utilization in an elderly population | 6.78 | 1.56 | 7 | 2 | High | Yes | 80.72 | ||
| High rates of drug survival | 7.73 | 1.15 | 8 | 1 | High | Yes | Yes | 95.18 | |
| Pharmacology | |||||||||
| Product that has a differential structure, mechanism of action and therapeutic target | 7.52 | 1.46 | 8 | 1 | High | Yes | Yes | 95.18 | |
| Product origin (murine-human) | 6.66 | 1.82 | 7 | 2 | High | Yes | 78.31 | ||
| Half-life (in some cases it would preferably be longer and in others shorter) | 6.52 | 1.73 | 7 | 2 | High | Yes | 75.90 | ||
| Product that is associated with a low incidence of neutralizing antibodies | 7.63 | 1.30 | 8 | 1 | High | Yes | Yes | 98.80 | |
| Product that does not require a long-term increase in dose due to loss of efficacy (tolerance) | 7.61 | 1.46 | 8 | 1 | High | Yes | Yes | 95.18 | |
| Efficacy | |||||||||
| Product that shows efficacy in patients who have had no previous exposure to treatment with a synthetic DMARD | 7.00 | 1.35 | 7 | 1 | High | Yes | Yes | 84.34 | |
| Product that shows efficacy after an inadequate response to or failure of a synthetic DMARD | 7.96 | 0.96 | 8 | 0 | High | Yes | Yes | Yes | 98.80 |
| Product that shows efficacy after failure of another biological | 8.18 | 0.59 | 8 | 1 | High | Yes | Yes | Yes | 100.00 |
| Product effective in monotherapy | 7.70 | 0.96 | 8 | 1 | High | Yes | Yes | Yes | 97.59 |
| Product effective in early RA | 7.81 | 1.21 | 8 | 0 | High | Yes | Yes | 91.57 | |
| Product effective in established RA | 8.51 | 0.55 | 9 | 1 | High | Yes | Yes | Yes | 97.59 |
| Product with demonstrated efficacy in combination with MTX (or another DMARD) | 7.88 | 0.92 | 8 | 0 | High | Yes | Yes | Yes | 96.39 |
| Treatment accompanied by an improvement in inflammatory activity, according to established indices | 8.75 | 0.56 | 9 | 0 | High | Yes | Yes | Yes | 97.59 |
| Treatment accompanied by a reduction in the progression of joint damage | 8.81 | 0.43 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Treatment accompanied by an improvement in physical function | 8.66 | 0.57 | 9 | 1 | High | Yes | Yes | Yes | 98.80 |
| Treatment accompanied by an improvement in quality of life | 8.52 | 0.72 | 9 | 1 | High | Yes | Yes | Yes | 97.59 |
| Treatment that reduces the comorbidities associated with RA | 8.11 | 0.91 | 8 | 1 | High | Yes | Yes | Yes | 100.00 |
| Treatment effective in extra-articular manifestations | 7.99 | 0.88 | 8 | 0 | High | Yes | Yes | Yes | 98.80 |
| Treatment characterized by its rapid action (early clinical response) | 7.81 | 1.08 | 8 | 1 | High | Yes | Yes | 92.77 | |
| Treatment characterized by a high rate of remission | 8.86 | 0.35 | 9 | 0 | High | Yes | Yes | Yes | 98.80 |
| Treatment characterized by a long-term response | 8.77 | 0.48 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Drug showing evidence of efficacy in different immunological subgroups of RA patients | 7.76 | 1.12 | 8 | 1 | High | Yes | Yes | 89.16 | |
| Product that facilitates treatment adherence | 7.96 | 0.77 | 8 | 2 | High | Yes | 85.54 | ||
| Safety | |||||||||
| Safe product (overall) | 8.86 | 0.39 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Safe product (overall) compared with other biological DMARDs | 8.58 | 1.00 | 9 | 1 | High | Yes | Yes | Yes | 98.80 |
| Demonstrated long-term safety | 8.80 | 0.44 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Product with no history of generating changes in prenatal or postnatal development (although it is not recommended in pregnant or breastfeeding women, and contraception is even recommended) | 7.22 | 1.47 | 7 | 1 | High | Yes | Yes | 90.36 | |
| Product associated with few contraindications | 7.92 | 0.84 | 8 | 0 | High | Yes | Yes | Yes | 98.80 |
| Treatment with infrequent allergic reactions | 8.01 | 0.59 | 8 | 0 | High | Yes | Yes | Yes | 98.80 |
| Treatment with a low incidence of severe infections | 8.86 | 0.39 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Treatment that does not increase the incidence of tuberculosis | 8.49 | 0.89 | 9 | 1 | High | Yes | Yes | Yes | 97.59 |
| Product that does not reactivate hepatitis B virus | 8.14 | 1.08 | 8 | 1 | High | Yes | Yes | 98.80 | |
| Product that does not reactivate hepatitis C virus | 8.07 | 1.06 | 8 | 1 | High | Yes | Yes | 98.80 | |
| Product that does not reactivate human immunodeficiency virus infection | 7.49 | 1.48 | 8 | 1 | High | Yes | Yes | 92.77 | |
| Product that does not increase the incidence of progressive multifocal leukoencephalopathy | 7.32 | 1.60 | 8 | 1 | High | Yes | Yes | 90.36 | |
| Product that does not increase the incidence of malignant solid tumors | 8.73 | 0.52 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Product that does not increase the incidence of hematologic malignancies | 8.76 | 0.46 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Product that does not increase the incidence of cutaneous psoriasis | 7.14 | 1.26 | 7 | 1 | High | Yes | Yes | 93.98 | |
| Product that does not increase the induction of lupus | 7.23 | 1.06 | 7 | 1 | High | Yes | Yes | 91.57 | |
| Product that is safe in patients with cardiovascular disease | 8.05 | 0.56 | 8 | 0 | High | Yes | Yes | Yes | 100.00 |
| Product that is safe in patients with interstitial lung disease | 8.05 | 0.64 | 8 | 0 | High | Yes | Yes | Yes | 100.00 |
| Product that is safe in patients with demyelinating disease | 7.66 | 1.13 | 8 | 1 | High | Yes | Yes | 90.36 | |
| Treatment that does not alter the immune response to vaccines | 7.67 | 1.21 | 8 | 1 | High | Yes | Yes | 87.95 | |
| Product compatible with and having no interaction with the standard treatments of RA: MTX, sulfasalazine, hydroxychloroquine, or leflunomide, nonsteroidal anti-inflammatory drugs, corticosteroids, etc. | 8.16 | 0.80 | 8 | 1 | High | Yes | Yes | Yes | 97.59 |
| Product that is safe with a sufficient dosing interval | 7.04 | 1.58 | 7 | 1 | High | Yes | Yes | 85.54 | |
| Associated with a decrease in mortality | 8.07 | 0.93 | 8 | 1 | High | Yes | Yes | Yes | 100.00 |
| Treatment produces no change in the atherogenic lipid profile | 7.01 | 1.43 | 7 | 1 | High | Yes | Yes | 90.36 | |
| Treatment produces no change in the liver enzymes | 7.58 | 0.95 | 8 | 1 | High | Yes | Yes | Yes | 98.80 |
| Treatment produces no change in blood counts | 7.81 | 0.63 | 8 | 1 | High | Yes | Yes | Yes | 98.80 |
| Product in which the excipients avoid potential intolerances | 6.93 | 1.45 | 7 | 1 | High | Yes | Yes | 89.16 | |
| In self-administration, the treatment is not accompanied by pain or reaction at the injection site (when administered subcutaneously [SC]) | 7.57 | 1.14 | 8 | 1 | High | Yes | Yes | 87.95 | |
| Treatment with a low rate of infusion reactions (when administered intravenously [IV]) | 7.94 | 0.79 | 8 | 0 | High | Yes | Yes | Yes | 98.80 |
| Product with a low incidence of adverse events | 8.57 | 0.91 | 9 | 1 | High | Yes | Yes | Yes | 98.80 |
| Product with a low incidence of severe adverse events | 8.93 | 0.26 | 9 | 0 | High | Yes | Yes | Yes | 100.00 |
| Administration of the product | |||||||||
| It has alternative routes of administration (IV and SC). | 6.48 | 1.77 | 7 | 2 | High | Yes | 73.49 | ||
| Does not require a loading dose | 5.70 | 1.99 | 6 | 3 | Intermediate | 48.19 | |||
| Does not require a long perfusion time | 6.61 | 1.68 | 7 | 2 | High | 80.72 | |||
| Convenient conditions and period of stability of the reconstituted drug | 6.37 | 1.88 | 7 | 1 | High | Yes | 84.34 | ||
| Presented in a simple and ergonomic vial | 6.90 | 1.43 | 7 | 2 | High | Yes | 79.52 | ||
| Absence of latex in the vial | 5.84 | 1.68 | 6 | 2 | Intermediate | 59.04 | |||
| Costs | |||||||||
| The direct costs of treatment are low | 7.88 | 0.92 | 8 | 0 | High | Yes | Yes | Yes | 96.39 |
| Cost should be low due to preparation in pharmacy (optimization of vials) | 7.11 | 1.53 | 7 | 1 | High | Yes | Yes | 85.54 | |
| Low cost of the administration of the drug | 7.59 | 1.10 | 8 | 1 | High | Yes | Yes | 91.57 | |
| Low cost of monitoring | 7.23 | 1.22 | 7 | 1 | High | Yes | Yes | 91.57 | |
| Demonstrates a favorable incremental cost-effectiveness | 7.86 | 0.95 | 8 | 1 | High | Yes | Yes | Yes | 98.80 |
| Has the support of pharmacoeconomic studies versus other biological alternatives | 7.14 | 1.52 | 7 | 1 | High | Yes | Yes | 86.75 | |
| Is accompanied by a reduction in work disability | 7.98 | 1.04 | 8 | 1 | High | Yes | Yes | 96.39 | |
| There is a reduction in indirect costs | 7.41 | 1.15 | 7 | 1 | High | Yes | Yes | 95.18 | |
| It has been shown to have a flexible regimen that enables reducing or prolonging the dose. The interval in patients in remission (dose optimization) | 7.93 | 1.25 | 8 | 1 | High | Yes | Yes | 97.59 | |
| The presentation coincides with the therapeutic regimen | 7.53 | 1.50 | 8 | 1 | High | Yes | Yes | 89.16 | |
ACR, American College of Rheumatology; DMARD, disease-modifying antirheumatic drug; EULAR, European League Against Rheumatism; IQR, interquartile range; IV, intravenously; MTX, methotrexate; RA, rheumatoid arthritis; SC, subcutaneously; SD, standard deviation; SER, Spanish Society of Rheumatology.
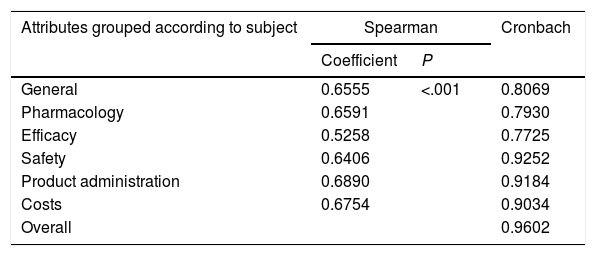
The Spearman correlation coefficients between the 2 rounds grouped according to subject and Cronbach's alpha are shown in Table 2.
Spearman Correlation Coefficients and Cronbach Alpha.
| Attributes grouped according to subject | Spearman | Cronbach | |
|---|---|---|---|
| Coefficient | P | ||
| General | 0.6555 | <.001 | 0.8069 |
| Pharmacology | 0.6591 | 0.7930 | |
| Efficacy | 0.5258 | 0.7725 | |
| Safety | 0.6406 | 0.9252 | |
| Product administration | 0.6890 | 0.9184 | |
| Costs | 0.6754 | 0.9034 | |
| Overall | 0.9602 | ||
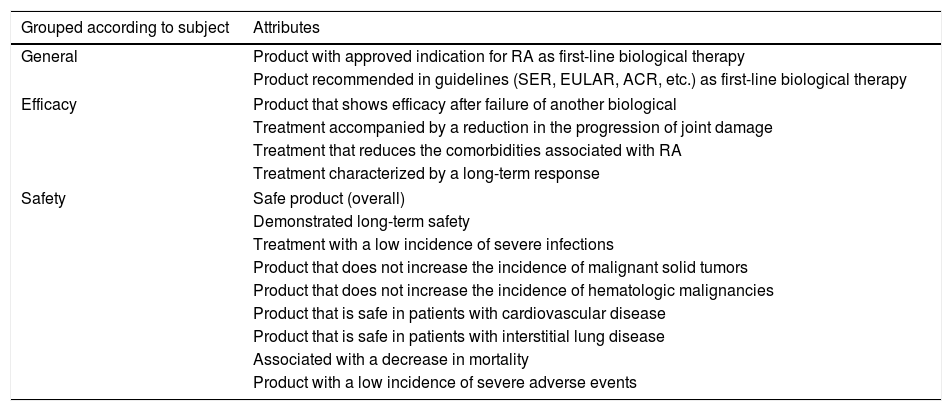
Fifteen attributes had an agreement of 100% and were considered to be those with the greatest importance (Table 3).
Attributes With an Agreement of 100% in the 2 Delphi Rounds of the Study.
| Grouped according to subject | Attributes |
|---|---|
| General | Product with approved indication for RA as first-line biological therapy |
| Product recommended in guidelines (SER, EULAR, ACR, etc.) as first-line biological therapy | |
| Efficacy | Product that shows efficacy after failure of another biological |
| Treatment accompanied by a reduction in the progression of joint damage | |
| Treatment that reduces the comorbidities associated with RA | |
| Treatment characterized by a long-term response | |
| Safety | Safe product (overall) |
| Demonstrated long-term safety | |
| Treatment with a low incidence of severe infections | |
| Product that does not increase the incidence of malignant solid tumors | |
| Product that does not increase the incidence of hematologic malignancies | |
| Product that is safe in patients with cardiovascular disease | |
| Product that is safe in patients with interstitial lung disease | |
| Associated with a decrease in mortality | |
| Product with a low incidence of severe adverse events | |
ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; RA, rheumatoid arthritis; SER, Spanish Society of Rheumatology.
The purpose of this article is to make an effort in support of personalized medicine to define the ideal drug for RA patients who need to receive a bDMARD after the failure of a first bDMARD or of a sDMARD. In the absence of biological markers that can define the most suitable drug for a patient, studies of this type may be helpful in the attempt to achieve greater efficacy in the use of agents with a high impact.
The participants in this project were rheumatologists from different Spanish autonomic communities that accounted for 91.45% of the total population. The number of participating experts was around 10% of the rheumatologists practicing in Spanish hospitals. There was a heterogeneity of 50% and, at the 95% confidence level, the margin of sampling error would be ±10.19%.
The Delphi process was chosen as it is a widely accepted scientific method that facilitates reaching consensus.28 It also enables the participation of a large number of experts through the utilization of telematics.
The Spearman correlation is acceptable and the P value is very small. Thus, it can be considered that there was agreement between the 2 Delphi rounds. On the other hand, the Cronbach alphas were always more than 0.7, demonstrating the internal consistency of the questionnaire.
As far as we know, this is the first endeavor to attempt to establish the ideal attributes to look for in choosing a bDMARD. From the results obtained, we can observe that it appears that, for the group of specialists, it was important that there be an approved indication for first-line treatment for RA, that there be a varied range of mechanisms of action and therapeutic targets, and that the drug did not generate neutralizing antibodies or need an increase in the dose to achieve efficacy. The availability of efficacy data on certain immunological subgroups of patients such as, for example, the presence or absence of anti-cyclic citrullinated peptide antibodies, is also widely supported in the study. Aspects like safety are also highly valued by the group of specialists.
In questions like the administration of the drug is where we find a wider dispersion in the responses. The need for a loading dose of the agent was, specifically, the aspect that was considered the least important in the study.
The strength of the study lies, in our opinion, in the originality of attempting to define, by means of a Delphi study, the ideal characteristics for choosing a bDMARD, which are those displayed in Table 3. Studies should be conducted specifically on each of these attributes to demonstrate that its presence is associated with a greater efficacy of each drug. However, carrying out clinical studies of this type would be costly and difficult, and it does not seem feasible that they come to be performed. Therefore, this type of Delphi study can contribute to helping clinicians in the choice of the best agent for each patient.
In conclusion, we can point out that:
- –
There was a high level of agreement on the attributes selected from the literature.
- –
The majority of the experts considered that 75 of the 77 aspects (97.40%) were of high importance, whereas none of them were assigned a low level of importance.
- –
There are 15 attributes ratified by 100% of the specialists, which could define the ideal bDMARD after the failure of a sDMARD or another bDMARD. All of them refer to general aspects, efficacy and safety.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis project was financed by Bristol-Myers Squibb, with the support of Scientia Salus, who provided the technical secretariat.
Conflict of InterestThe authors declare they have no conflicts of interest.
Participants and authors, in alphabetical order by first surname:
Abad Hernandez, MA, H. Virgen del Puerto (Extremadura)
Alcalde Villar, M., H. U, Severo Ochoa (Community of Madrid)
Álvarez Pio, A., H. G. de La Palma (Canary Islands)
Aragón Diez, A., H. U. de Getafe (Community of Madrid)
Atanes Sandoval, A. D., C. H. U. A Coruña (Galicia)
Bernad Pineda, M., C. U. La Paz (Community of Madrid)
Blanco Rodríguez, J. S., C. H. U. de Santiago (Galicia)
Bustabad Reyes, S., C. H. U. de Canarias (Canary Islands)
Caliz Caliz, R., C. H. R. Virgen de las Nieves (Andalusia)
Calvo Alén, J., H. de Sierrallana (Cantabria)
Campos Fernández, C., H. G. U. de Valencia (Valencian Community)
Caro Fernandez, N., H. G. Ntra. Sra. del Prado (Castile-La Mancha)
Carrasco Cubero, M. C., C. H. U. de Badajoz (Extremadura)
Castaño Sánchez, M., H. C. U. Virgen de la Arrixaca (Region of Murcia)
Castro Oreiro, S., H. U. Joan XXIII de Tarragona (Catalonia)
Chalmeta Vermejo, I., H U. i Politècnic La Fe (Valencian Community)
Chamizo Carmona, E., C. H. del Área de Salud de Mérida (Extremadura)
Cobo Ibañez, M. T., H. U. Infanta Sofía (Community of Madrid)
Conesa Mateos, M. A., H. C. U. de Valencia (Valencian Community)
Corominas Macias, H., H. de Sant Joan Despí Moisés Broggi (Catalonia)
Díaz Torne, C., H. de la Santa Creu i Sant Pau (Catalonia)
Fernández Domínguez, L., C. H. U. de Ourense (Galicia)
Fernández Nebro, A., C. H. R. de Málaga (Andalusia)
Fernández Ortiz, A. M., H. G. de Almansa (Castile-La Mancha)
Fernández Prada, M., H. U. de Guadalajara (Castile-La Mancha)
Fernández-Llanio Comella, N., H. Arnau de Vilanova (Valencian Community)
Ferraz Amaro, I. A., C. H. U. de Canarias (Canary Islands)
Ferrer Gonzalez, M. A., C. H. R. Virgen de las Nieves (Andalusia)
Francisco Hernández, F. M., C. H. U. de Gran Canaria Dr. Negrín (Canary Islands)
García Gonzalez, J., H. U. 12 de Octubre (Community of Madrid)
García Llorente, J. F., H. de Basurto (Basque Country)
García Aparicio, A. M., C. H. de Toledo (Castile-La Mancha)
García Feito, J., C. H. de E. Torrecárdenas (Andalusia)
García Porrua, C., H. U. Lucus Augusti (Galicia)
García Vadillo, J.A., H. U. de La Princesa (Community of Madrid)
García Vivar, M. L., H. de Basurto (Basque Country)
García-Villalba Sánchez, F., H. G. U. Reina Sofía (Region of Murcia)
Garmendia Sánchez, M. E., H. U. de Cruces (Basque Country)
Gomez Centeno, A. D., H. de Sabadell (Catalonia)
González Álvarez, B. C., H. U. Ntra. Sra. de Candelaria (Canary Islands)
González Gómez, M. L., H. El Escorial (Community of Madrid)
Gonzalez Hernandez, M. T., C. H. Gregorio Marañón (Community of Madrid)
Gutierrez Polo, R., C. H. de Navarra (Chartered Community of Navarre)
Hernandez Beriain, J. A., C. H. U. Insular-Materno Infantil (Canary Islands)
Hidalgo Calleja, M. C., C. A. U. de Salamanca (Castile and León)
Maceiras Pan, F. J., C. H. U. de Vigo (Galicia)
Magallares López, B., H. de la Santa Creu i Sant Pau (Catalonia)
Martinea Taboada, V., H. U. Marqués de Valdecilla (Cantabria)
Martínez Gonzales, O., C. A. U. de Salamanca (Castile and León)
Martinez López, J. A., H. U. Fundación Jiménez Díaz (Community of Madrid)
Mateo Soria, M. L., H. U. Germans Trias y Pujol (Catalonia)
Miguelez Sanchez, J. R., H. U. de Móstoles (Community of Madrid)
Minguez Blasco, S., Althaia Xarxa de Manresa (Catalonia)
Minguez Vega, M., H. U. San Juan de Alicante (Valencian Community)
Morcillo Valle, M., H. El Escorial (Community of Madrid)
Morell Hita, J. L., H. Ramón y Cajal (Community of Madrid)
Moreno Martinez, M. J., H. Rafael Mendez (Murcia)
Moreno Gil, M. P., C. H. de Cáceres (Extremadura)
Moreno Morales, J., H. G. U. Santa Lucía (Region of Murcia)
Moya Alvarado, P., H. de Sant Pau i Santa Tecla (Catalonia)
Muñoz Fernandez, S., H. U. Infanta Sofía (Community of Madrid)
Muñoz Jiménez, A., C. H. regional Virgen del Rocío (Andalusia)
Narvaez García, F. J., H. U. de Bellvitge (Catalonia)
Noguera Pons, J. R., H. G. U. de Elche (Valencian Community)
Oliva Ruiz, M. R., H. Comarcal del Noroeste (Region of Murcia)
Paredes Gonzalez-Alto, S. R., H. U. de Sant Joan de Reus (Catalonia)
Peiteado López, D., C. U. La Paz (Community of Madrid)
Pérez Sandobal, T., C. Universitario de León (Castile and León)
Pérez Venegas, J. J., H. de Jerez de la Frontera (Andalusia)
Ponce Fernández, A., H. G. de Granollers (Catalonia)
Raya Álvarez, E. G. C., H. R. Virgen de las Nieves (Andalusia)
Reyner Echevarria, P., H. Santa Caterina (Catalonia)
Rivera Redondo, J., C. H. Gregorio Marañón (Community of Madrid)
Rodriguez Heredia, J. M., H. U. de Getafe (Community of Madrid)
Rodríguez Montero, S. A., C. H. Virgen de Valme (Andalusia)
Romero Yuste, S. M., C. H. U. de Pontevedra (Galicia)
Rubio Moreno, E., C. H. Regional Virgen del Rocío (Andalusia)
Rueda Cid, A., H. G. U. de Valencia (Valencian Community)
Ruiz Esquide, V., H. Clínic i Provincial de Barcelona (Catalonia)
Ruíz Jimeno, M. T., H. Sierrallana (Cantabria)
Salvador Alarcon, G., H. U. Mútua de Terrassa (Catalonia)
Urena Garnica, I. C., C. H. Regional de Málaga (Andalusia)
Valls Pascual, E., H. U. Doctor Peset (Valencian Community)
The members of the ACORDAR 2015 Working Group are listed in Appendix A.
Please cite this article as: Muñoz-Fernández S, Bustabad Reyes MS, Calvo Alén J, Castaño Sánchez M, Chamizo Carmona E, Corominas H, et al. Atributos del fármaco antirreumático modificador de la enfermedad biológico en las primeras líneas de tratamiento de la artritis reumatoide. Proyecto ACORDAR 2015. Reumatol Clin. 2018;14:90–96.