To describe the prevalence of extra-articular disease (uveitis, psoriasis and inflammatory bowel disease [IBD]), in a cohort of patients with spondyloarthritis (SpA).
Patients and methodsAQUILES is an observational, prospective and multicentric study of three cohorts of patients with one of the following immune-mediated inflammatory diseases (IMID): SpA, psoriasis, or IBD. In the present cohort, patients ≥18 years of age with SpA were enrolled from Rheumatology clinics. The main objective was to assess the coexistence of these diseases and of uveitis, based on the patients’ clinical history up to the study entry.
ResultsA total of 601 patients with SpA (men: 63.1%; women: 36.9%) were enrolled. The specific diagnoses were: ankylosing spondylitis (55.1%), psoriatic arthritis (25.1%), undifferentiated spondyloarthritis (16.1%), enteropathic arthritis (2.5%), and others (1.3%). In 43.6% (95% CI: 39.7–47.6) of the patients, at least one of the three abovementioned diseases was encountered, predominantly psoriasis (prevalence 27.8%, 95% CI: 24.4–31.5), uveitis (13.6%, 95% CI: 11.1–16.6) and IBD (5.1%, 95% CI: 3.7–7.2). In patients with ankylosing spondylitis the proportion of other disease was 25.3% (IBD: 3.9%, psoriasis: 5.4%, uveitis: 19.0%) whilst it was 94.7% in psoriatic arthritis, due to the presence of psoriasis (94.0%). The coexistence of these diseases was associated with age, female gender and the presence of other extra-articular manifestations associated with SpA.
ConclusionsExtra-articular disease in patients with SpA is common and, in this study, it was associated to age, female gender and the presence of other SpA-related extra-articular manifestations.
Evaluar la prevalencia de enfermedad extraarticular (uveítis, psoriasis y enfermedad inflamatoria intestinal [EII]) en una cohorte de pacientes con espondiloartritis (EsA).
Pacientes y métodosAQUILES es un estudio observacional, prospectivo y multicéntrico, en 3 cohortes de pacientes con una de las siguientes enfermedades inflamatorias mediadas por inmunidad (EIMI): EsA, psoriasis y EII. En la cohorte presente se incluyó a pacientes ≥18 años con EsA atendidos en consultas hospitalarias de Reumatología. El objetivo principal fue evaluar la coexistencia de estas enfermedades y de uveítis, valorada sobre la base de la historia clínica del paciente hasta el momento de entrar en el estudio.
ResultadosSe incluyó a 601 pacientes con EsA (varones: 63,1%, mujeres: 36,9%). Los diagnósticos fueron: espondilitis anquilosante (55,1%); artritis psoriásica (25,2%); espondiloartritis indiferenciada (16,1%); artritis enteropática (2,5%), y otros (1,3%). En el 43,6% (IC del 95%, 39,7–47,6) coexistió al menos una de las 3 enfermedades mencionadas, predominando psoriasis (prevalencia: 27,8%, IC del 95%, 24,4–31,5), uveítis (13,6%, IC del 95%, 11,1–16,6) y EII (5,1%, IC del 95%, 3,7–7,2). En pacientes con espondilitis anquilosante, la prevalencia fue del 25,3% (EII: 3,9%, psoriasis: 5,4%, uveítis: 19,0%) y en pacientes con artritis psoriásica fue del 94,7%, debido a la presencia de psoriasis (94,0%). La coexistencia de estas manifestaciones se asoció a mayor edad, sexo femenino y presencia de otras manifestaciones extraarticulares de las EsA distintas de las estudiadas.
ConclusionesLa enfermedad extraarticular en pacientes con EsA es frecuente y en este estudio se asoció a la edad, el sexo femenino y la presencia de otras manifestaciones extraarticulares de EsA.
Spondyloarthritis (SpA) is a group of chronic inflammatory rheumatic diseases whose common characteristic is the involvement of the axial skeleton, although in different forms and progression it may affect peripheral joints, with a prevalence of approximately 1.5%–2% of the general population.1–4 Furthermore, SpA often have extra-articular manifestations such as ocular, mucocutaneous and cardiovascular and renal lesions, the detection of which is important in order to plan treatment and follow-up.5–9
The pathogenic features that distinguishes this group of rheumatic diseases are altered levels of cytokines, sharing some traits with other pathogenic diseases, such as psoriasis and inflammatory bowel disease (IBD).10 While psoriasis and uveitis may be considered extra-intestinal manifestations of SpA, both these and IBD occur or evolve independently to SpA and therefore this group of diseases is often referred to as “immune-mediated inflammatory diseases” (IMID).10–12 It has been observed that both genetic and environmental factors play a decisive role in the development of these conditions.13,14
Given the implications for treatment and monitoring of patients with SpA that present extra-articular manifestations such as uveitis or psoriasis, or other associated diseases such as IBD, it is of interest to describe their prevalence in our environment. The ACHILES study is a 2 year prospective observational study following 3 cohorts of patients with IMID (SpA, psoriasis or IBD), designed to assess the prevalence and incidence of these diseases in patients with SpA, psoriasis or IBD. The objective of this study was to describe the prevalence of the 3 diseases (psoriasis, uveitis and IBD) in patients with SpA at the time of recruitment into the study. For the purposes of the study, all 3 events were grouped under the concept of IMID, although both psoriasis and uveitis can be considered as extra-articular manifestations of SpA.
Materials and MethodsACHILES Study DesignThe ACHILES study was planned as 3 independent cohorts of patients (SpA, psoriasis and IBD), defined by the main diagnosis at the time of enrollment. The study was conducted in 15 Spanish hospitals with the participation of three departments: Rheumatology, Dermatology and Gastroenterology. Each department included patients aged ≥18 years diagnosed with one of the following IMID: SpA (including ankylosing spondylitis, psoriatic arthritis, undifferentiated spondyloarthritis, enteropathic arthritis or other), psoriasis and IBD, thus constituting the 3 independent cohorts. Patients with 2 or more diseases followed by 2 or more specialists could only be included into one of the cohorts. Patients were followed up for a period of 2 years by the same specialist who included them in the study.
The objective of this work is to describe the baseline characteristics of the cohort of patients with a diagnosis of SpA at the time of inclusion into the study and the prevalence of psoriasis, uveitis and IBD (primary endpoint). These patients were recruited from the rheumatology clinics of the participating hospitals.
Patient Selection and Data CollectionIn this cohort study researchers included adult patients (age ≥18 years) with an established or de novo diagnosis of SpA. The diagnosis of SpA was made in all cases by a rheumatologist. Patients who, in the opinion of the investigator, presented any circumstance that would prevent their proper follow up for 2 years were excluded. The protocol was approved by the Ethics Committees of the participating centers and was conducted in compliance with the standards of good clinical practice.
Researchers could include patients with a previous diagnosis of SpA by random assignment or consecutively. In the first case, a list of patients with SpA who had been seen in rheumatology in the 6 past months prior to study start was generated, and on that basis patients were randomly selected. In the SpA cohort, however, most patients already diagnosed (∼90%) were included consecutively. Patients with a new diagnosis of SpA (seen de novo in Rheumatology) who met criteria were included consecutively into the study.
The inclusion period lasted from March 2008 until December 2010. The information was gathered from personal interviews with the patient and review of the clinical history, following a protocol agreed upon by the 3 specialties. The diagnosis of each disease was made on the basis of the diagnoses listed in the medical record until the time of study entry and to determine prevalence at baseline, the diseases associated with flares (Uveitis) were counted as a case not only when they were present at baseline, but also when the patient presented a flare in the past, reflected as such in the medical record. In the study no laboratory test or radiological test was performed and baseline data and diagnoses were collected from medical records.
Statistical AnalysisThe sample size calculation was based on the primary endpoint, i.e. the prevalence of these diseases in each of the cohorts. For each cohort of patients (SpA, IBD and psoriasis), an expected prevalence for each disease of 3%, a confidence level of 95% and an accuracy of 1% was assumed. The sample size was calculated, for each cohort, at 1075 patients. Recruitment was lower than expected and the final sample of the SpA cohort (601 patients) allows an accuracy of 3% for the prevalence found.
Continuous variables were described as mean±standard deviations, and medians and interquartile ranges (IQR). Categorical variables were presented using frequencies and percentages. The population was divided according to various demographic and clinical variables to assess differences in the prevalence of various diseases. These differences were analyzed with the chi-square test. The Student's t test of for independent groups was used to compare means of the 2 groups. When categorical variables were related, the proof of the chi-square test was used. Subsequently, a logistic regression model was adjusted to study what demographic and clinical variables were independently associated with the coexistence of uveitis, psoriasis and IBD, presenting odds ratios (OR) and 95% confidence interval (CI). In all the contrasts, the null hypothesis was rejected when the alpha error was less than 0.05. For processing data, the SPSS 15.0 software package was employed.
ResultsBaseline Characteristics of Patients601 patients with SpA were included in the study, 379 men (63.1%) and 222 women (36.9%). Their mean age±SD was 47.9±12.9 years. Only 79 patients (13.1%) were newly diagnosed; the majority (86.9%) had an established diagnosis of SpA. In the latter, the median duration of disease was 8.7 years (IQR 25–75: 3.3–16.5). Baseline diagnoses of SpA were: ankylosing spondylitis: 55.1% (n=332), psoriatic arthritis: 25.1% (n=151), undifferentiated spondyloarthritis: 16.1% (n=97), enteropathic arthritis: 2.5% (n=15) and other 1.3% (n=8). 15% presented extra-articular manifestations associated to SpA (other than those studied [uveitis, psoriasis or IBD], mainly conjunctivitis [5.0%], cystitis [2.3%], nail hyperkeratosis [6.0%], balanitis [1.5%] and a lower prevalence of 1%, pulmonary fibrosis, aortic and renal amyloidosis). 19.5% had a family history of SpA. The most frequent comorbidities were depression (8.3%), anemia (5.3%), dermatitis and other skin lesions (8.0%) as well as cardiovascular risk factors (obesity [20.4%], smoking [26.6%], hypertension [22.1%], hypercholesterolemia [16.0%] and diabetes mellitus [6.0%]).
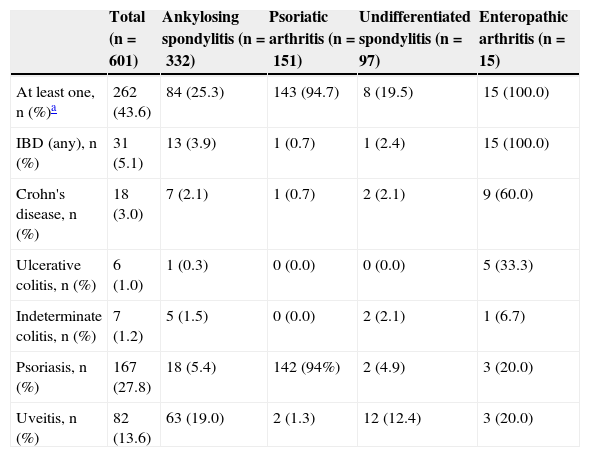
Prevalence of Extra-articular Immune Mediated Inflammatory Diseases (Psoriasis, Uveitis and Inflammatory Bowel Disease)In the SpA cohort, 262 patients had at least one of these IMID, with a prevalence of 43.6% (95% CI, 39.7–47.6). The most common IMID was psoriasis, present in 167 patients (prevalence: 27.8%, 95% CI, 24.4–31.5); most of them showed plaque psoriasis (88.6%). A total of 82 patients had uveitis (prevalence: 13.6%, 95% CI, 11.1–16.6). Finally, 31 patients were diagnosed with IBD (prevalence: 5.1% [95% CI, 3.7–7.2]), with the following distribution: Crohn's disease: 18 patients (3.0%, 95% CI, 1.9–4.7), ulcerative colitis, 6 patients (1.0%, 95% CI, 0.5–2.2) and undifferentiated colitis: 7 patients (1.2%, 95% CI, 0.6–2.4). In 18 patients (3.0%; 95% CI, 1.9–4.7), 2 IMID coexisted concomitantly to SpA.
The prevalence of the major diagnostics is presented in Table 1. In patients with ankylosing spondylitis, uveitis was the most common extraarticular IMID (prevalence 19.0% [95% CI, 15.1–23.5]). Virtually all patients with psoriatic arthritis had psoriasis (prevalence 94.7% [95% CI, 89.9–97.3]). When we excluded patients with psoriatic arthritis, the prevalence of psoriasis in patients with other types of SpA was 5.6% (95% CI, 3.8–8.1). Moreover, 100% of the patients with enteropathic arthritis presented IBD and, by excluding patients with enteropathic arthritis, the prevalence of IBD in patients with the rest of SpA was 2.7% (95% CI, 1.7–4.4) (Table 1).
Percentage of Patients With Extra-articular Immune Mediated Inflammatory Disease (Psoriasis, Inflammatory Bowel Disease and Uveitis) Stratified by Type of Spondyloarthritis.
| Total (n=601) | Ankylosing spondylitis (n=332) | Psoriatic arthritis (n=151) | Undifferentiated spondylitis (n=97) | Enteropathic arthritis (n=15) | |
|---|---|---|---|---|---|
| At least one, n (%)a | 262 (43.6) | 84 (25.3) | 143 (94.7) | 8 (19.5) | 15 (100.0) |
| IBD (any), n (%) | 31 (5.1) | 13 (3.9) | 1 (0.7) | 1 (2.4) | 15 (100.0) |
| Crohn's disease, n (%) | 18 (3.0) | 7 (2.1) | 1 (0.7) | 2 (2.1) | 9 (60.0) |
| Ulcerative colitis, n (%) | 6 (1.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 5 (33.3) |
| Indeterminate colitis, n (%) | 7 (1.2) | 5 (1.5) | 0 (0.0) | 2 (2.1) | 1 (6.7) |
| Psoriasis, n (%) | 167 (27.8) | 18 (5.4) | 142 (94%) | 2 (4.9) | 3 (20.0) |
| Uveitis, n (%) | 82 (13.6) | 63 (19.0) | 2 (1.3) | 12 (12.4) | 3 (20.0) |
The table has excluded 8 patients with other diagnoses.
IBD: inflammatory bowel disease.
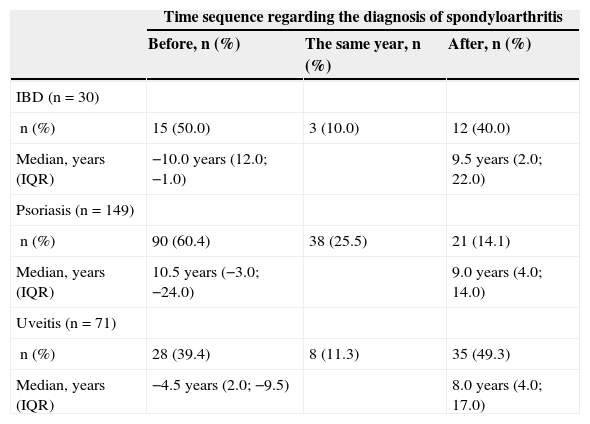
The chronology of diagnoses is described in Table 2. The diagnosis of IBD was prior to that of SpA in 50% of the cases, with a median of 10 years earlier. In 60% of the patients with psoriasis, this was diagnosed before SpA (median 10.5 years earlier) and only 14% had been diagnosed later. 39% of the uveitis were diagnosed prior to SpA (median: 4.5 years earlier).
Time From Diagnosis of Spondyloarthritis to That of Other Diseases and Median (IQR 25–75) Temporary Difference Between the Two Diagnoses.
| Time sequence regarding the diagnosis of spondyloarthritis | |||
|---|---|---|---|
| Before, n (%) | The same year, n (%) | After, n (%) | |
| IBD (n=30) | |||
| n (%) | 15 (50.0) | 3 (10.0) | 12 (40.0) |
| Median, years (IQR) | −10.0 years (12.0; −1.0) | 9.5 years (2.0; 22.0) | |
| Psoriasis (n=149) | |||
| n (%) | 90 (60.4) | 38 (25.5) | 21 (14.1) |
| Median, years (IQR) | 10.5 years (−3.0; −24.0) | 9.0 years (4.0; 14.0) | |
| Uveitis (n=71) | |||
| n (%) | 28 (39.4) | 8 (11.3) | 35 (49.3) |
| Median, years (IQR) | −4.5 years (2.0; −9.5) | 8.0 years (4.0; 17.0) | |
The number of cases and the percentage of each disease are presented. The date of diagnosis in a patient with inflammatory bowel disease, 18 with psoriasis and 11 with uveitis were unavailable.
IBD: inflammatory bowel disease; IQR, interquartile range 25–75.
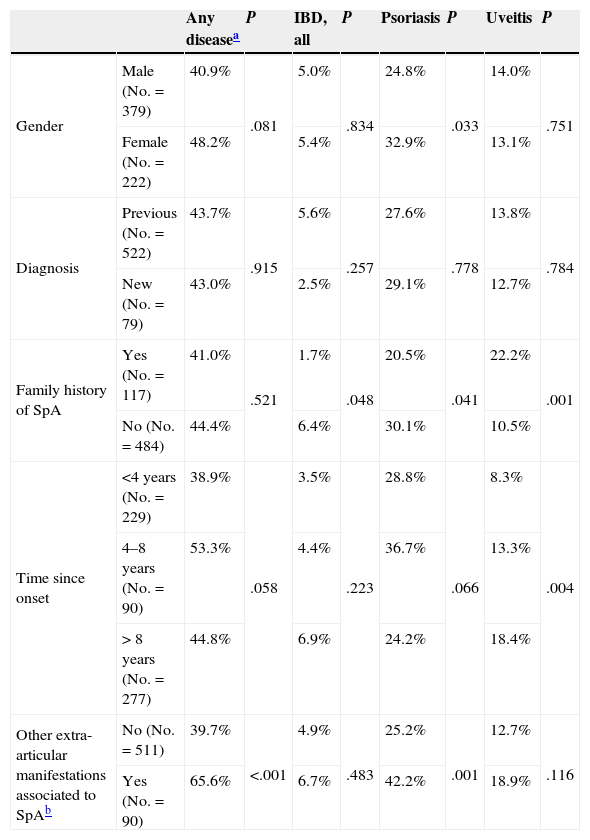
Prevalence in different subgroups. The prevalence of each diagnosis per subgroup is shown in Table 3. Overall, the prevalence was numerically higher in women due to a significantly higher prevalence of psoriasis (32.9% vs 24.8% in women in men, P=.033). No differences were seen in patients with a diagnosis of established or de novo SpA. The overall prevalence of uveitis, psoriasis and IBD was also significantly higher in patients with the presence of other extraarticular manifestations associated to SpA different than those studied (65.6% versus 39.7% in patients without other extra-articular manifestations associated to SpA, P<.001). The prevalence of IBD was similar across subgroups analyzed. Psoriasis was more prevalent in women, in patients with no family history of SpA and patients with other extra-articular manifestations associated with SpA. Finally, uveitis was more frequent in patients with a longer history of the disease and in patients with a family history of SpA (Table 3).
Prevalence of Extraarticular Disease per Each Subgroup of Patients.
| Any diseasea | P | IBD, all | P | Psoriasis | P | Uveitis | P | ||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male (No.=379) | 40.9% | .081 | 5.0% | .834 | 24.8% | .033 | 14.0% | .751 |
| Female (No.=222) | 48.2% | 5.4% | 32.9% | 13.1% | |||||
| Diagnosis | Previous (No.=522) | 43.7% | .915 | 5.6% | .257 | 27.6% | .778 | 13.8% | .784 |
| New (No.=79) | 43.0% | 2.5% | 29.1% | 12.7% | |||||
| Family history of SpA | Yes (No.=117) | 41.0% | .521 | 1.7% | .048 | 20.5% | .041 | 22.2% | .001 |
| No (No.=484) | 44.4% | 6.4% | 30.1% | 10.5% | |||||
| Time since onset | <4 years (No.=229) | 38.9% | .058 | 3.5% | .223 | 28.8% | .066 | 8.3% | .004 |
| 4–8 years (No.=90) | 53.3% | 4.4% | 36.7% | 13.3% | |||||
| > 8 years (No.=277) | 44.8% | 6.9% | 24.2% | 18.4% | |||||
| Other extra-articular manifestations associated to SpAb | No (No.=511) | 39.7% | <.001 | 4.9% | .483 | 25.2% | .001 | 12.7% | .116 |
| Yes (No.=90) | 65.6% | 6.7% | 42.2% | 18.9% |
IBD: inflammatory bowel disease; SpA: spondyloarthritis.
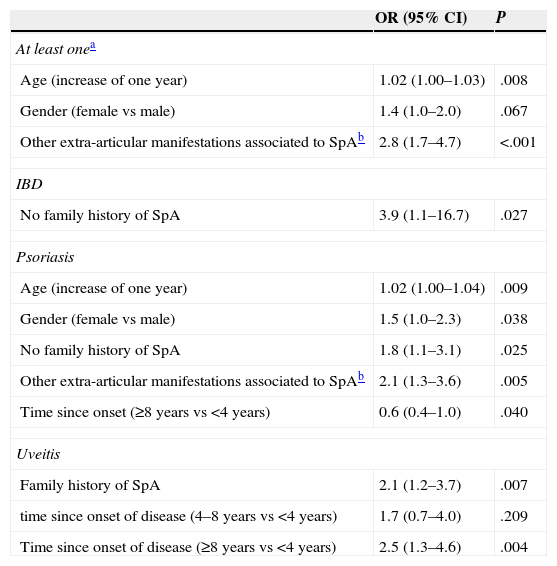
Multivariate analysis. To assess the variables related to the presence of the 3 diseases combined, multivariate analysis that included the variables age, gender, prior or de novo diagnosis, family history of SpA, duration (<4 years, 4–8 years, >8 years was performed) and the presence of extra-articular manifestations associated with SpA. In the resulting model, the overall prevalence of IMID is independently associated with age (OR=increase of 1.02 per year of age), female gender (OR=1.4 with respect to males) and the presence of other extra-articular manifestations associated to SpA different to those studied (OR=2.8) (Table 4). Models were later performed for each of the diseases studied. IBD is associated with the absence of family history of SpA. Psoriasis is associated with age, female gender, absence of family history of SpA, presence of extra-articular manifestations associated with SpA and shorter evolution of SpA. Finally, uveitis was associated independently with a greater time since onset of SpA and family history of SpA.
Variables Associated With the Presence of Extra-articular Disease: Multivariable Analysis.
| OR (95% CI) | P | |
|---|---|---|
| At least onea | ||
| Age (increase of one year) | 1.02 (1.00–1.03) | .008 |
| Gender (female vs male) | 1.4 (1.0–2.0) | .067 |
| Other extra-articular manifestations associated to SpAb | 2.8 (1.7–4.7) | <.001 |
| IBD | ||
| No family history of SpA | 3.9 (1.1–16.7) | .027 |
| Psoriasis | ||
| Age (increase of one year) | 1.02 (1.00–1.04) | .009 |
| Gender (female vs male) | 1.5 (1.0–2.3) | .038 |
| No family history of SpA | 1.8 (1.1–3.1) | .025 |
| Other extra-articular manifestations associated to SpAb | 2.1 (1.3–3.6) | .005 |
| Time since onset (≥8 years vs <4 years) | 0.6 (0.4–1.0) | .040 |
| Uveitis | ||
| Family history of SpA | 2.1 (1.2–3.7) | .007 |
| time since onset of disease (4–8 years vs <4 years) | 1.7 (0.7–4.0) | .209 |
| Time since onset of disease (≥8 years vs <4 years) | 2.5 (1.3–4.6) | .004 |
IBD: inflammatory bowel disease; IMID: immune-mediated inflammatory disease; SpA: spondylitis; CI: confidence interval; CI: confidence interval; OR: odds ratio.
Extra-articular manifestations associated with SpA: conjunctivitis, cystitis, nail hyperkeratosis, balanitis, pulmonary fibrosis, aortic insufficiency or renal amyloidosis. Variables included in the models: age (years), gender, de novo or previous diagnosis, family history of SpA (yes/no), duration (<4 years, 4–<8 years, ≥8 years) and extra-articular manifestations associated to SpA (yes/no).
The results of this study show that a substantial percentage of patients with SpA have coexisting diseases (psoriasis, uveitis or IBD, grouped under the name IMID), predominantly psoriasis followed by uveitis, while the prevalence of IBD is also relatively high. Some of these IMID (psoriasis, and uveitis) may nevertheless be considered extraarticular manifestations of SpA, although for the purposes of study they were grouped alongside IBD, under the term IMID since sometimes their presence or progression may be independently of SpA. In the recruited cohort, ankylosing spondylitis was the most frequent study entry SpA (55.1%), followed by psoriatic arthritis (25.1%) and undifferentiated spondyloarthritis (16.1%). The distribution was virtually identical to that of the eMAR II study, also conducted in hospital-based rheumatology clinics, in which 55.2% were patients with ankylosing spondylitis, 22.2% psoriatic arthritis, and 16.1% undifferentiated spondyloarthritis,15 so this cohort seems to reflect the profile of patients seen in routine practice of rheumatology hospital clinics.
Of the 3 conditions studied, psoriasis was the most prevalent disease (27.8%) due to the inclusion of patients with psoriatic arthritis, but the prevalence of uveitis (13.6%) and IBD (5.1%) was also significantly higher and similar to those found in the REGISPONSER study (23.2, 16.2 and 5.0%, respectively).16 The association between psoriasis and psoriatic arthritis is evident, although, in a percentage of patients, psoriatic arthritis or psoriasis can be diagnosed before the psoriasis is apparent. In our study, in fact, 5% of the patients with psoriatic arthritis had a diagnosis that was not associated with psoriasis. A higher prevalence of psoriasis in patients with ankylosing spondylitis compared to the general population has also been described.17
The prevalence of uveitis was 13.6%, mainly due to a high prevalence in patients with ankylosing spondylitis (19.0%). A recent systematic review showed higher prevalences of uveitis than those found in our study in 33.2% of the patients with ankylosing spondylitis and 25.2% of those with psoriatic arthritis.18 Our study did not include active uveitis detection by routine eye examination, so the prevalence rate may be lower than actual. Eye exams increase the rate of diagnosis of uveitis compared to clinical assessment alone, and since there is a described reduction in visual acuity associated with uveitis in 8.3% of the cases, it is important to actively detect symptoms18 in patients with SpA, as it may have implications for treatment.19
The prevalence of IBD (5.1%) in the AQUILES study was also higher than that expected in the general population.20,21 Excluding enteropathic arthritis, we found a prevalence of 2.7% (3.0% in patients with ankylosing spondylitis), several times higher than the expected prevalence in the general population, described around 0.2%–0.3%.20,21 Overall, IBD occurs most frequently as Crohn's disease and ulcerative colitis was documented on fewer occasions. The interrogation of gastrointestinal symptoms should be part of routine evaluation of these patients for early diagnosis and treatment of this disease.
In the ACHILES study, factors associated with the presence of IMID were age, female gender, family history of SpA and the presence of extra-articular manifestations of SpA. Psoriasis was associated with female gender. A recent study showed that patients with ankylosing spondylitis had a risk of developing psoriasis 2 times higher in men and 4 times higher in women compared to the general population.17 Uveitis showed a clear association with the duration of the SpA, an aspect mentioned in the review cited above.18 An interesting aspect of the multivariate analysis is the association of IMID studied together (psoriasis, uveitis and IBD) with the presence of other extra-articular manifestations associated with SpA (conjunctivitis, cystitis, nail hyperkeratosis, balanitis, pulmonary fibrosis, aortic insufficiency, kidney amyloidosis). It appears that the clinical expression of SpA is more severe in patients who develop these diseases. It has been reported that patients with ankylosing spondylitis and one comorbidity are more likely to develop a second or third comorbidity; the severity of the expression of ankylosing spondylitis (based on commonly used activity parameters) is also influenced by comorbidities and affected family members also have a higher risk of comorbidities.22 We also found an association with family history in the case of uveitis, but in psoriasis and IBD the association was with a lack of family history.
The present study has several limitations. It is important to point out that the data collection was conducted primarily by patient interviews and a review of medical history and that there was no active search for these diseases at baseline through additional diagnostic tests. Therefore, although all patients in this cohort had a diagnosis of SpA, we cannot rule out that some had unrecognized comorbidities and their prevalence was underestimated. For example, an eye examination included in the analysis can detect uveitis that may be unsymptomatic.18 Regarding the extrapolation of the results to the general population of patients with SpA, it bears mention that these patients were collected in a hospital setting, so they are not representative of the general population of patients with SpA, but only those who are seen in a hospital setting. In addition, patients were selected mostly (∼90%) consecutively and not randomly, representing a selection bias toward patients who visited the hospital more often, usually those with more symptomatic disease or more comorbidities. There were also a number of patients (very small) that was not included because they were unsuitable for a 2-year follow-up, and who did not provide baseline information. The study, however, shows a normal Rheumatology hospital population and indeed the prevalence of different diagnoses was similar to those of the emAR II15 and REGISPONSER16 studies. Finally, the study enrolled fewer patients than expected, the final sample size being of 601 patients allowing us to interpret the prevalence found with an accuracy of 3%, which is reasonably accurate for this type of study, while reducing the possibility of detecting potential differences in patient subgroups.
In conclusion, patients with SpA have a high prevalence of concomitant extra-articular immune mediated diseases (psoriasis, uveitis, IBD), and in addition there is an association between these diseases and other extra-articular manifestations of SpA, forming a patient profile with disease expression at various organ and system levels. It is therefore very important that in patients with SpA, which usually start young, rheumatologist conduct a comprehensive patient management, including questioning about events that may indicate the presence of disease at an extraarticular level, a situation that can influence patient treatment and monitoring. The management of patients with extra-articular manifestations of SpA may require coordination with other specialists.
Ethical ResponsibilitiesProtection of people and animalsThe authors state that the study procedures conformed to the ethical standards of the committee responsible for human experimentation and were in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients, and all patients included in the study have received sufficient information and gave written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
FundingThe ACHILES study was funded by Merck Sharp & Dohme of Spain.
Conflict of InterestMary J. Arteaga and Luis Cea-Calvo (employees at Merck Sharp & Dohme of Spain), Ignacio Marín-Jiménez (presentations or educational projects for Merck Sharp & Dohme, Abbie, FalkPharma and Shire, advisor for Merck Sharp & Dohme, Ferring, FalkPharma, Abbie, FAES and Shire), Rosario García-Vicuña (consultancy for the OpenSER-Allied Health Professionals project), Luis Linares (advisor for Abbie). The other authors declare no conflicts of interest.
The following Departments of Rheumatology included patients with spondyloarthritis in the ACHILES cohort study: Alcorcón (Madrid), La Princesa (Madrid), 12 de Octubre (Madrid), Carlos Haya (Málaga), Reina Sofía (Córdoba), Gregorio Marañón (Madrid), Sant Pau i Santa Creu (Barcelona), Donostia (San Sebastian), Marques de Valdecilla (Santander), Principe de Asturias (Madrid), Virgen de la Arrixaca (Murcia), Puerta de Hierro (Madrid) and Infanta Sofia (Madrid). We thank the investigators for their collaboration on the inclusion and monitoring of patients in the study. Statistical analysis was performed by Cristina Fernández-Pérez (Alalas, SA). We thank Sofia Perea (Pipeline Biomedical Resources, SL) for her contribution in drafting the manuscript.
Please cite this article as: Zarco P, González CM, Rodríguez de la Serna A, Peiró E, Mateo I, Linares L, et al. Manifestaciones extraarticulares en pacientes con espondiloartritis. Características basales de la cohorte de pacientes con espondiloartritis del estudio AQUILES. Reumatol Clin. 2015;11:83–89.