To standardize clinical evaluation of patients with axial spondyloarthritis (SpA) and psoriatic arthritis (PsA) using a checklist.
MethodsQualitative study that included: (1) nominal group (18 experts); (2) literature reviews of measures used in the assessment of patients with axial SpA or PsA; and (3) focus groups, one with rheumatologists and another with patients, organized to become familiar with their opinion on medical assistance. Taking this into account, the experts selected the measures to be included in the checklist based on their relevance, feasibility, and the outcome type.
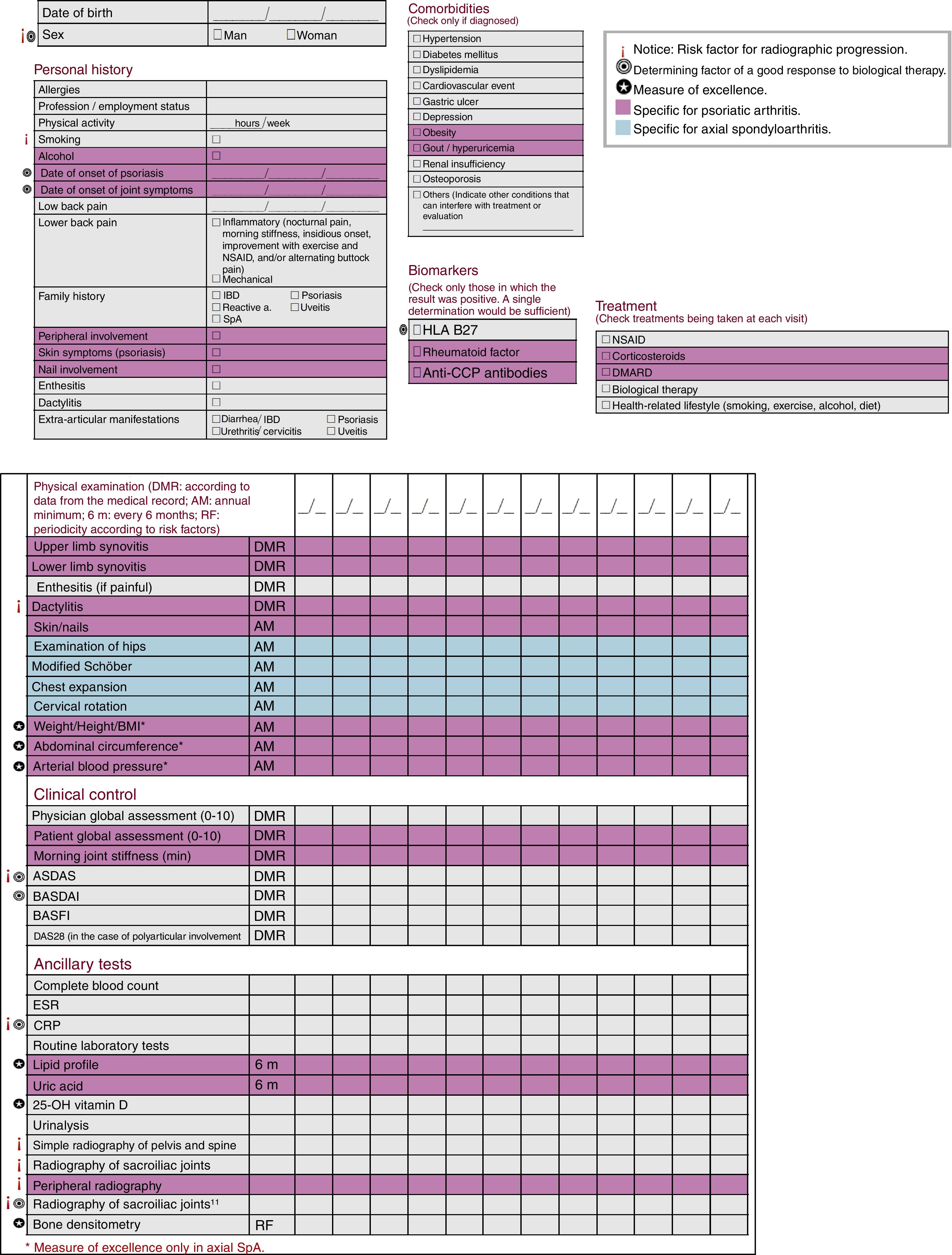
ResultsThe checklist includes measures for the evaluation of personal history, physical examination, activity and function, laboratory tests, imaging studies and treatments. It also defines risk factors of radiographic progression, predictors of the response to biological therapies, and comprises measures of excellence.
ConclusionsThis checklist for patients with axial SpA and PsA could help standardize daily clinical practice and improve clinical management and patient prognosis.
Estandarizar la evaluación clínica de pacientes con espondiloartritis (EspA) axial y artritis psoriásica (APs).
MétodosEstudio cualitativo que incluyó: 1) grupo nominal (18 expertos); 2) revisión de la literatura sobre variables empleadas en la evaluación de los pacientes con EspA axial o APs, y 3) grupo focal con reumatólogos y otro con pacientes con EspA axial o APs para analizar la evaluación de las EspA en las consultas de reumatología. Los expertos seleccionaron las variables a incluir en el cuadro de actuación con base en su relevancia, factibilidad en consulta y método/s de medición.
ResultadosEl cuadro de actuación incluye las variables para valorar antecedentes personales, exploración física, actividad y función, pruebas complementarias y tratamientos. Detalla factores de riesgo de progresión radiográfica, factores predictores de respuesta a terapia biológica, e incluye variables de excelencia.
ConclusionesEste cuadro de actuación para pacientes con EspA axial y APs podrá ayudar a homogeneizar la práctica clínica diaria y a mejorar el manejo y el pronóstico de estos pacientes.
In routine clinical practice, it is recommended that patients with spondyloarthritis (SpA) be evaluated according to clinical signs, symptoms and acute-phase reactants,1,2 for example the Bath Ankylosing Spondylitis Disease Activity Index, C-reactive protein or the Ankylosing Spondylitis Disease Activity Score, which combines the 2 aspects (both subjective assessment of the patient and the acute-phase reactants).3 The same occurs with psoriatic arthritis (PsA).2
However, despite the availability of a number of Spanish and international guidelines for the evaluation of SpA including PsA, the EmAR II study, conducted in Spain,4 demonstrated that, in the assessment of SpA patients, approximately 60% of their medical records do not include an assessment of possible joint involvement or overall evaluation of the patient. In 87%, no joint score is recorded and, in 84%, there is no mention of a functional score. Distinct factors may contribute to this situation. At the present time, there is great pressure on health professionals that does not favor the evaluation and systematic collection of data. Moreover, there is a great variability in the assessment variables recorded for these patients (number, characteristics, feasibility, validation, etc.). This means that the outpatient follow-up of these individuals may not be optimal.5
These facts justify the need to design realistic strategies that contribute to improving the quality of clinical practice in order that these patients receive integrated care.4 On the basis of the above aspects, the purpose of this project was to draw up a normalization tool (a framework for action, in the form of a checklist of items to be considered in visits to the rheumatologist, for an adequate assessment of the patient) to enhance the evaluation in routine practice of patients with axial SpA and PsA, for the aim of standardizing and achieving a stricter control of the disease, favoring the identification of high-risk factors and responses, as well as control of comorbidities.
Material and MethodsStudy DesignWe designed a qualitative study based on the methodology of the nominal group and a review of the literature promoted by the Work Group for the Study of Spondyloarthritis of the Spanish Society of Rheumatology, which comprises 2 projects: APROXIMA (“Approximate”) and PERSONALIZA (“Personalize”).
Participant SelectionWe selected a group of 18 experts (with interest and demonstrated experience in the subject of the project) from all the regions of Spain.
Drafting the ChecklistWe performed a literature review that included PubMed, as well as Spanish and international guidelines and consensus documents. For this, we selected all of the variables employed for the evaluation of axial SpA and PsA patients (sociodemographic, clinical and treatment-related). We then organized 2 focus groups, 1 with rheumatologists (to explore the barriers and facilitators in the management of patients with axial SpA and PsA, as well as the most relevant aspects of their routine evaluation in the rheumatology department) and another with patients (to assess the needs that they detected in their visits to their physician, so that they subsequently be evaluated by the experts). The list of variables and the results of the focus groups were presented and discussed in a nominal group of experts, which prepared a provisional list of variables, both for the first visit and for successive appointments. The experts the assessed each of these variables in terms of their: (1) relevance (impact on the patient, decision making, prognostic factor, etc.) from 1 (little relevant) to 10 (very relevant); (2) feasibility in the outpatient office from 1 (difficult to implement) to 10 (very feasible); (3) periodicity of the evaluation; and 4) method/s of measurement (direct question, questionnaire, scale, etc.). Once all the variables had been evaluated, the experts selected them for inclusion in the first visit and for successive appointments (together with their periodicity) those that had achieved the best scores and that they considered important for the evaluation of these patients. Next, they defined: (1) measures of excellence (those that, by taking into account the characteristics of routine clinical practice, may be more complicated to measure); variables considered prognostic factors, on the basis of the literature; (3) variables that predict the response to biological therapies, according to published reports; and 94) common variables and those specific for axial SpA and PsA. These specifications were included in the checklist.
Finally, this checklist and its specifications were presented and analyzed in local meetings throughout Spain by an extensive and representative group of rheumatologists (see ONLY TOOLS work group in Appendix A). All of the recommendations proposed in these local meetings were conveyed to the experts, who drafted the definitive checklist and specifications. This entire process was done with the aid of methodological advice, that helped both in the organization of the focus groups and in the subsequent discussion of the elements to be included in the checklist.
Statistical AnalysisThis analysis was based on a descriptive study.
ResultsWe drafted a checklist to evaluate SpA patients, with differentiated markers for patients with axial SpA and PsA, and their periodicity, as well as other specifications (Fig. 1). It includes sociodemographic variables, like the date of birth and sex (risk factor for the risk of radiographic progression and a determinant of a good response to biological therapy.
Concerning personal history, we documented, among other data, allergies, profession/employment status and use of tobacco (another risk factor affecting radiographic progression). Likewise, we recorded the date of symptom onset, the date of the diagnosis, related family history, enthesitis, dactylitis, extra-articular manifestations and the presence of inflammatory lower back pain. In the group of PsA patients, we included peripheral involvement, cutaneous symptoms and nail involvement.
With respect to comorbidities, we considered only those that had been diagnosed, such as hypertension, gastric ulcers and osteoporosis. As biomarkers, we included human leukocyte antigen (HLA) B27 and, specifically, in PsA, rheumatoid factor and anti-cyclic citrullinated peptide antibodies.
For patients with PsA, the physical examination included a study of synovitis in an upper and a lower limb and dactylitis, as well as a study of the skin and nails. Those with axial SpA underwent examinations of hip mobility, modified Schöber test, chest expansion and cervical rotation.
On the other hand, patient weight, height, body mass index, abdominal circumference and arterial blood pressure should be determined yearly in PsA patients and, as a measure of “excellence”, in the case of those with SpA.
With respect to clinical control, for axial SpA, the checklist includes the physician global assessment, the Bath Ankylosing Spondylitis Functional Index and the Disease Activity Score in 28 joints in the case of polyarticular involvement, aside from the Ankylosing Spondylitis Disease Activity Score and the Bath Ankylosing Spondylitis Disease Activity Index as determinants of a good response to biological therapy, and the former, moreover, as a risk factor of radiographic progression. In the case of PsA, the patient global assessment and morning joint stiffness are also evaluated.
Ancillary tests that should be performed in the control of axial SpA are complete blood count, erythrocyte sedimentation rate, routine laboratory tests, urinalysis and C-reactive protein (a determining factor of a good response to biological therapy and of radiographic progression). As a measure of excellence, it was decided to include 25-OH vitamin D and bone densitometry. In PsA patients, urinary uric acid should also be determined and, as a measure of excellence, the lipid profile, as well. With regard to imaging studies, plain radiography of the pelvis, spine and sacroiliac joints (and peripheral joints in patients with PsA) helps to monitor risk factors for radiographic progression. A radiograph of the pelvis enables a proper assessment of the sacroiliac joints and the hips, making it unnecessary to X-ray these structures. Magnetic resonance of the sacroiliac joints serves as a marker of radiographic progression, and is also related to the response to biological therapy. Thus, the decision was made to include it.
Finally, this checklist incorporates a section on treatments.
DiscussionThe work group for the study of SpA of the Spanish Society of Rheumatology has developed a practical and simple checklist, achieved by consensus. It is adapted to the characteristics of Spanish outpatient clinics, and is based on a review of the literature and the opinion of experts for the assessment of patients with axial SpA and PsA in routine clinical practice.
A number of studies have shown that adherence to the recommendations for the evaluation of patients with SpA and PsA is highly variable, either because of a lack of time, motivation or knowledge, or because of the considerable number of the variables and their characterists.2,5–8
In addition to standardizing routine clinical practice, the drafting of this checklist was to facilitate the identification of different risk profiles in patients (response to biological therapies,9 radiographic progression,10,11, etc.) and aid in improving our approach to their comorbidities.12 With these “warning signs” the effort is made to stress the need for greater awareness and control in these patients. It includes all the variables recommended in the Spanish and international guidelines and is presented in a simple form so that its use is feasible, either printed out or incorporated into the electronic medical record.1,2 Thus, although these items are familiar to rheumatologists, providing them in an orderly and accessible way that is easy to employ should be an incentive for its implementation. This should contribute to a better control, adaptation of the resources utilized and facilitation of an integrated management of complex patients and serve as a tool to resolve the detected needs.5
The project has several limitations. For example, a possible limitation could be derived from the representativeness of the panelists, making it difficult to generalize and compare the suggested ideas. To avoid these limitations, we contacted rheumatologists from different centers in Spain to ensure demographic representativeness. Another limitation could be derived from the election of the items included in the checklist; although the failure to mention a relevant variable does not appear to be probable, new ones can be defined during every patient visit. We have not performed a real implementation of the checklist to determine whether or not its use is truly feasible and whether it enhances the care of patients with axial SpA, but this will be the object of future projects.
The design of a checklist as a tool for standardization might improve normalization and evaluation in the outpatient clinic. It could lead to a stricter control of the disease, identification of risk profiles and the control of comorbidities in routine clinical practice. At the present time, a project is underway for the introduction and assessment of the use of this checklist.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FinancingThe project was financed by the Spanish Foundation of Rheumatology.
Conflict of InterestEL has received funding for research projects from AbbVie, Roche, MSD, Pfizer, Gebro, UCB, Grünenthal, Sobi, Celgene and Eisai. The remaining authors declare they have no conflicts of interest.
We thank the axial spondyloarthritis and psoriatic arthritis patients who participated in the focus groups.
Carlos Faced Olmos, Elia Valls Pascual, Javier Calvo Catalá, Cristina Campos Fernández, Amelia Rueda Cid, Rosa Negueroles Albuixech, Luis González Puig, Roxana González Mazario, M. Teresa Buades Soriano, Juan Antonio Castellano Cuesta, Alejandra Begazo Cruz, Juan Alberto Paz Solarte, Jorge Fragio Gil, Ana Urruticoechea Arana, Teresa Font Gayá, Luis Espadaler Poch, Inmaculada Ros Vilamajó, Andrés Ponce Fernández, Lourdes Mateo Soria, Ana Laiz Alonso, Patricia Moya Alvarado, Jerónima Cañellas Oliver, Melania Martínez Morillo, Sandra Farietta Varela, Meritxell Salles Lizarzaburu, María Bonet Llorach, Estefanía Moreno Ruzafa, Alba Erra Duran, M. Elena Martínez Castro, M. José González Fernández, Marta Valls Roc, Patricia Reyner Echevarria, Eulalia de Cendra Morera, Ramón Valls García, Juan de Dios Cañete Crespillo, Andres Cuervo Aguilera, Silvia Martínez Pardo, Georgina Salvador Alarcón, Carmen García Gómez, Delia Reina, Dolores Beteta Fernández, M. Rocío González Molina, Antonia Hernández Balibrea, Javier José Martínez Ferrín, M. José Moreno Martínez, Juan Moreno Morales, M. Rosario Oliva Ruiz, Encarnación Pagán García, Deseada Palma Sánchez, Elena Peñas Martínez, M. Francisca Pina Pérez, Fernando José Rodríguez Martínez, Encarnación Saiz Cuenca, Edgar Enrique Soriano Navarro, Esther Toledano Martínez, M. Concepción Morado Quiñoa, Javier Quirós Donate, Alberto Diaz Oca, Alicia Humbría, Marta Valero Expósito, Carmen de la Cruz Tapiador, M. Teresa Gonzalez Hernandez, Javier Rivera Redondo, M. Hildegarda Godoy Tundidor, Carmen Barbadillo Mateo, Laura Cebrián Méndez, Leticia Lojo Oliveira, Beatriz Joven, M. Cruz Fernández Espartero, María Ahijón Lana, Alejandro Jesús González Gutiérrez, Cristina Redondo Romero, Bryan Josue Flores Robles, Julia Martínez Barrio, Cristina Fernández Carballido, Vega Jovani Casano, Teresa Pedraz Penalva, Cintia Romera López, Gregorio Santos Soler, Mariano Andrés Collado, José Antonio Bernal Vidal, Raúl Noguera Pons, Gaspar Panadero Tendero, Elisa Trujillo Martín, Juan José Bethencourt Baute, M. Vanesa Hernández Hernández, Iván Alejandro Ferraz Amaro, M. García González, Esmeralda Delgado Frías, Beatriz Tejera Segura, M. Ángeles Gantes Mora, Lorena Expósito Pérez, Valeriano Miguel Flores Rodríguez, Fátima Álvarez Reyes, Cristina Luna Gómez, Laura Magdalena Armas, Laura Casas Hernández, Aaron Fariña González, Luis Coronel Tarancón, José Luis Álvarez Vega, Raúl Veroz González, Juan José Aznar Sánchez, José García Torón, Esther del Rincón Padilla, Puerto Moreno Gil, Fernando Gamero Ruíz, Antonio Cardenal Escarcena, Antonia Ferreiro Conejo, Piter José Cossio Jiménez, Sara M. Rojas Herrera, Manuel Fernández Prada, José Rey Rey, Simón Sánchez Fernández, Jimena Zalazar, Andrés Ariza Hernández, Rebeca Belmonte Gómez, Pastora Granados Bautista, Ángel García Aparicio, David Castro Corredor, Carmen Amelia Ordas Calvo, Jesús Babio Herráiz, M. Edilia García Fernández, M. Trinidad Pérez Sandoval, Carolina Álvarez Castro, M. Elvira Díez Álvarez, Alejandra López Robles, Clara Moriano Morales, Miriam Retuerto Guerrero, Marta Garijo Bufort, Lucía Pantoja Zarza, M. Carolina Díez Morrondo, Iñigo Hernández Rodríguez, Luis Fernández Rodríguez, José Antonio Pinto Tasende, Ceferino Barbazán Álvarez, Francisco Maceiras Pan, Marina Rodríguez López, José M. Pego Reigosa, Jesús Ibáñez Ruan, Rafael Melero González, Susana Romero Yuste, José Antonio Mosquera Martínez, Manuel Rodríguez Gómez, José Luis Ferreiro Seoane, Antonio Fernández Nebro, José Javier Pérez Venegas, Francisco Gabriel Jiménez Nuñez, Carmen Castro Villegas, Yolanda Cabello Fernández, Carmen Romero Barco, M. del Carmen Ordónez Cañizares, Inmaculada Ureña Garnica, M. Victoria Irigoyen Oyarzabal, Angelines Belmonte López, Virginia Coret Cagigal, Concepción Aranda Varela, Marta Rojas Jiménez, Clara Cienfuegos García, Antonio Ponce Vargas, Concepción Castillo Gallego, Montserrat Gómez Romero, Jerusalén Calvo Gutiérrez, Pilar Font Ugalde, Rafaela Ortega Castro, Clementina López Medina, Laura Bautista Aguilar, Clara Ojeda García, Isabel García Hernández, Carmen Vargas Lebrón, Julio García Feito, Juan Salvatierra Ossorio, Pilar Morales Garrido, Inmaculada Jiménez Moleón, Mar López-Ibáñez, Antonio García Sánchez, Susana Quirosa Flores, Teresa García Contreras, Alfonso González Utrilla, Antonio Romero Pérez, Irati Urionaguena Onaindia, Ioana Atxotegi Saez de Buruaga, M. Luz García Vivar, Eva Galíndez Agirregoikoa, M. Elena Garmendia Sánchez, José Francisco García Llorente, Rosa M. Morla Novell, Jesús Rodríguez Moreno, María Aparicio Espinar, Elena Sirvent Alierta, Sonia Castro Oreiro, Milagros Ricse Salcedo, Merçe López de Recalde, Helena Borrell Paños, Patricia Corzo García, María Pascual Pastor and Fabiola Ojeda Morillo.
Please cite this article as: Almodovar R, Torre Alonso JC, Batlle E, Castillo C, Collantes-Estevez E, de Miguel E, et al. Desarrollo de un cuadro de actuación para la evaluación de pacientes con espondiloartritis axial y artritis psoriásica en la práctica diaria: proyecto ONLY TOOLS. Reumatol Clin. 2018;14:155–159.