To establish a set of recommendations for the management of patients diagnosed with rheumatoid arthritis (RA) who cannot be treated with methotrexate (MTX) due to contraindications, drug toxicity or lack of adherence, and to establish therapeutic strategies more effective and safer in these RA patients. A qualitative analysis of the scientific evidence available to June 2015. The 2-round Delphi technique of consensus was used to collect and establish expert opinion based on the participants’ clinical experience when only low quality evidence was available.
A total of 18 recommendations were developed for the management of this patient profile. Fourteen of these recommendations were related to drug safety aspects. Recommendations on contraindication and toxicity of MTX have been updated. The experts recommend the use of biological monotherapy, a preferred treatment option, in patients whose profiles reveal a contraindication, intolerance or circumstances that prevent us against the use of MTX. There is some high-quality scientific evidence that supports contraindication and establishes certain conditions of MTX use in RA patients with specific clinical profiles.
El objetivo es establecer recomendaciones para el manejo del paciente con artritis reumatoide (AR) que no puede utilizar metotrexato (MTX) por contraindicación, toxicidad o falta de adherencia farmacológica, y establecer las estrategias terapéuticas más eficaces y seguras. Se realizó un análisis cualitativo de la evidencia científica disponible hasta junio de 2015. Se utilizó un Delphi con un panel de 17 reumatólogos para consolidar la opinión de expertos en aquellas recomendaciones con ausencia o baja calidad científica.
Se elaboraron 18 recomendaciones, y 14 de ellas abordan aspectos de seguridad. Se han actualizado las recomendaciones sobre la contraindicación del MTX y su toxicidad, y se recomienda como una opción terapéutica preferente la utilización de monoterapia biológica en pacientes con contraindicación, intolerancia o circunstancias que desaconsejan el uso de MTX. Existe evidencia científica de buena calidad que contraindica y extrema la utilización de MTX en pacientes con AR con determinados perfiles clínicos.
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects 0.5% of the Spanish population and, if not treated, causes pain and disability.1 In 2010, RA was 42nd of the 291 disorders analyzed in the study of the global burden of disease,2 representing 0.49% (0.36%–0.62%) of the total of years lived with disability. The years lived with disability adjusted for age and the growth in the population between 1990 and 2010 went from 48/100,000 population in 1990 to 55/100,000 population in 2010. In terms of years of life adjusted to disability, in 2010, RA was in 74th place of the ranking, which means 0.19% of the total years of life adjusted to disability.2 In economic terms, RA also originates a considerable cost to the health system. In 2001, the costs derived from RA in Spain surpassed 2250 million euros, and the calculated direct costs attributable to RA reached 1575 million euros, representing 70% of the total.3
The major objective in treating RA patients is to achieve clinical remission or the lowest possible disease activity,4,5 which has been associated with a better medium-term functional prognosis, although the reduction in the life expectancy of these patients does not seem to have improved throughout the last 20 years.6 The recommendations of the European League Against Rheumatism (EULAR) for the treatment of RA include the use of methotrexate (MTX) as the first therapeutic option in active patients.4 Nevertheless, in 10%–36% of the patients who receive MTX, the treatment must be discontinued because of an adverse drug reaction (ADR).7–10
Another reason why RA patients cannot take MTX is its contraindication. In the end, the lack of adherence to a treatment regimen, as has been demonstrated in other chronic diseases, originates worse health outcomes and increases the costs of medical care.11
This indicates that some patients are limited with regard to treatment with MTX, making it necessary to consider other therapeutic alternatives.
The objective of the present report is to outline recommendations based on scientific evidence, and on the opinion of experts, for the management of patients with RA who cannot receive MTX because of contraindication, toxicity or lack of adherence to the drug regimen, and to establish efficient and safe therapeutic strategies that contribute to achieving a better control of the disease and quality care in these patients.
Materials and MethodsWe prepared a qualitative summary of the scientific evidence actually available up to June 2015. We utilized a 2-round Delphi consensus technique to gather and consolidate the opinion of experts on the recommendations in which there was no scientific evidence or the evidence was of low quality.
Literature SearchWe searched for literature in the MEDLINE (PubMed) (1950–2015), EMBASE (1980–2015) and Cochrane Library (up to 2015) databases (the latter via Wiley Online), for 5 systematic reviews (SR) that required: (1) contraindication for MTX; (2) MTX toxicity; (3) lack of adherence to MTX; (4) therapeutic strategies with synthetic disease-modifying antirheumatic drugs (DMARD) other than MTX in patients who cannot utilize MTX; and (5) therapeutic strategies using biological drugs in patients who cannot take MTX. The literature searches were completed with a manual search of the references cited in articles that the reviewer considered to be of interest. The strategies for the literature searches of the 5 SR can be consulted in Supplementary Material.
The following definitions were used for the SR: (1) contraindication: specific situation in which a drug should not be utilized, as it can be harmful to the patient; (2) drug toxicity: the potential of a drug to produce harmful effects in a person; (3) adverse reaction: the World Health Organization (WHO) defines adverse reactions as a harmful or undesired effect that occurs after the administration of a drug at the doses normally utilized in humans to prevent, diagnose and/or treat a disease; (4) drug intolerance: type B ADR, according to the WHO classification, characterized by not being related to the drug action and to be unexpected; these reactions only develop in susceptible individuals and are due to 2 mechanisms: immunological and pharmacogenetic, and are independent of the dose of the medication and can even develop at subtherapeutic doses; and (5) drug adherence: degree in which the comportment of the patient, with respect to taking the medication, corresponds to the recommendations agreed to with the physician.
Analysis and Synthesis of the Scientific EvidenceWe evaluated the quality of the included studies through the critical appraisal of the articles selected for full-text review following a data collection notebook using the templates for critical appraisal provided by the Scottish Intercollegiate Guidelines Network (SIGN).
For the evaluation and synthesis of the scientific evidence, we considered the internal validity of the studies, whether or not there was statistical significance, the accuracy of the outcome and its applicability. The system chosen to classify the scientific evidence is that proposed by SIGN (available in Supplementary Material).
Delphi ConsensusThe objective of this consensus technique was to determine the level of agreement among the group of 17 expert rheumatologists with respect to recommendations supported by a low level of scientific evidence (SIGN<2++) (available in Supplementary Material). Those in which there was a high level of agreement would directly become part of the final set of recommendations.
We conducted a 2-round Delphi process. The first questionnaire included 11 recommendations that were arranged into 4 blocks: contraindication of MTX, MTX toxicity, adherence to MTX and therapeutic strategies in RA patients who cannot take MTX. The items were formulated as brief and independent statements. The response to each could be expressed using a Likert scale that measured the level of agreement with the statement in question, using a range of 1 (complete disagreement) to 5 (complete agreement). Aside from that evaluation, the panelists could comment on each of the items proposed on the basis of their routine clinical practice or scientific evidence. We did a qualitative and quantitative analysis of the responses in the first round. After that, a second questionnaire was sent to the panelists who participated in the first round, taking into consideration the overall results of the first, that is, the joint opinion of the panelists, and the information provided. We requested the evaluation of the 4 recommendations in which the group was least in agreement: median ≤4, interquartile range (IQR: Q1–Q3) ≥2 and level of agreement (≥4) ≤80% and the statements corresponding to the final recommendations. We also conveyed the comments made by the panelists during the first round. The remarks remained anonymous and were sent verbatim, with the exact reproduction of the sentence, phrase or reference.
Formulation of Final RecommendationsThe final recommendations were framed on the basis of the results from the 5 SR and the Delphi consensus. Each recommendation was provided with its corresponding level of evidence and grade of recommendation, utilizing the system employed by SIGN (available in Supplementary Material).
Results and DiscussionA total of 18 recommendations were drawn up for the clinical management of RA patients who cannot receive MTX (Table 1).
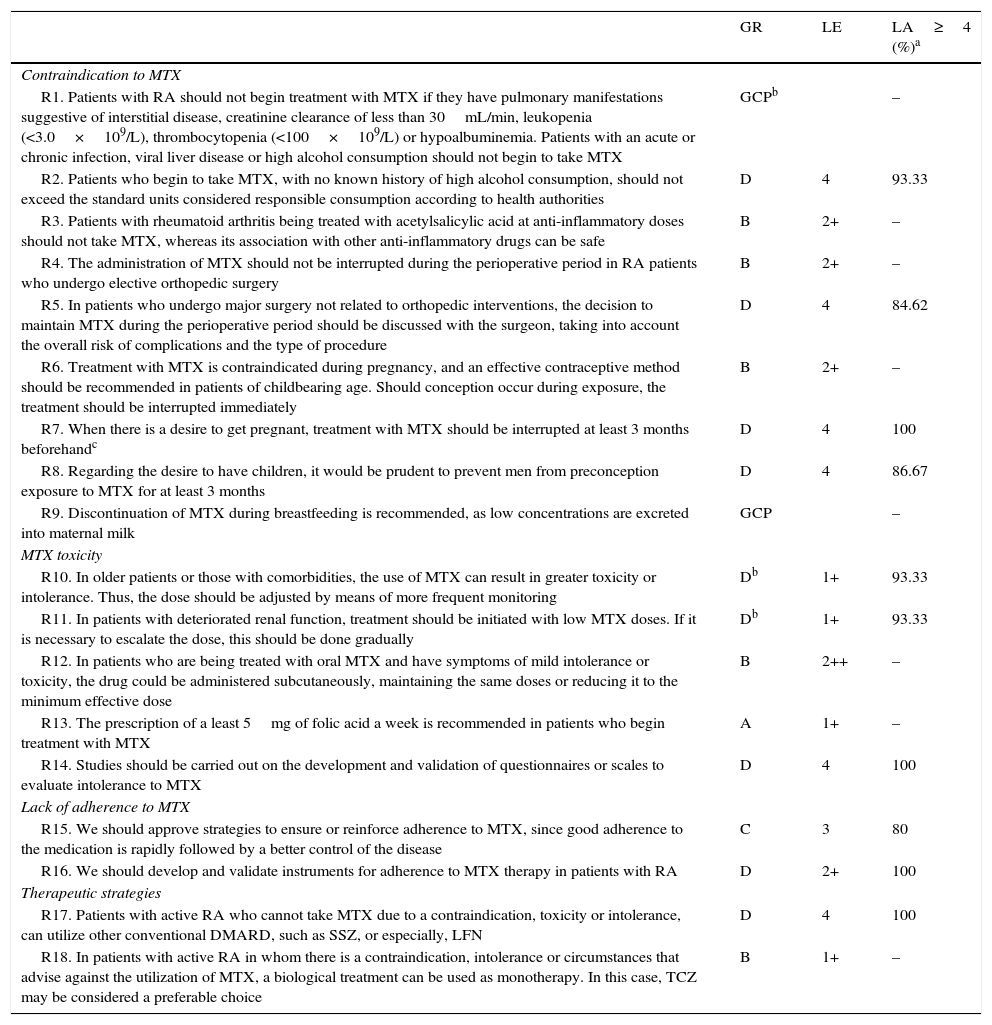
Recommendations From the Spanish Society of Rheumatology for the Management of Patients With Rheumatoid Arthritis who Cannot Take Methotrexate.
| GR | LE | LA≥4 (%)a | |
|---|---|---|---|
| Contraindication to MTX | |||
| R1. Patients with RA should not begin treatment with MTX if they have pulmonary manifestations suggestive of interstitial disease, creatinine clearance of less than 30mL/min, leukopenia (<3.0×109/L), thrombocytopenia (<100×109/L) or hypoalbuminemia. Patients with an acute or chronic infection, viral liver disease or high alcohol consumption should not begin to take MTX | GCPb | – | |
| R2. Patients who begin to take MTX, with no known history of high alcohol consumption, should not exceed the standard units considered responsible consumption according to health authorities | D | 4 | 93.33 |
| R3. Patients with rheumatoid arthritis being treated with acetylsalicylic acid at anti-inflammatory doses should not take MTX, whereas its association with other anti-inflammatory drugs can be safe | B | 2+ | – |
| R4. The administration of MTX should not be interrupted during the perioperative period in RA patients who undergo elective orthopedic surgery | B | 2+ | – |
| R5. In patients who undergo major surgery not related to orthopedic interventions, the decision to maintain MTX during the perioperative period should be discussed with the surgeon, taking into account the overall risk of complications and the type of procedure | D | 4 | 84.62 |
| R6. Treatment with MTX is contraindicated during pregnancy, and an effective contraceptive method should be recommended in patients of childbearing age. Should conception occur during exposure, the treatment should be interrupted immediately | B | 2+ | – |
| R7. When there is a desire to get pregnant, treatment with MTX should be interrupted at least 3 months beforehandc | D | 4 | 100 |
| R8. Regarding the desire to have children, it would be prudent to prevent men from preconception exposure to MTX for at least 3 months | D | 4 | 86.67 |
| R9. Discontinuation of MTX during breastfeeding is recommended, as low concentrations are excreted into maternal milk | GCP | – | |
| MTX toxicity | |||
| R10. In older patients or those with comorbidities, the use of MTX can result in greater toxicity or intolerance. Thus, the dose should be adjusted by means of more frequent monitoring | Db | 1+ | 93.33 |
| R11. In patients with deteriorated renal function, treatment should be initiated with low MTX doses. If it is necessary to escalate the dose, this should be done gradually | Db | 1+ | 93.33 |
| R12. In patients who are being treated with oral MTX and have symptoms of mild intolerance or toxicity, the drug could be administered subcutaneously, maintaining the same doses or reducing it to the minimum effective dose | B | 2++ | – |
| R13. The prescription of a least 5mg of folic acid a week is recommended in patients who begin treatment with MTX | A | 1+ | – |
| R14. Studies should be carried out on the development and validation of questionnaires or scales to evaluate intolerance to MTX | D | 4 | 100 |
| Lack of adherence to MTX | |||
| R15. We should approve strategies to ensure or reinforce adherence to MTX, since good adherence to the medication is rapidly followed by a better control of the disease | C | 3 | 80 |
| R16. We should develop and validate instruments for adherence to MTX therapy in patients with RA | D | 2+ | 100 |
| Therapeutic strategies | |||
| R17. Patients with active RA who cannot take MTX due to a contraindication, toxicity or intolerance, can utilize other conventional DMARD, such as SSZ, or especially, LFN | D | 4 | 100 |
| R18. In patients with active RA in whom there is a contraindication, intolerance or circumstances that advise against the utilization of MTX, a biological treatment can be used as monotherapy. In this case, TCZ may be considered a preferable choice | B | 1+ | – |
DMARD, disease-modifying antirheumatic drugs; GCP, good clinical practice; GR, grade of recommendation; LA, level of agreement; LE, level of evidence; LFN, leflunomide; MTX, methotrexate; RA, rheumatoid arthritis; SSZ, sulfasalazine; TCZ, tocilizumab.
Level of agreement is shown only for the recommendations for which consensus was reached by a Delphi-like process.
Although there is a study of high scientific quality that shows that preconception exposure does not increase the risk of spontaneous abortions, the authors conclude that more studies are necessary to confirm those findings. Thus, the group of experts recommends a prudent attitude concerning the interruption of MTX therapy before conception.
High alcohol consumption corresponds to that which exceeds the limit established for responsible use by the health authorities.
The flow chart for the SR selection that deals with this block of recommendations can be consulted in systematic review 1 of Supplementary Material. Duplicates were eliminated from the 193 initial citations (9), leaving a total of 184 references. We excluded 137 on the basis of the title and 5 after reading the abstract. Of the 42 remaining citations, we excluded 32 after reading the entire text, and 4 were incorporated from the manual search. A total of 14 articles were included in the SR.
Recommendation 1. Patients with RA should not begin treatment with MTX if they have pulmonary manifestations of interstitial lung disease, creatinine clearance less than 30mL/min, leukopenia (<3.0×109/L), thrombocytopenia (<100×109/L) or hypoalbuminemia. Patients with acute or chronic infection or viral liver disease, or those who consume large amounts of alcohol should not start to take MTX.
Evidence summary. The intake of MTX increases the risk of developing pulmonary disease, cytopenia, infections and liver disease.
Scientific evidence to decide whether a patient can begin a treatment with MTX does not come from ad hoc studies, but is based on indirect evidence of studies with confirmed scientific quality that analyze the risk factors for developing severe toxicity. The results of a SR with a meta-analysis (MA) of double-blind, randomized, controlled trials (RCT)12 indicated a small increase in the risk of developing pulmonary disease (relative risk [RR]: 1.1; 95% CI: 1.02–1.19) and of infectious pulmonary disease (RR: 1.11; 95% CI: 1.02–1.20) in patients with receiving MTX vs patients with other DMARD or biological therapy, although no increase was observed in the risk of mortality due to pulmonary disease (RR: 1.53; 95% CI: 0.46–5.01). In that same report,12 patients taking MTX had an increase in the risk of developing pneumonitis (RR: 1.78; 95% CI: 1.76–34.72), although these results were derived from, only, 4 of the 22 studies included in the SR and all of the cases of pneumonitis occurred prior to 2002. The authors mention that the characteristics of the patients recruited for the RCT, before and after 2002, may be different and that information is lacking to properly assess selection biases; therefore, in patients included in older studies, the attribution of pneumonitis to MTX could have to do with the presence of other risk factors for pulmonary complications, and the MA is not conclusive in this aspect. In the SR by Salliot and van der Heijde, pneumonitis due to MTX was analyzed in patients included in 21 prospective studies, and there were only 15 cases in the 3463 RA patients who received MTX over a period of 36.5 months. In the SR by Salliot and van der Heijde,13 5.2% of the 3463 patients analyzed had cytopenia of a single cell line among the patients who received MTX. The 2014 update of the SR and MA of RCT by López-Olivo et al.,14 which includes 7 studies with moderate-to-high scientific quality, shows that a reason for interrupting treatment with MTX was the abnormal increase in liver enzymes (8% vs 2%; RR: 3.8; 95% CI: 1.6–8.8).15–18 The patients receiving treatment with MTX had a higher risk of an elevation of liver enzymes than patients taking placebo (15% vs 3%; RR: 4.8; 95% CI: 2.3–10.0).16,19 The international group of rheumatologists of the 3E initiative prepared in 200920 a set of recommendations based on the scientific evidence for the use of MTX in patients with rheumatic diseases, in which they recommend a series of tests to identify the risk factors for the development of toxicity to MTX when a patient is going to initiate the treatment. The tests include determining alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, creatinine and complete blood count, and having a chest radiograph done during the year prior to starting treatment. In that work, the authors provide the list of absolute contraindications for beginning MTX in the patients included in RCT in recent years: creatinine clearance less than 20mL/min, leukopenia (<3.0×109/L), thrombocytopenia (<100×109/L), hypoalbuminemia, and patients with acute or chronic infection or viral liver disease.20,21
With all this information, the expert group adjusted the recommendation to “good clinical practice” and would not question avoiding the initiation of treatment with MTX in patients with certain clinical profiles.
Recommendation 2. Patients who start treatment with MTX, with no known history of high alcohol consumption, should not exceed the standard units considered responsible consumption according to health authorities.
Evidence summary. The consumption of alcohol in patients receiving MTX does not increase ALT levels.
A MA22 that provides the results of sequential liver biopsies performed systematically in patients with RA and psoriatic arthritis being treated with MTX concludes that 3% developed severe fibrosis or cirrhosis after 55 months of treatment, especially those who consumed more than 100g of alcohol a week. Nevertheless, these results should be interpreted with caution given that the authors report that there were no baseline liver biopsies for some patients. In their study of 2008, Rajakulendran et al.23 had the objective of determining alcohol consumption and its influence on ALT in RA patients treated with leflunomide (LFN) or MTX, and they found no effect of alcohol consumption on ALT concentrations. However, despite that finding, the group of experts considers that studies should be performed with more solid designs to confirm these data, as they are from studies of low quality, such as surveys.
Recommendation 3. Patients with RA being treated with acetylsalicylic acid at anti-inflammatory doses should not be given MTX, whereas the association with other anti-inflammatory drugs can be safe.
Evidence summary. The combination of MTX and acetylsalicylic acid at anti-inflammatory doses produces changes in the liver and kidney function, whereas the combination with other nonsteroidal anti-inflammatory drugs (NSAID) at standard doses can be safe.
With respect to interaction with other drugs, a SR that includes 17 RCT and nonrandomized studies24 that analyzes the safety of NSAID in RA patients being treated with MTX concludes that treatment combined with acetylsalicylic acid at anti-inflammatory doses should not be administered, as there have been reports of changes in liver and kidney function, whereas the combination with other anti-inflammatory drugs at standard doses can be safe. Most of the studies included in the SR by Colebatch et al.24 have a low-to-moderate scientific quality and, none of these reports mentions the combination of MTX and paracetamol, although the experts consider that it can be safe.
Recommendation 4. The administration of MTX should not be interrupted during the perioperative period in RA patients who undergo elective orthopedic surgery.
Recommendation 5. In patients who undergo major surgery not related to orthopedic interventions, the decision to maintain MTX during the perioperative period should be discussed with the surgeon, taking into account the overall risk of complications and the type of procedure.
Evidence summary. The utilization of MTX during the perioperative period of elective orthopedic surgery maintains RA under control and does not increase the risk of postoperative complications.
With respect to the interruption of MTX during the perioperative period of elective orthopedic surgery, the SR of high quality cohort studies, by Loza et al.,25 performed in RA patients in 2009, concludes that administering MTX at low doses (≤10mg/week) is safe during the postoperative period and maintains the disease under control, at least in patients without complications. Grennan et al.26 demonstrated in 2001 that maintaining MTX at any dose prior to surgery or interrupting it is not associated with an increase in postoperative complications (OR: 1.15; 95% CI: 0.34–1.40). Similar results were observed in the work of Sany et al.,27 in which no significant differences were found in the development of infection of the surgical wound (19% in the group in which treatment was discontinued vs 13% in the group in which it was maintained).
Recommendation 6. Treatment with MTX is contraindicated during pregnancy, and an effective contraceptive method should be recommended in patients of childbearing age. Should conception occur during exposure, the treatment should be interrupted immediately.
Recommendation 7. When there is a desire to get pregnant, treatment with MTX should be interrupted at least 3 months before.
Evidence summary. Maternal exposure to MTX (≤30mg/week) after conception increases the risk of spontaneous abortion; however, it does not seem to increase the risk of teratogenicity. Preconception maternal exposure to MTX (≤30mg/week) does not increase the risk of spontaneous abortion.
According to the guidelines drawn up by the British Society for Rheumatology and British Health Professionals in Rheumatology for the prescription of drugs during pregnancy or breastfeeding in patients with rheumatic diseases,28 the risk associated with MTX in pregnancy is dose-dependent (high risk: >20mg/week; low risk: ≤20mg/week). In general, the dose of MTX for the treatment of rheumatic diseases is low, and no teratogenic effects have been associated with MTX at doses lower than 10mg/week, although the number of cases from which this information was obtained is still too small to establish the weekly MTX dose that can safely be utilized. In this prospective cohort study with low risk of bias, recently published by Weber-Schoendorfer et al.,29 it was observed that the patients with postconception exposure to MTX (≤30mg/week) increased by 2.1-fold the risk of having a spontaneous abortion (adjusted hazard risk [HR]: 2.1; 95% CI: 1.3–3.2) with respect to the disease-matched group of patients, and 2.5-fold with respect to a group of women with no autoimmune disease (adjusted HR: 2.5; 95% CI: 1.4–4.3). In this report, the risk of patients having spontaneous abortions was also evaluated, but only considering preconception exposure, and there was no statistically significant increase in comparison with the other 2 groups: disease-matched and with no autoimmune diseases, respectively (adjusted HR: 0.8; 95% CI: 0.4–1.4, and adjusted HR: 1.3; 95% CI: 0.6–3.0). Regarding congenital disorders, there was a significant increase in major defects in the women exposed to MTX after conception vs the group with no autoimmune diseases (6.6% vs 2.9%) (adjusted OR: 3.1; 95% CI: 1.03–9.5). After adjusting for confounding variables, treatment with conventional DMARD and/or glucocorticoids did not increase the risk of teratogenic effects (adjusted OR: 1.2; 95% CI: 0.4–4.1). In the study by Martínez-López et al., published in 2009,30 a SR that included 6 articles (series of cases and surveys completed by physicians and patients), the objective was to analyze the safety of MTX in RA with respect to the reproductive system (fertility, pregnancy and breastfeeding). The authors conclude that, given the low scientific quality available, it is not clear that induced abortion is a better option than to continue the pregnancy, and they insist on the need to design studies that confirm the findings obtained. With this information, the group of experts recommends a prudent attitude concerning the interruption of MTX therapy before conception.
Recommendation 8. Regarding the desire to have children, it would be prudent to prevent men from preconception exposure to MTX for at least 3 months.
Evidence summary. Paternal preconception exposure to low doses of MTX (≤30mg/week during the 3 months prior to conception) is not associated with an increase in spontaneous abortions or major congenital disorders.
In the case of men being treated with MTX there is no high-level scientific evidence relating this drug at low doses with male infertility or anomalies during gestation or fetal development.31 There is no evidence indicating that male fertility, under exposure, is absolutely normal. A study was retrieved that uses prospective cohorts in the design32 and confirms that preconception paternal exposure to MTX at low doses (≤30mg/week during the 3 months prior to conception) is not associated with an increase in spontaneous abortions or major congenital disorders. Nevertheless, these results should be interpreted with caution, given that the sample size was insufficient to detect the effect of paternal exposure on the fetus, including defects or genetic alterations. Another limitation of the study is that the outcomes were not adjusted for possible paternal confounding factors, such as age, the consumption of tobacco, alcohol or drugs, or occupational exposure.
With these data, the group drawing up the recommendations suggests a prudent attitude concerning preconception exposure in treatment with MTX.
Recommendation 9. Discontinuation of MTX during breastfeeding is recommended, as low concentrations are excreted into maternal milk.
Evidence summary. Low concentrations of MTX are excreted into maternal milk.
There are no studies with a sufficiently high scientific quality to examine the safety of breastfeeding in patients being treated with MTX,33 but given that low concentrations of MTX can be excreted into maternal milk, the experts involved in this report consider discontinuing MTX therapy during nursing a good clinical practice.
Methotrexate ToxicityThe process of article selection is reflected in systematic review 2 of Supplementary Material. We read the titles of the 890 citations initially selected and 99 articles that were duplicated in the databases were excluded. We eliminated 517 that did not fulfill the objective or meet the selection criteria of the study. We read 274 complete abstracts and excluded 254 as either the intervention or the comparator was not suitable, or because the format was a narrative review. Of the 20 articles that were read in their entirety, 12 were excluded because the comparator was not suitable. Two articles that were read after the manual search were not included, either because the comparator was not suitable or they did not respond to the objective of the review. In all, 8 articles were included in the SR.
Recommendation 10. In older patients or those with comorbidities, the use of MTX can result in greater toxicity or intolerance. Thus, the dose should be adjusted by means of more frequent monitoring.
Recommendation 11. In patients with deteriorated renal function, treatment should be initiated with low MTX doses. If it is necessary to escalate the dose, this should be done gradually.
Evidence summary. Exposure to MTX produces lung involvement (interstitial disease) and increases liver enzymes, and is associated with nonsevere infections, stomatitis, oral aphthae, alopecia and gastrointestinal distress. Control of the disease is better with MTX treatment, and the risk of interruption due to toxicity is higher than when placebo is utilized. In comparison with other DMARD, the rate of interruption of MTX is similar to or lower than that of LFN, sulfasalazine (SSZ), d-penicillamine and gold salts and greater than that of hydroxychloroquine (HCQ).
The recommendations have been drafted taking into account indirect high-quality scientific evidence, as we found no reports in which the study population consisted of patients with specific sociodemographic and clinical characteristics, such as being elderly or groups of patients with certain comorbidities. In this case, in contrast to the recommendations formulated in the block on contraindications to MTX, these do not make it impossible for the patient to begin to receive MTX, but the attitude is more conservative.
In a recent study for the Cochrane Foundation,14 a SR was done using MA to evaluate the efficacy and adverse effects of MTX compared with placebo for a study period of 12–52 weeks, and it was concluded that MTX at a dose of 5–25mg a week showed a clinical and statistically significant benefit with respect to placebo, although patients taking MTX had 2-fold higher risk of interrupting it due to ADR in comparison with the placebo group (16% vs 8%; RR: 2.1; 95% CI: 1.3–3.3).15,16 The total rate of ADR was 3 times higher in the group taking MTX vs the placebo group (45% vs 15%; RR: 3.0; 95% CI: 1.4–6.4),15,16 but there were no statistically significant differences when stratified for serious ADR (3% vs 2%; RR: 1.44; 95% CI: 0.36–5.74).17,18 Other frequent ADR in patients receiving MTX were stomatitis and oral ulcers (9% vs 4%; RR: 2.0; 95% CI: 1.0–4.0), and infections (49% vs 35%; RR: 1.3; 95% CI: 1.0–1.6), the most common of which were those involving the upper airway, bronchitis and pneumonia. Alopecia (6% vs 1%; RR: 6.51; 95% CI: 1.20–35.33)16,18 and gastrointestinal distress (9% vs 4%; RR: 2.20; 95% CI: 1.03–4.68)18,19 were also more frequent in patients being treated with MTX.
In other studies in which the comparator was not placebo but another DMARD, the rate of interruption was lower in patients on MTX, except when compared with HCQ. In 2009, Salliot and van der Heijde13 published a SR that included cohort studies and others of low quality to evaluate the safety of long-term MTX monotherapy in RA patients (12.7 treatment years), in which they demonstrate that the rate of discontinuing MTX due to toxicity (10%–37%) is lower than that observed in patients receiving SSZ (17%–52%), d-penicillamine (24%–55%) and gold salts (22%–64%), but higher than that found with HCQ (10%–14%). There are 3 good quality RCT that compare MTX with LFN in RA patients. In the RCT by Emery et al.,34 with a 2-year follow-up, the authors conclude that there are no differences between the two drugs, either in the percentage of ADR or in the rate of withdrawal. The RCT of Cohen et al.,35 which also lasted 2 years, reports the ADR known for MTX, and concludes that the safety profile is similar and acceptable in both groups, although the rate of interruption was higher for LFN. The third RCT, which had a duration of 1 year, compares LFN with MTX,36 describes the ADR and concludes that the rate of ADR was similar in the 2 groups and that they were mild, although low doses of MTX were used and the sample size was small.
With regard to the ADR of MTX, in the SR of Salliot and van der Heijde,13 8.3% of the patients in the 6 studies included in the SR developed infections (treatment duration, 3 years). The authors conclude that MTX does not seem to be a risk factor for infections in general, for serious ones (including herpes zoster) or for those that develop after surgery to place a knee or hip prosthesis.
With respect to liver toxicity, the elevation of liver enzymes was the second most frequent ADR after gastrointestinal distress in patients taking MTX. The results of 27 prospective studies that evaluated 3808 RA patients who received low-dose MTX (10.5mg/week) over an average of 55.8 months demonstrated that 20.2% of the patients (n=769) had at least one episode of liver enzyme elevation, 12.9% had elevation of enzymes over 2-fold the upper limit and 3.7% had to interrupt treatment due to liver toxicity.
The RCT of Genovese et al.,37 in which MTX (20mg/week) vs treatment with etanercept (ETN) (25mg/week), with a follow-up lasting over 2 years, describes the known effects of MTX and it is shown that patients taking MTX had a larger percentage of ADR (nausea, alopecia and oral ulcers). Moreover, there were 2 cases of pneumonitis in the group treated with MTX. In the RCT of Edwards et al.,38 that compares rituximab (RTX) in 3 arms with MTX, the authors refer mainly to ADR attributable to RTX and conclude that there are no differences, although the rate of serious infections is greater in the groups taking RTX. Finally, the RCT published by Jones et al.39 compares MTX vs tocilizumab (TCZ) for 24 weeks. The ADR of MTX are those reported and there were no differences between the 2 groups in terms of safety, although the authors conclude that TCZ was more effective.
Recommendation 12. In patients who are being treated with oral MTX and have symptoms of mild intolerance or toxicity, the drug could be administered subcutaneously, maintaining the same doses or reducing it to the minimum effective dose.
Evidence summary. Beginning with lower MTX doses, making more gradual dose escalations and changing the route of administration, from oral to subcutaneous, are therapeutic strategies that enable us to reduce the toxicity of MTX.
Three RCT40–42 demonstrated that the efficacy and toxicity of MTX are dose-dependent. Thus, beginning with a dose of 12.5–20mg/week vs 5–10mg/week shows greater efficacy in the first group, with a significant increase in toxicity.41 Utilizing an initial dose of 25mg/week vs 15mg/week is more effective, but is associated with a greater toxicity.40 On the other hand, making rapid dose escalations, from 5mg/week to 25–30mg/week, is associated with a greater efficacy but also with greater toxicity, in comparison with more gradual increases of 5mg/3 months.42 With respect to the route of administration, a number of retrospective studies suggest that parenteral administration of MTX is associated with a greater efficacy and less gastrointestinal toxicity in comparison with oral administration,43,44 which could be due to a greater bioavailability of the parenteral route.45,46 The only RCT of 2008,47 which compares orally administered MTX and that administered subcutaneously (15mg/week), demonstrated a greater clinical efficacy in patients that utilized subcutaneous MTX, but they also had a higher percentage of interruption due to toxicity.
Recommendation 13. The prescription of a least 5mg of folic acid a week is recommended in patients who begin treatment with MTX.
Evidence summary. The administration of folic acid in patients being treated with MTX reduces the secondary effects of the drug.
A SR based on RCT, published by Shea et al.48 in 2013, includes 6 studies with 624 RA patients. It demonstrates that the exogenous administration of folate (≤7mg/week of folic or folinic acid) reduces by 26% the incidence of gastrointestinal effects such as nausea, vomiting and abdominal pain (RR: 0.74; 95% CI: 0.59–0.92). It also reduces the elevation of liver enzymes by 76.9% (RR: 0.23; 95% CI: 0.15–0.34) and the interruption of MTX for any cause (RR: 0.39; 95% CI: 0.28–0.53). They analyzed the incidence of stomatitis and oral ulcers and, although they saw a trend toward a reduction it was not statistically significant (RR: 0.72; 95% CI: 0.49–1.06). The international group of rheumatologists in the 3E initiative20 includes, among their recommendations, the prescription of at least 5mg/week of folic acid49,50 to reduce the toxicity of MTX for its utilization in patients with rheumatic diseases.
Recommendation 14. Studies should be carried out on the development and validation of questionnaires or scales to evaluate intolerance to MTX.
Evidence summary. We did not identify studies for the design and validation of scales or questionnaires to evaluate MTX toxicity in RA patients.
Lack of Therapeutic Adherence to MethotrexateOf a total of 258 articles initially selected for the SR, we excluded duplicates (n=28) and those in which reading the title (n=102) and the abstract (n=118) indicated that they did not respond to the research question. We excluded 3 articles after reading the entire text and included 3 articles after the manual search. A total of 10 articles were included in the review. The flow chart on article selection is provided in systematic review 3 of Supplementary Material.
Recommendation 15. We should approve strategies to ensure or reinforce adherence to MTX, since good adherence to the medication is rapidly followed by a better control of the disease.
Evidence summary. Good adherence to treatment with MTX will shortly be associated with better results in the Disease Activity Score in 28 joints (DAS28) and Health Assessment Questionnaire (HAQ) and with a lower C-reactive protein (CRP).
One of the main reasons for the lack of adherence or the interruption of treatment with MTX is the development of adverse reactions associated with the drug.
Concerning the evaluation of adherence and the impact on the control of the disease of the 10 articles included in the SR, 8 dealt with this relationship,51–57 although all of them had a high risk of bias or were nonanalytical studies, like series of cases. The study by Waimann et al.56 is a series of cases in which the authors studied 201 patients with established RA being treated with glucocorticoids and/or DMARD for a period of 2 years. A total of 107 patients accepted monitoring by the Medication Event Monitoring System (MEMS) and 94 accepted only the Compliance Questionnaire for Rheumatology (CQR). Although the primary objective was to assess adherence after implementing an electronic device that reminds the user to take a medicine, a secondary goal was to explore the determining factors and consequences. The authors observed that a good adherence to DMARD (81% took MTX) was associated with lower DAS28 than the baseline score after 24 months (r=−0.26, P=.01), regardless of age, sex, disease duration, cumulative prednisone dose and number of synthetic or biological DMARD employed. The authors conclude that these patients had lower DAS28 throughout the entire study and had a less marked radiological progression than those who were nonadherent, although the statistical significance was lost at 24 months. The results of this study must be interpreted with caution, since the design has important limitations, as the sample size is not estimated and there is no analysis of losses. On the other hand, the study population has very specific sociodemographic characteristics, such as having a low socioeconomic level, which means that it is not representative of the target population of the present work.
In 2010, Contreras-Yanez et al.,53 in a series of cases of 112 patients with recent-onset RA, evaluated the effect of DMARD medication persistence (assessed in a structured interview) on outcome variables. The authors observed that this persistence was associated with favorable results appearing in the Patient-Reported Outcomes of functioning and quality of life (PRO): Rheumatoid Arthritis Disease Activity Index (RADAI), Short Form (SF)-36, HAQ, visual analog scale (VAS: pain and general, morning stiffness and fatigue), PHYRO (DAS28 and medical VAS) and LARO (CRP and erythrocyte sedimentation rate) in comparison with nonpersistent patients. On the other hand, in Cox models, the variables that best predicted sustained compliance with DMARD were the components of PRO (except fatigue), PHYRO and LARO (only CRP). The study population is not representative of that for which the present report is intended, as they are patients with recent-onset RA.
Recommendation 16. We should develop and validate instruments for adherence to MTX therapy in patients with RA.
Evidence summary. The CQR questionnaire accurately predicts compliance with the correct taking and dosing of DMARD in RA patients.
In relation to the instruments for evaluating adherence to treatment that have been validated for MTX in RA, the only validated tool is CQR, although it has been little used for the studies retrieved. Others more frequently utilized are based on structured interviews or local questionnaires and the recount of drugs that have been acquired from the pharmacy, when there is access to the pharmaceutical database. De Klerk et al.58 studied a prospective cohort of different diseases (81 RA, 17 polymyalgia rheumatica and 29 gout) and antirheumatic therapies to validate CQR, taking MEMS as a reference pattern for 6 months. The authors observed that the items in CQR accurately predict taking compliance and correct dosing, being more specific to detect low taking compliance (≤80%) and more sensitive for incorrect dosing (≤80%). The scientific quality of the study is good, but the estimation of the sample size was not specified and losses were not taken into account.
Therapeutic Strategies in Patients With Rheumatoid Arthritis who Cannot Utilize MethotrexateThe total of articles retrieved on DMARD as a therapeutic strategy for the management of patients who cannot take MTX was 500, after eliminating 20 duplicates from the 3 databases. We excluded 492 after reading the abstract and 8 after reading the full text (the flow chart for article selection is provided in Supplementary Material: systematic review 4). The recommendations of the Spanish Society of Rheumatology (SER)59 on biological therapies in RA patients was retrieved by means of a manual search.
Recommendation 17. Patients with active RA who cannot take MTX due to a contraindication, toxicity or intolerance, can utilize other conventional DMARD, such as SSZ, or especially, LFN.
Evidence summary. Leflunomide, above all, or SSZ can be comparable to MTX in efficacy.
Given the absence of articles to include after the selection of reports retrieved from the literature search and taking, as a reference, the recommendations of the SER on biological therapies in patients with RA,59 which concludes that LFN, in particular, or SSZ can be comparable to MTX in efficacy, the authors of the present study have decided that it be a part of the body of evidence for drafting these recommendations.
The process of selecting the articles on biological therapy is shown in systematic review 5 of Supplementary Material. Of the 483 initial citations, we eliminated the duplicates in the 3 databases,38 which left 445 articles. After reading the title, 249 were excluded, as were 161 after we had read the abstract. Ultimately, of the remaining 35 citations, 12 were eliminated once the full text had been read. In all, 23 articles were included in the SR.
Recommendation 18. In patients with active RA in whom there was a contraindication, intolerance or circumstances that advise against the utilization of MTX, a biological treatment can be used as monotherapy. In this case, TCZ may be considered a preferable choice.
Evidence summary. Monotherapy with TCZ is more effective than monotherapy with MTX. Alone, TCZ tends to achieve better responses than ADL monotherapy. In monotherapy, TCZ has responses similar to combined TCZ+MTX therapy, unlike anti-tumor necrosis factor α (anti-TNFα) agents, which always have a better response when combined with MTX.
It is usual in rheumatology to begin the administration of biological agents in association with MTX,60 as available scientific information suggests, as it is shown to be more effective and potent. Moreover, the directions for use of many biological therapies (infliximab [INF], golimumab [GL], ADL, RTX, abatacept [ABT]) recommend use combined with MTX in RA.38,61–66 There are several reasons for this: (a) in itself, MTX reduces joint inflammation and arrests radiological progression67; (b) it increases the bioavailability of the biological agent68; (c) it reduces the formation of antibodies against the biological drug69,70; and (d) it reduces the formation of antinuclear autoantibodies.71
In patients who need a biological but have contraindications or intolerance, or other circumstances that advise against the utilization of MTX, there are 2 alternatives: using the biological agent as monotherapy or combining it with a DMARD other than MTX.72
The use of a biological as monotherapy is defined by fulfilling 3 properties: to be better than placebo, at least comparable to MTX/another DMARD and similar to the combination with a DMARD. Moreover, the biological agent should have a good tolerability profile and the duration of its therapeutic effect should be maintained over time.60
In relation to the different biological drugs, TCZ is the one whose use as monotherapy in the treatment of RA has been most extensively analyzed. It has been shown that:
- 1.
The use of TCZ as monotherapy is more effective than treatment as monotherapy with MTX/another synthetic DMARD (including SSZ, bucillamine and d-penicillamine).73,74 The SATORI75 and AMBITION39 clinical trials demonstrate that treatment with TCZ as monotherapy provides American College of Rheumatology (ACR) 20, 50 and 70 responses greater than treatment with MTX as monotherapy; moreover, the AMBITION study39 demonstrates that the responses are maintained over time. The objective of that study was to compare the efficacy of TCZ at a dose of 8mg/kg body weight (bw), administered intravenously, with treatment with MTX in adult patients with active RA, without a previous failure of MTX or other biologicals. Most of the patients included had early RA (>40% were less than 2 years old) and were MTX-naïve (nearly 66%), and the treatment with TCZ was not inferior to the treatment with MTX with regard to clinical efficacy (ACR 20) at 24 weeks of follow-up. The SAMURI study76,77 confirmed that TCZ administered as monotherapy is superior to treatment with DMARD for reducing the progression of radiological damage, even in patients with a poor prognosis.
- 2.
In contrast to what occurs with anti-TNFα agents,78 the combined use of TCZ with MTX is not always superior to TCZ as monotherapy, in RA patients with an inadequate response to MTX.74,79–81 In the ACT-RAY study,81 a group of patients with RA with inadequate response to MTX were randomized to receive intravenous TCZ (8mg/kg bw/month) associated with MTX, previously or as monotherapy. At 24 weeks of treatment, there were no statistically significant differences in the percentage of patients with a DAS28<2.6 in the two groups.
The proportion of patients with neutralizing antibodies was similar among the patients who continued the treatment as monotherapy (4.4%) and those who received the combined therapy (3.7%). In the ACT-STAR clinical practice and pharmacovigilance study,82 it was found that the improvement between the two groups was similar (TCZ as monotherapy and combined [TCZ with DMARD]). The STREAM study83 showed that treatment with monotherapy with TCZ was not associated with a decrease in the clinical response throughout follow-up; the ACR responses and DAS 28 were stable after 5 years of treatment with monotherapy. The ADACTA trial84 compared the efficacy of TCZ (8mg/kg bw/month) vs ADL (40mg/2 weeks), both as monotherapy, in patients who were intolerant to MTX. The patients included in the study had a mean disease duration of 7 years and had not received previous biological treatment. The decrease in the DAS 28 from baseline to week 24 was greater in the group of patients taking TCZ.
A number of SR with RCT have confirmed73,85,86 that treatment with TCZ as monotherapy provides superior ACR 20, 50 and 70 responses than treatment with MTX alone. A MA of 6 studies conducted in a Japanese population and their respective open-label extensions confirmed ACR 20, 50 and 70 responses of 91.3%, 73% and 51.3%, respectively, and a remission (determined by the DAS 28 [59.7%]) maintained after TCZ monotherapy for 5 years.87 Another recent MA demonstrates that, when TCZ is taken as monotherapy, it has an efficacy superior to that observed with anti-TNFα as monotherapy and an efficacy similar to that found with treatment of TCZ associated with MTX.74
A Cochrane SR73,85 affirms that treatment TCZ in monotherapy is superior to MTX monotherapy in improving the clinical control of RA (ACR 20 and 50 and remission according to DAS 28). Monotherapy TCZ is 1.45-fold more likely to achieve that the disease ceases its radiological progression, and is beneficial in that it reduces the clinical activity of RA, especially in RA patients who are MTX-naïve and in those who have an inadequate response to this agent.
There are studies that analyze treatment with TNFα antagonists as monotherapy. With respect to ADL, it has been used as monotherapy and, in different dosing regimens, is superior to placebo in patients with RA and an inadequate response to DMARD.88 Treatment with ADL as monotherapy has been seen to have the same clinical efficacy as treatment with MTX, although it is associated with a greater effect on arresting the radiological damage.89 Patients with a low disease activity at the end of clinical trials in which they received ADL as monotherapy remained in the open-label extension after 6 years of good control of the disease and a minimal radiological progression.89 Nevertheless, in both recent onset and established RA with an inadequate response to DMARD, in general, ADL as monotherapy is less efficacious. The most effective combination is ADL with MTX,61 followed by ADL in combination with antimalarial agents and, finally, ADL plus LFN.90
Studies that analyze treatment with ETN as monotherapy demonstrate similar results. In the Early Rheumatoid Arthritis (ERA) study, which involved patients who had been diagnosed less than 3 years earlier and were MTX-naïve, it was observed that treatment with ETN as monotherapy had advantages in arresting the radiological damage; subcutaneous ETN monotherapy with 25mg (twice a week), is significantly superior to MTX in controlling the signs and symptoms of RA, but only after 2 years of follow-up.37,91 The RCT TEMPO92 included patients with a mean disease duration of 6 years. Although some clinical and radiological scores showed a more favorable course with monotherapy with ETN rather than MTX, the most effective therapeutic arm was that which combined ETN with MTX, clearly superior to ETN as monotherapy.93
In the open-label ADORE study,94 after 16 weeks of treatment, it was observed that the effect of ETN as monotherapy was very similar to the effect of the combination of ETN plus MTX. The open- label RADIUS II observational registry also observed a similar response in treatment with ETN as monotherapy and combined ETN and MTX therapy, evaluated using the Clinical Disease Activity Index (CDAI) to assess remission.95
The use of GL as monotherapy has not been approved. However, there are studies that demonstrate that the clinical efficacy of intravenous GL is comparable to that of MTX. However, combined therapy using GL and MTX has been shown to be more effective than GL as monotherapy.96,97 Patients with RA who receive monotherapy consisting of INF have a shorter drug survival and more adverse events than patients who take INF in combination with MTX.78,98 Monotherapy with certolizumab pegol was superior to placebo in the FAST4WARD study,99 and similar to concomitant treatment with DMARD in the REALISTIC,82 regardless of the anti-TNFα used previously. Monotherapy with other biological agents that do not inhibit TNFα, aside from TCZ, has not been thoroughly researched, although there are a few studies that evaluate the clinical efficacy of treatment with ABT (ARRIVE) and RTX as monotherapy.100
Available data74,101,102 indicate that treatment with anti-TNFα, agents, ABT and TCZ utilized in combination with MTX have responses that are comparable (through comparisons, mostly indirect) in RA patients and an inadequate response to DMARD. However, administered as monotherapy, TCZ is associated with a better clinical response than anti-TNFα drugs.102,103 The responses in terms of efficacy are similar in TCZ associated with MTX and TCZ as monotherapy, whereas, anti-TNFα agents combined with MTX generally show a greater therapeutic efficacy than anti-TNFα monotherapy. These findings suggest that treatment with TCZ as monotherapy73,85,104 should be considered an effective therapeutic alternative in patients with active RA that is refractory to MTX, who should receive a biological agent but do not tolerate MTX or do not adhere to a treatment that includes it.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingSpanish Society of Rheumatology.
Conflict of InterestRosario García-Vicuña has received funds for meetings/congresses from Pfizer, Roche, UCB and MSD; fees as a speaker from BMS, UCB, Pfizer, Roche, Sandoz/Novartis from the Spanish Foundation of Rheumatology; funding for participating in research from Tigenix, Roche, AMGEN, Actelion, Roche and MSD, and has acted as a consultant for BMS, Pfizer, UCB, Roche, Actelion and Hospira.
María Auxiliadora Martín-Martínez declares she has no conflicts of interest.
María Rosa González-Crespo has received funds for meetings/congresses from Pfizer, Abbvie and MSD, and fees as a speaker from Pfizer, Abbvie y MSD.
Jesús Tornero-Molina has received funds for meetings/congresses from Pfizer; fees as a speaker from Gebro, Pfizer, UCB, Roche and Grunenthal; funding as an instructor from AMGEN, and funding for participating in a research project from Roche.
Antonio Fernández-Nebro has received funding from Pfizer, MSD and Roche; fees as a speaker from Pfizer, MSD, Roche and Abbvie; funding for participating in a research project from Pfizer, MSD, Roche, Abbvie, UCB and BMS, and has acted as a consultant for Pfizer, Roche, Abbvie, UCB and BMS.
Francisco Javier Blanco-García has received fees as a speaker from Bioibérica and BMS; funding for participating in a research project from Roche, and has acted as a consultant for Gebro.
Ricardo Blanco-Alonso has received funds for meetings/congresses from Roche, Abbvie, MSD, Bristol and Pfizer; funding for participating in a research project from Roche and Abbvie, and has acted as a consultant for Roche, Abbvie, Bristol and Pfizer.
Sara Marsal-Barril has received funds for meetings/congresses from Pfizer, Roche, UCB, Abbvie, BMS y MSD; fees as a speaker from de BMS, Roche and CELGENE; funding for participating in a research project from Roche, and has acted as a consultant for Roche, UCB, BMS and Hospira.
The group of experts from the present report wishes to express their gratitude to the rheumatology physicians who have participated in the Delphi consensus phase, for their interest in and commitment to the study subject. They also wish to thank Dr. Federico Díaz González, director of the Research Unit of the Spanish Society of Rheumatology (SER), for his participation by reviewing the final manuscript and contributing to preserving the independence of this document, as well as Mercedes Guerra, documentalist of the SER, and Daniel Seoane, methodologist of the SER, for their collaboration in the present report.
Please cite this article as: García-Vicuña R, Martín-Martínez MA, Gonzalez-Crespo MR, Tornero-Molina J, Fernández-Nebro A, Blanco-García FJ, et al. Documento de Recomendaciones de la Sociedad Española de Reumatología para el manejo clínico del paciente con artritis reumatoide que no puede utilizar metotrexato. Reumatol Clin. 2017;13:127–138.