Hypertriglyceridemia is common in children with systemic lupus erythematosus (SLE). A retrospective analysis of the baseline clinical–pathological presentation and treatment outcome (status of lipid profiles) was performed in two children with SLE, who presented with extreme hypertriglyceridemia over a follow-up period of four weeks. The children were treated with prednisolone, mycophenolate mofetil (MMF), hydroxychloroquine and hypolipidemic agents, depending on their disease status. On serial follow-up, the first child showed a significantly raised serum triglyceride level after receiving one week of oral prednisolone therapy. Anti-lipoprotein-lipase (LPL) autoantibody was absent. Lipid profile levels of this child gradually improved after replacing oral prednisolone with another immunosuppressant, namely MMF. The second child presented with extreme hypertriglyceridemia with positive anti-LPL autoantibody. She responded to plasmapheresis followed by increasing the dose of immunosuppressant. So, extreme hypertriglyceridemia in children with SLE may be steroid induced or due to presence of anti-LPL auto antibody. Management should be individualized depending on the etiology.
La hipertrigliceridemia es común en niños con lupus eritematoso sistémico. Un análisis retrospectivo de la presentación clínico-patológica al inicio del estudio y los resultados del tratamiento (estado de los perfiles lipídicos) se llevó a cabo en 2 niños con lupus eritematoso sistémico, que presentaron hipertrigliceridemia extrema durante un período de seguimiento de 4 semanas. Los niños fueron tratados con prednisolona, micofenolatomofetil, hidroxicloroquina y agentes hipolipidemiantes, dependiendo de su estado de salud. En el seguimiento, el primer niño mostró un nivel de triglicéridos en suero significativamente elevado después de recibir una semana de tratamiento con prednisolona oral. No existían anticuerpos antilipoproteína lipasa. Los niveles de perfil de lípidos de este niño mejoraron gradualmente después de sustituir la prednisolona oral con otro inmunosupresor, a saber micofenolatomofetil. El segundo niño presentó hipertrigliceridemia extrema con autoanticuerpos antilipoproteína lipasa-positivos. Ella respondió a la plasmaféresis seguida del incremento de la dosis de inmunosupresores. Por lo tanto, la hipertrigliceridemia extrema en niños con lupus eritematoso sistémico puede ser inducida por esteroides o debido a la presencia de autoanticuerpos antilipoproteína lipasa. El manejo debe ser individualizado en función de la etiología.
Patients suffering from systemic lupus erythematosus (SLE) may present with extreme hypertriglyceridemia either due to disease itself or due to drug-related toxicity, though exact prevalence is unknown and extreme hypertriglyceridemia is rarely reported and generally well tolerated in children with SLE.1 Here, we describe two case scenarios belonging to two ends of the same spectrum, i.e., hypertriglyceridemia in children with SLE, necessitating completely different treatments. Appropriate approval of our institutes’ ethical committee was obtained. Informed consent was obtained from parents of all individual participants included in the study.
Case scenariosCase scenario 1An 11-year-old girl was admitted at our institute with anasarca, maculopapular rash over the face and hematuria (urine RBC plenty, urine protein–creatinine-ratio 1:7). Her blood pressure was normal for age, sex and height as per centile charts.2 Investigations revealed mildly deranged renal function tests (BUN 88mg/dl, creatinine 1.2mg/dl) along with low C3 levels (35mg/dl) and a positive ANA (titer >1:160), but complete blood count was unremarkable. She was also shown positive for anti-double-stranded DNA (dsDNA) (3+) and anti-nucleosome (3+) antibodies by immunofluorescence assay. Renal biopsy specimen confirmed changes of lupus nephritis stage II. Her lipid profile at presentation was serum triglyceride 172mg/dl and serum cholesterol 233mg/dl (HDL 44mg/dl, LDL 106mg/dl, VLDL 38mg/dl). She was started on oral prednisolone (2mg/kg/day), along with hydroxychloroquine (7mg/kg/day) and rosuvastatin (5mg/day) after obtaining due approval from her parents.
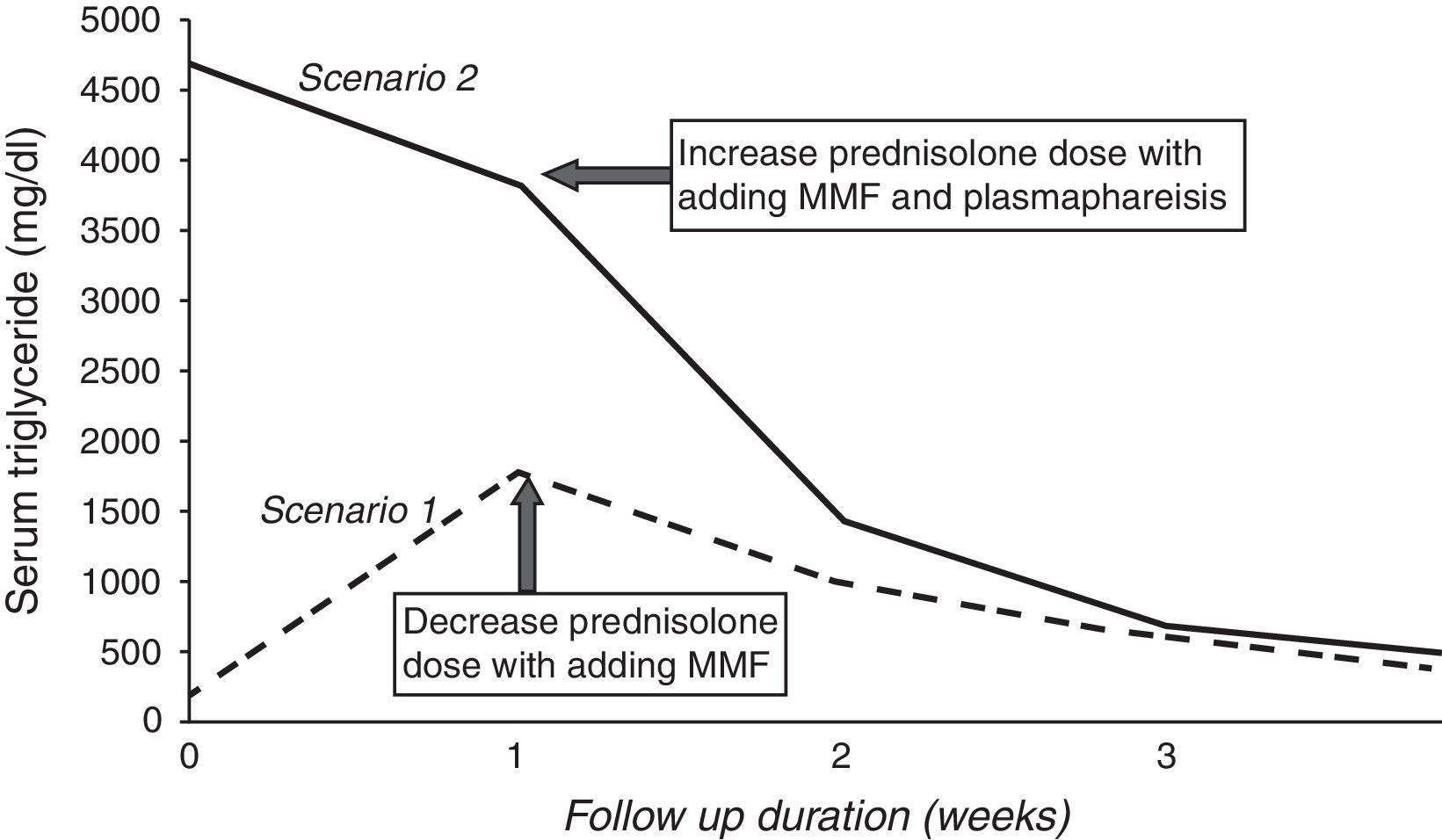
We repeated investigations after one week of oral prednisolone and rosuvastatin usage. Surprisingly, lipid profile levels were found to be markedly elevated (triglyceride 1786mg/dl, cholesterol 350mg/dl, LDL 275mg/dl, HDL 30mg/dl and VLDL 88mg/dl). She had also developed mild cushingoid features. Considering the possibility of an autoimmune phenomena, anti-lipoprotein-lipase (LPL) antibody (reactivity of anti-LPL immunoglobulin G was determined by enzyme-linked immunosorbent assay) estimation was done to find out the cause of hyperlipidemia. The report turned out to be negative. Hence, we were left with the possibility of steroid-induced hypertryglyceridemia. Prednisolone dose was rapidly tapered to 0.5mg/kg/day. On serial follow-up, triglyceride levels of this child gradually reduced with decreasing doses of prednisolone (Fig. 1). At 4 weeks, her triglyceride level was 356mg/dl and cholesterol was 222mg/dl (HDL 38mg/dl, LDL 146mg/dl, VLDL 48mg/dl). Mycophenolate mofetil (MMF) at a dose of 1000mg/m2/day was added to the schedule and prednisolone was continued at 0.5mg/kg/day.
Case scenario 2A 10-year-old girl was admitted to our pediatric emergency with hypertensive encephalopathy. She had also been suffering from low-grade intermittent fever, joint pains and a non-pruritic rash over exposed parts of the body, which worsened on exposure to sunlight. Strikingly, routine blood samples drawn in the emergency room were lipemic. Samples sent for determining lipid-profile revealed marked hypertriglyceridemia (serum triglyceride 4696mg/dl) with hypercholesterolemia (serum cholesterol 514mg/dl, HDL 24mg/dl, LDL 357mg/dl, VLDL 133mg/dl). Complete blood counts revealed anemia (hemoglobin 8.7mg/dl) with thrombocytopenia (1.2lakh/cumm). Anti-nuclear (ANA, titer >1:160) and anti-ds DNA (3+) antibodies were positive. An MRI of the brain showed changes suggestive of acute disseminated encephalomyelilitis (ADEM). The child became fully conscious after successful control of hypertension over 48h at our pediatric intensive care unit. In view of SLE with ADEM, pulse methyl-prednisolone (30mg/kg/dose) was given for five days followed by oral prednisolone (2mg/kg/day).
Hydroxychloroquine (7mg/kg/day), antihypertensives namely labetalol (1mg/kg/dose) and amlodipine (10mg/day), along with hypolipidemic agent rosuvastatin (5mg/day) were also started along with fat-free diet. Due approval was obtained from the parents. Renal biopsy specimen confirmed changes of lupus nephritis stage II.
In this case, repeat investigations after one week of prednisolone and rosuvastatin therapy revealed a mild improvement in lipid profile levels. Serum triglyceride reduced to 3851mg/dl, cholesterol to 514mg/dl, HDL to 24mg/dl, LDL to 357mg/dl and VLDL to 133mg/dl. Another hypolipidemic agent, namely fenofibrate (45mg/day) was added. Plasmapheresis was done to rapidly reduce the levels of circulating triglyceride levels to prevent inflammation of the pancreas. Anti-LPL antibody turned out to be positive (samples were considered positive if optical density was ≥3 standard deviations above the mean optical density obtained for the 20 control samples from healthy individuals included in each assay) this time. The dose of oral prednisolone was hiked to 3mg/kg/day and MMF (800mg/m2/day) was also added. The child showed significant improvement in her lipid profile levels at the end of the second week (serum triglyceride 1435mg/dl, cholesterol 234mg/dl, HDL 54mg/dl, LDL 237mg/dl, VLDL 93mg/dl) (Fig. 1). However, there was worsening of hypertension along some cushingoid-features due to the high-dose of prednisolone. So, we tapered the dose of prednisolone and increased the dose of MMF to 1200mg/m2/day. At 4-week follow-up, the child was normotensive on two antihypertensives and her lipid profiles significantly improved. Her serum triglyceride was 457mg/dl and serum cholesterol was 184mg/dl (HDL 74mg/dl, LDL 137mg/dl, VLDL 83mg/dl) with decrease in the titer of anti-LPL antibody. Prednisolone was continued at a maintenance dose of 0.8mg/kg/day.
DiscussionIn this report, we have described two case scenarios with extreme hypertriglyceridemia in children with SLE. The first scenario was the more commonly encountered situation of steroid-induced hypertryglyceridemia, which was managed by tapering steroids and adding another immunosuppressant, MMF. The initial mild hyperlipidemia in this case may have been due to increased hepatic lipoprotein synthesis secondary to albuminuria.3 Although it is common, the exact prevalence of steroid-induced hypertriglyceridemia is yet to be determined in the pediatric population. Studies show an increased tendency of lipid profiles in post-transplant patients treated with cyclosporine-prednisolone combination.4 An experimental study on primates suggests that glucocorticoid-induced hypertriglyceridemia is primarily a consequence of increased hepatic-triglyceride production rates, through elevated plasma-free fatty acid and glucose levels.5 However, exact mechanism of steroid-induced hypertriglyceridemia has not been definitely established.
The second scenario depicts a situation where hypertriglyceridemia developed secondary to anti-LPL antibody. De Carvalho et al. found a prevalence of 46.7% of anti-LPL-antibodies in SLE in their study.6 Anti-LPL antibodies have been closely linked to elevated triglyceride levels and implicated in mechanisms of atherosclerosis in SLE and other autoimmune inflammatory diseases such as rheumatoid arthritis and systemic sclerosis.7–9 This observation was explained on the basis of decreased LPL activity in these patients, which in turn corroborates with triglyceride levels.7 Experimental studies have demonstrated that the association between autoimmune disease and LPL can lead to circulating LPL inhibitors, resulting in hyperchylomicronemia in patients with such diseases.10 One study speculates that anti-LPL antibodies may be associated with disease activity and markers of inflammation in SLE.6
The present literature gives very little data on extreme hypertriglyceridemia in children with SLE. While most are iatrogenic and drug related, many can be due to developing autoantibodies against LPL. Our cases reveal two very different mechanisms as well as management for the same disease presentation. This report suggests that estimation of anti-LPL antibody is crucial in managing extreme hypertriglyceridemia with SLE. Detection of anti-LPL antibody level above the cut-off titer necessitates an increase in the dose of prednisolone or addition of another immunosuppressant. On the flip side, if the antibody titer is below cut-off value, then we may have to reduce the dose of prednisolone. Although extreme hypertriglyceridemia is generally well tolerated by most SLE patients, there is always the possibility of complications such as pancreatitis.1 One other point of interest is the difficulty in controlling hypertriglyceridemia in these children with classical oral hypolipidemic agents.
To conclude, hypertriglyceridemia in SLE can be due to two very different causes. An estimation of anti-LPL autoantibody level is useful in determining the next step of management.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Financial supportNone.
Authors’ contributionsDr. BG Babu, Dr. S Bhattacharyya & Dr. B Basu – case study, patient management, data collection and analysis, preparation of manuscript.
Conflict of interestNone.
Dr. M Nandi and Prof. TKS Mahapatra.