Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting diarthrodial joints, in which patients tend to perform less physical activity (PA) than recommended. This review focuses on the existing evidence about the relationship of PA and RA, specifically how the former influences joint inflammation, disability, quality of life and pain in RA patients, and also how disease activity potentially impacts PA in these patients.
MethodsA literature search of EMBASE and MEDLINE databases from January 2000 to January 2015.
ResultsThe evidence indicating that PA in RA patients is safe and the benefits from regularly performing, both aerobic and resistance exercises, in these patients include improvement in: quality of life, functionality, pain and number of swollen joints. Interestingly, recent studies suggest that changes in disease activity in RA patients inversely correlate with variations in PA, as assessed by accelerometry.
ConclusionsThe regular monitoring of PA in RA patients might facilitate a more objective evaluation of variations in disease activity, helping physicians to make general and therapeutic recommendations that will improve both the health status and the joint functionality of these patients.
La artritis reumatoide (AR) es una enfermedad inflamatoria crónica que afecta a las articulaciones diartrodiales, en la que los pacientes tienden a realizar menos actividad física (AF) de lo que se recomienda. Esta revisión se centra en la evidencia existente sobre la relación entre AF y AR, específicamente cómo la primera influye en la inflamación articular, la discapacidad, la calidad de vida y el dolor en los pacientes con AR, y también cómo la actividad clínica de la AR puede afectar a la AF de estos pacientes.
MétodosSe realizó una búsqueda bibliográfica desde enero del 2000 hasta enero del 2015 en las bases EMBASE y MEDLINE.
ResultadosLa evidencia indica que la AF en pacientes con AR es segura y que los beneficios de la realización periódica de ejercicios, tanto aeróbicos como de resistencia, en estos pacientes incluyen mejoras en: calidad de vida, funcionalidad, dolor y número de articulaciones inflamadas. Curiosamente, estudios muy recientes muestran que los cambios en la actividad de la enfermedad en pacientes con AR se correlacionan inversamente con variaciones en su AF, medida por acelerometría.
ConclusionesEl control sistemático de la AF en pacientes con AR podría facilitar una evaluación más objetiva de las variaciones en la actividad de la enfermedad, lo que ayudaría a los médicos a hacer recomendaciones generales y terapéuticas para mejorar tanto el estado de salud como la funcionalidad articular de estos pacientes.
Although there are extensive data showing the beneficial effects of physical activity (PA) on cardiovascular disease and all-cause mortality, physical inactivity is a major health problem worldwide.1 The precise measurement of PA is key to study its association with diseases, but the assessment of PA is complex and it is conditioned by a balance between accuracy and ease of use.2
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory disorder of unknown etiology. Because the central pathology of RA occurs in the synovial membrane, joint limitation is a typical manifestation of the disease. This, in addition to the fact that most RA patients also suffer from muscle loss, contributes to decreased physical function and quality of life in these patients. In this regard, it has been shown that RA patients tend to exercise less than healthy controls when PA is measured by both subjective3–5 and objective methods.5–8 Moreover, there remain two important unanswered questions concerning PA vis-à-vis RA: (1) Do patients who are sufficiently physically active suffer less aggressive disease? and (2) Does disease activity influence PA in RA patients? With regard to the former, there is considerably more evidence favoring the prescribing PA for RA patients than there is against.9–11 With regard to the latter, although there is less evidence, recent data suggest that disease activity influences negatively the PA in RA patients.5,7
This work reviews the current evidence about the relationship between PA and RA, specifically how the former influences joint inflammation, disability, pain and quality of life in these individuals, and also how RA disease activity potentially impacts on PA in these patients. We purpose that periodic monitoring of PA using objective methods, as the accelerometry would help to better handling of RA patients.
Strategy and results of literature searchingA review was conducted by the documentation department of the Spanish Rheumatology Society (SER) to identify all published literature relating to PA and/or energy expenditure in people with RA. The search strategy combined 2 sets of keywords. For Embase, EMTREE terms were used. In this case the search consisted of rheumatoid arthritis AND physical activity OR motor activity OR energy expenditure OR leisure activity AND accelerometry OR questionnaire OR calorimetry. For MEDLINE, the MeSH terms were: arthritis, rheumatoid AND leisure activities OR motor activity OR energy metabolism AND accelerometry OR questionnaires. The results included all publications published from 1 January 2000 to January 2015, that included at least 1 search term from each of the 2 categories. Only English language publications were included.
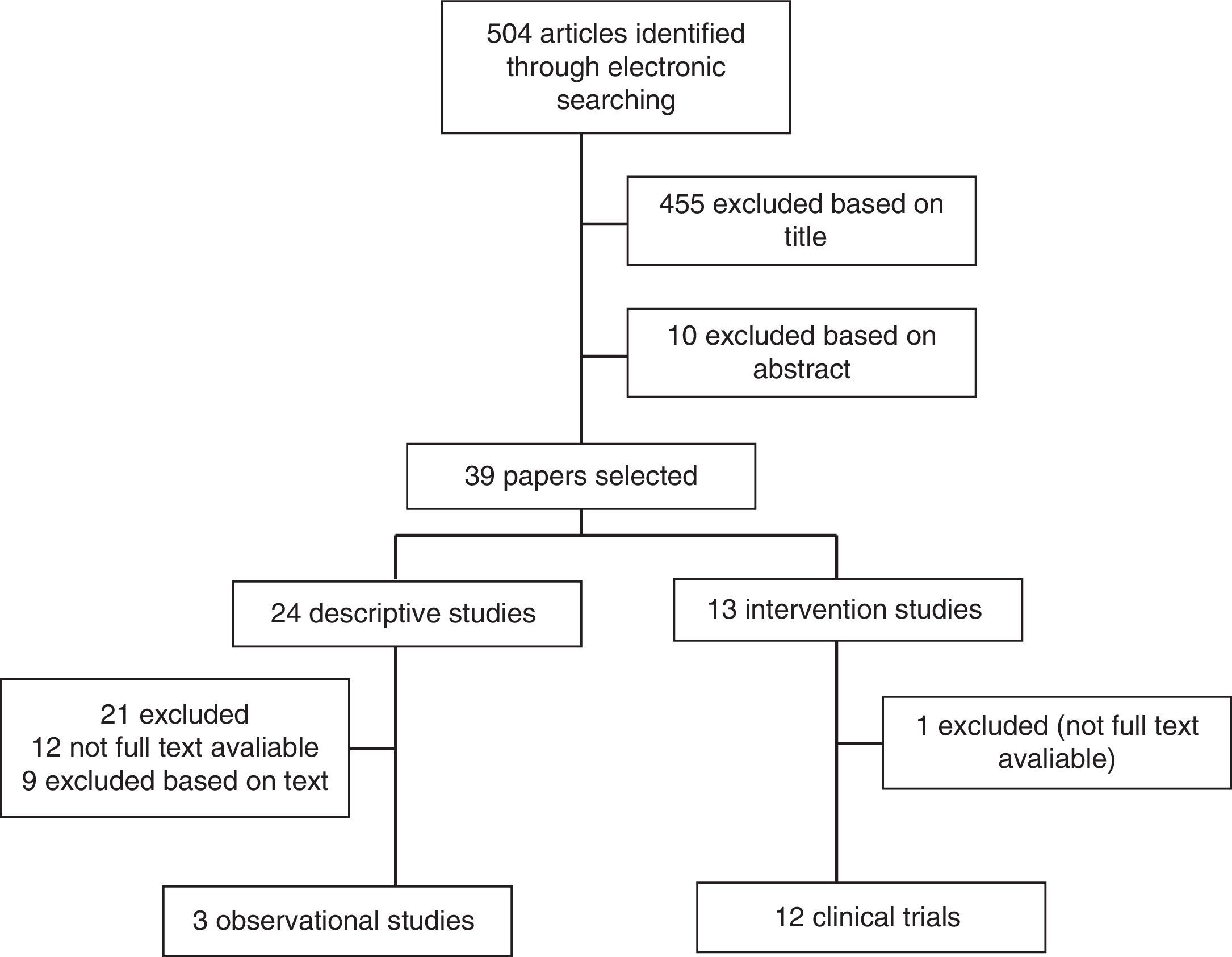
To be included in the review, studies had to be: (1) either observational studies or interventional studies with programs of exercises measuring free living PA or total/activity related energy expenditure levels, using either subjective or objective methods and (2) related to adult RA population with all subjects included in studies fulfilling the criteria set down by the American College of Rheumatology, 1987. Studies which were: (1) interventional in nature with new pharmacological treatments; (2) not in English; or (3) published only in abstract format were excluded. The article selection flowchart is described in Fig. 1.
Assessment of PAPA is defined as any bodily movement produced by skeletal muscles that result in energy expenditure.12 Under this broad concept, activities relating to leisure-time, exercise, sports, locomotion (e.g., walking, biking), and work must be considered parts of PA. Sedentarism has been defined as expenditure below 10% of total daily energy expenditure performing moderate- and high-intensity activities.13 Since, sedentarism has been associated with high incidences of obesity, type-2 diabetes mellitus, cardiovascular disease and other chronic illnesses,14 its prevention has become a priority of the public healthcare systems. To avoid the consequences of sedentarism, U.S. Department of Health and Human Services recommends that adults should do at least 150min a week of moderate-intensity exercise (e.g., brisk walking).15
A very accurate measurement of PA can be obtained by the assessing the total daily energy expenditure, albeit using expensive and cumbersome procedures such as the doubly-labeled water technique16 or calorimetry.2 Viable alternatives, involving less precise but more accessible methods, are available, however. These include questionnaires and triaxial accelerometers, approaches that are gaining acceptance for assessing PA both in healthy individuals and in patients suffering chronic diseases.17–19 Although questionnaires provide a evaluation limited by subjectivity, in the last 10 years a significant number of them have been developed to assess PA in different diseases; one of them, the International Physical Activity Questionnaire (IPAQ), has been used mainly for research purpose in rheumatic diseases.4,5,20 This self-report method is the cheapest and easiest way to quickly compile PA data from a large number of people providing an assessment of PA by domains. Alternatively, accelerometers are easily portable devices that offer one important advantage in objectively measuring PA based on the fact that it can capture continuously, for days and weeks distinctive characteristics of movement including the direction, frequency, intensity and duration of PA, as well as resting periods.21 Although accelerometry has already been employed in clinical osteoarthritis trials,22–24 it was not until recently that this technique was used to evaluate PA patterns in inflammatory joint disorders such as RA.5,7,8,25
PA in RA patientsRegular exercise with a moderate to high level of intensity has proven to be effective in improving muscle strength and cardiovascular fitness in healthy populations and in patients with chronic illnesses, including RA.11,26–28 RA, if left uncontrolled, leads to joint deformity and destruction due to the erosion of cartilage and bone. Consequently, it has been assumed than RA patients are less active than the general population because of such joint manifestations. An additional factor that may have contributed to this inactivity tendency stems from recommendations, classically given by physicians, which restrict exercise due to concerns that excessive PA might aggravate both joint inflammation29 and pain,30 and accelerate joint damage in RA patients. However, currently, evidence suggests that exercise has no deleterious effects either on disease activity or on joint damage27,31 and improves muscle strength32 in RA. In this regard, recently it has been suggested that RA patients who are physically active before clinical disease onset present with a milder disease, both in terms of inflammation, pain and function.33
Patients with RA have an increased risk of developing cardiovascular diseases, resulting from a proatherogenic profile driven by systemic inflammation.34 In healthy populations there is an inverse relationship between PA and cardiovascular risk (CVR) factors such as BMI, body composition (increased whole body fat and visceral fat)35 and blood lipid levels.36 Although different studies on the relationships between PA, BMI, fat mass and lipid levels in RA populations have yielded controversial data,5,37,38 the evidence clearly suggests that PA improves the CVR profile in RA patients.5,11,39
Different questionnaire-based surveys have indicated that RA patients tend to exercise less than what is currently recommended.3,40,41 When PA is objectively assessed by doubly-labeled water or accelerometry, the main disparity in PA between RA patients and healthy individuals can be seen in the time devoted to different intense activities. RA patients dedicate less time than control to high-intensity PA and to moderately intensive activities5,7,8,25 probably due to local limitations causing joint pain and disability and systemic effects like fatigue and sarcopenia. A recent systematic review of the literature about this issue, encompassing both objective and subjective methods for evaluating PA, suggests that PA levels among RA individuals tends to be lower than in healthy controls.42 Although, this review concluded that owing to methodological limitations, a definitive conclusion could not be drawn, currently it is generally accepted that RA patients spend less time than controls doing moderate and vigorous PA.
Effect of PA on joints and disease activity in RA patientsAs in the general population, regular PA in RA patients may, apart from providing general health gains, also yield disease-specific benefits such as reduced pain, improved muscle function, and delayed onset of disability,10,31,43–45 without deleterious effects on joint.27,31
Interestingly, moderate-intensity PA exerts anti-inflammatory effects both in healthy individuals and in those affected by various chronic illnesses.46 Indeed, regular PA has been associated with a diminution in C-reactive protein (CRP)11,39 and erythrocyte sedimentation rate (ESR)47 levels in RA patients. Although the exact mechanism by which these positive effects arise remains unknown, it seems linked to reductions in blood pressure, lipids, and fatty mass48. Taking into account that the main tissular source of IL-6 is the adipose tissue,49 and that several lipids have proinflammatory effects, the reduction of fat accumulation in the skeletal muscle and liver is likely one of the most relevant anti-inflammatory benefits of exercise. Furthermore, it has been well established that PA releases IL-6 from skeletal muscle, a potent inducer of IL-1 receptor antagonist (IL-1ra) by monocytes and macrophages, which constitutes an important anti-inflammatory mechanism of exercise.50
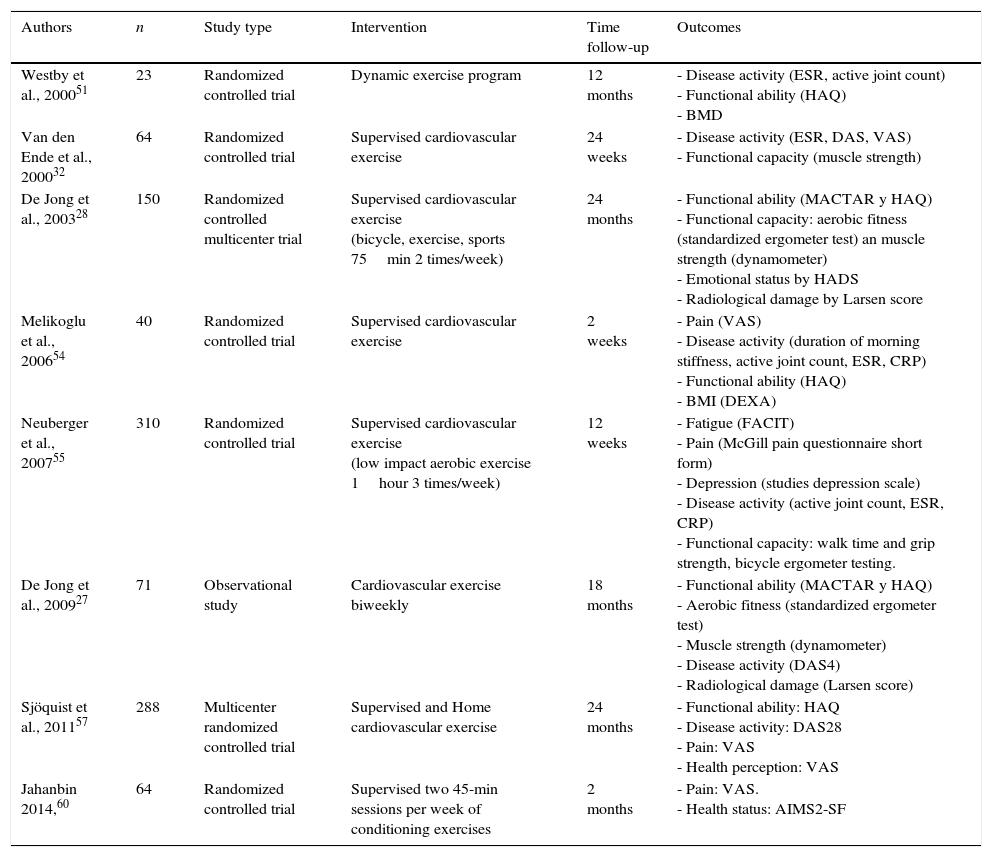
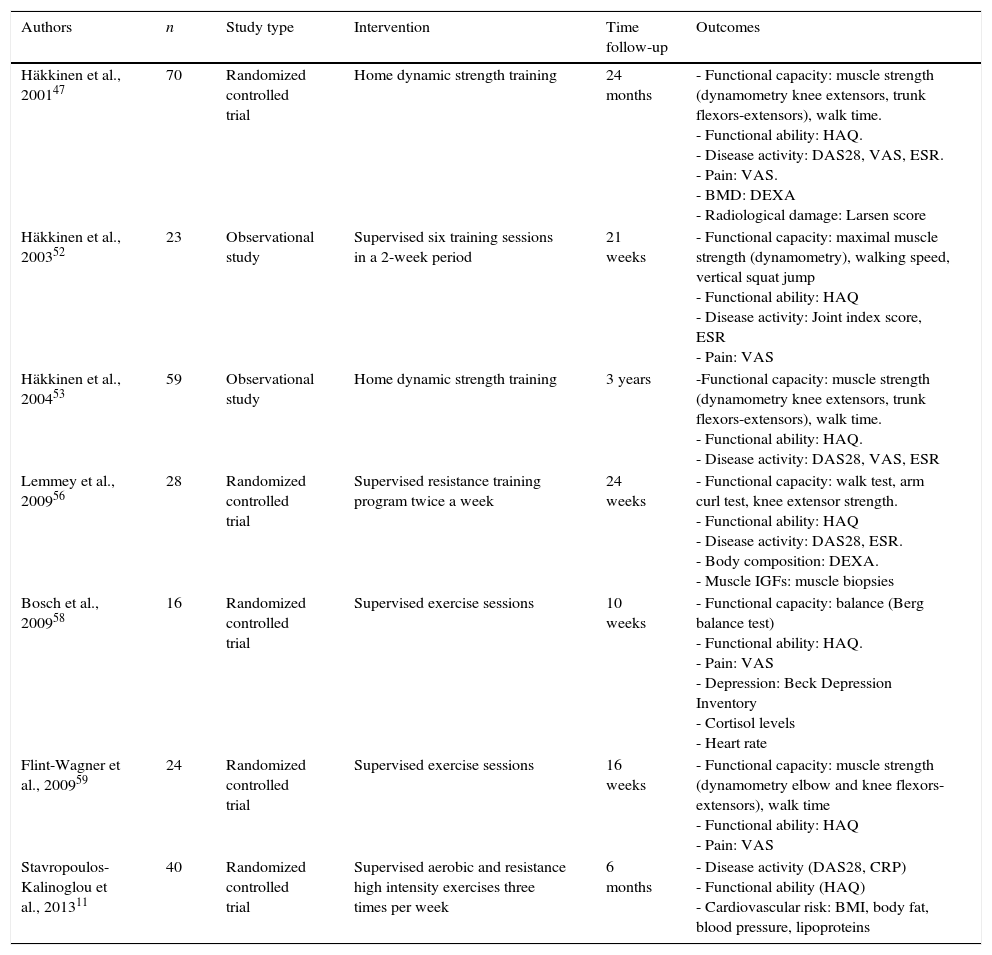
A number of clinical trials evaluating the effects of PA on RA patients have been developed. While much evidence suggests that some exercise is better than none at all, specific exercise parameters (intensity, duration, frequency, and type) resulting in real benefits have not been clearly defined. Some short-term and several long-term clinical trials have evaluated the effects of both aerobic and resistance exercises on outcome measures in RA patients11,27,28,32,47,51–60 (see Tables 1 and 2).
Studies of aerobic exercise programs in RA.
| Authors | n | Study type | Intervention | Time follow-up | Outcomes |
|---|---|---|---|---|---|
| Westby et al., 200051 | 23 | Randomized controlled trial | Dynamic exercise program | 12 months | - Disease activity (ESR, active joint count) - Functional ability (HAQ) - BMD |
| Van den Ende et al., 200032 | 64 | Randomized controlled trial | Supervised cardiovascular exercise | 24 weeks | - Disease activity (ESR, DAS, VAS) - Functional capacity (muscle strength) |
| De Jong et al., 200328 | 150 | Randomized controlled multicenter trial | Supervised cardiovascular exercise (bicycle, exercise, sports 75min 2 times/week) | 24 months | - Functional ability (MACTAR y HAQ) - Functional capacity: aerobic fitness (standardized ergometer test) an muscle strength (dynamometer) - Emotional status by HADS - Radiological damage by Larsen score |
| Melikoglu et al., 200654 | 40 | Randomized controlled trial | Supervised cardiovascular exercise | 2 weeks | - Pain (VAS) - Disease activity (duration of morning stiffness, active joint count, ESR, CRP) - Functional ability (HAQ) - BMI (DEXA) |
| Neuberger et al., 200755 | 310 | Randomized controlled trial | Supervised cardiovascular exercise (low impact aerobic exercise 1hour 3 times/week) | 12 weeks | - Fatigue (FACIT) - Pain (McGill pain questionnaire short form) - Depression (studies depression scale) - Disease activity (active joint count, ESR, CRP) - Functional capacity: walk time and grip strength, bicycle ergometer testing. |
| De Jong et al., 200927 | 71 | Observational study | Cardiovascular exercise biweekly | 18 months | - Functional ability (MACTAR y HAQ) - Aerobic fitness (standardized ergometer test) - Muscle strength (dynamometer) - Disease activity (DAS4) - Radiological damage (Larsen score) |
| Sjöquist et al., 201157 | 288 | Multicenter randomized controlled trial | Supervised and Home cardiovascular exercise | 24 months | - Functional ability: HAQ - Disease activity: DAS28 - Pain: VAS - Health perception: VAS |
| Jahanbin 2014,60 | 64 | Randomized controlled trial | Supervised two 45-min sessions per week of conditioning exercises | 2 months | - Pain: VAS. - Health status: AIMS2-SF |
AIMS2-SF: Arthritis Impact Measurement Scales 2 short form; BMD: bone mineral density; BMI: body mass index; CRP: c-reactive protein; DEXA: dual energy X-ray absorptiometry; ESR: erythrocyte sedimentation rate; HADS: Hospital Anxiety and Depression Scale; HAQ: Health Assessment Questionnaire; MACTAR: McMaster Toronto Arthritis patient preference disability questionnaire; VAS: visual analog scale.
Studies of strengthening programs in RA.
| Authors | n | Study type | Intervention | Time follow-up | Outcomes |
|---|---|---|---|---|---|
| Häkkinen et al., 200147 | 70 | Randomized controlled trial | Home dynamic strength training | 24 months | - Functional capacity: muscle strength (dynamometry knee extensors, trunk flexors-extensors), walk time. - Functional ability: HAQ. - Disease activity: DAS28, VAS, ESR. - Pain: VAS. - BMD: DEXA - Radiological damage: Larsen score |
| Häkkinen et al., 200352 | 23 | Observational study | Supervised six training sessions in a 2-week period | 21 weeks | - Functional capacity: maximal muscle strength (dynamometry), walking speed, vertical squat jump - Functional ability: HAQ - Disease activity: Joint index score, ESR - Pain: VAS |
| Häkkinen et al., 200453 | 59 | Observational study | Home dynamic strength training | 3 years | -Functional capacity: muscle strength (dynamometry knee extensors, trunk flexors-extensors), walk time. - Functional ability: HAQ. - Disease activity: DAS28, VAS, ESR |
| Lemmey et al., 200956 | 28 | Randomized controlled trial | Supervised resistance training program twice a week | 24 weeks | - Functional capacity: walk test, arm curl test, knee extensor strength. - Functional ability: HAQ - Disease activity: DAS28, ESR. - Body composition: DEXA. - Muscle IGFs: muscle biopsies |
| Bosch et al., 200958 | 16 | Randomized controlled trial | Supervised exercise sessions | 10 weeks | - Functional capacity: balance (Berg balance test) - Functional ability: HAQ. - Pain: VAS - Depression: Beck Depression Inventory - Cortisol levels - Heart rate |
| Flint-Wagner et al., 200959 | 24 | Randomized controlled trial | Supervised exercise sessions | 16 weeks | - Functional capacity: muscle strength (dynamometry elbow and knee flexors-extensors), walk time - Functional ability: HAQ - Pain: VAS |
| Stavropoulos-Kalinoglou et al., 201311 | 40 | Randomized controlled trial | Supervised aerobic and resistance high intensity exercises three times per week | 6 months | - Disease activity (DAS28, CRP) - Functional ability (HAQ) - Cardiovascular risk: BMI, body fat, blood pressure, lipoproteins |
BMD: bone mineral density; DEXA: dual energy X-ray absorptiometry; EQ-5D: EuroQol-5D; ESR: erythrocyte sedimentation rate; HAQ: Health Assessment Questionnaire; MHQ: Michigan Hand outcomes Questionnaire; SF-12: short form 12; VAS: visual analog scale.
Although intuitively, long-term regular exercise should be beneficial for RA patients because of the increased cardiovascular mobility and mortality in these patients, reports about aerobic exercise interventions in RA populations are scarce. A systematic review and meta-analysis of aerobic exercise,10 which examined 14 randomized controlled trials, showed that cardiorespiratory aerobic conditioning in stable RA appears to be safe and improves quality of life, functionality and pain levels, but not DAS28 or joint count. This meta-analysis suggests that the sooner the exercise program is initiated, the better are the results obtained in terms of quality of life and functionality. Other clinical trials published afterwards also found an improvement in functionality57 and pain57,60 in RA patients who were participating in aerobic exercise programs.
Both, program duration and disease duration impacted on pain, with better results in cases of RA established, and short-term programs. However, data concerning to quality of life suggested than exercise was more benefits in patients with early RA than in established RA patients. When patients were followed up for a long period of time,27 it was found that no significant decrease in aerobic fitness occurred after a relatively brief period of detraining; rather, long-term exercises produced a prolonged positive effect on functional ability. Therefore, the maintenance of a structured PA program after intervention seems to be critical for long-term benefits.57,61
Of all the studies referenced in this review that focused on aerobic exercise in RA patients (Table 1), three assessed disease activity changes using composite disease activity measurements.27,32,57 These studies concluded that aerobic exercise has a positive impact on disease activity, reducing DAS28, albeit without reaching statistical significance.
Resistance exercise in RA populationThe value of resistance exercise for RA patients remains controversial because its effects on cardiovascular risk are not clear,11,62 and its real impact on disability seems to be very small.56 However, despite design differences, the results of previous studies suggest that high-intensity strength training is feasible and safe for many patients with RA.52,56,63
A systematic review by Baillet et al.9 showed that resistance exercise programs in RA patients can provide a mild, but significant, improvement in terms of functional capacity, the number of tender and swollen joints and a decrease in ESR. Resistance exercise had no impact on DAS28 or on structural damage in any of the studies analyzed. The HAQ response has proven more variable in several reports,11,47,53 which may reflect the fact that this outcome was designed for monitoring patients in pharmacologic trials and is not be suitable for evaluating physical interventions in patients with mild disabilities. Although the short-term effects of strength training in RA patients have been described in several studies64–66 the length of time that positive results achieved after cessation remains controversial.32,53,67
There is no clear evidence of the superiority of supervised strengthening exercises versus home-based exercises, since the results are varied.
Effects of disease activity on PA in RA patientsSome studies indicate that PA levels (as measured by questionnaires or by accelerometry) have an inverse relationship with disease activity (as assessed by DAS28 or SDAI) in RA patients.5,7,25,68 One of these studies involved RA patients lacking lower-limb disease activity, which suggests that it is the state of inflammation as a whole that influences patients’ tendency to reduce their levels of PA.5 In these studies, accelerometry was sufficiently sensitive to detect PA-changes related with disease activity both in RA patients, who showed clinical improvements in response to treatment7 and in patients who suffered a disease flare.5
Several disease activity indexes – such as the DAS, CDAI, SDAI or ACR – are available and allow physicians to quantify changes in the disease activity of RA over time. These indexes are liable to certain limitations related to both patient subjectivity and inter- and intraobserver variability.69 As stated above, by measuring variations in PA, accelerometry appears to be sufficiently sensitive to register changes in the clinical activity of RA,5,7 and could be used as a complementary objective method for assessing changes in disease activity. Consequently, variation in accelerometry might aid physicians in optimizing the pharmacologic management of RA patients. In addition, accelerometry can help to determine whether or not PA level in a given patient is appropriate to reduce their cardiovascular risk, main cause of premature mortality in these patients.
Practical applications and exercise recommendationsAlong the last 15 years, a number of interventional clinical trials have been developed in order to obtain recommendations on aerobic (Table 1) and resistance exercises (Table 2) in RA patients. The high variability in the type, intensity and duration of PA studied, as well as limitations in trial quality and size, suggest that the conclusions of these studies should be still considered to be tentative and further research in the field of PA and RA is warranted. However, a number of practical recommendations can be elaborated respect to exercise in patients with RA.
All RA patients benefit from a balanced program of strengthening and endurance or aerobic exercise. Therefore, current data indicate that the benefits from performing moderate and high-intensity PA frequently (both aerobic and resistance exercises 2–3 times per week for 30–60min) in RA patients include: improved quality of life, functionality, reduced pain, improvement in the number of swollen joints, and lower levels of radiologic damage.11,28,31
Low-impact exercises, such as walking, swimming or biking are aerobic exercises recommended for arthritis patients. Aerobic exercise has been shown to improve cardiovascular fitness and patient quality of life, while reducing RA-associated disability and pain.10,54,55,60 As a result of the little number of studies, it has been suggested that the intensity levels in aerobic exercise should be moderate to hard (i.e., 60–85% of maximum heart rate), and such exercise should be performed 3 times weekly in sessions of 30–60min, divided in 3–4 periods of 15–20min a day. Progressive adjustment of the intensity level is recommended.70
The strengthening exercises are designed to increase the joint stability. Movements should be smooth, performed 8–10 times twice a day and without joint pain. The weight must be increased slowly and always that it does not cause pain. Information derived from the reviewed studies70 suggest that the load level objective for strengthening exercises should be moderate to hard (i.e., 50–80% of a maximal voluntary contraction), and said exercises should be performed along 20–30min 2–3 times per week. Exercises may be static or dynamic, done against body weight or with different kinds of equipment (resistance training equipment, pulley apparatuses, dumbbells, or elastic bands). Progressive adjustment of the load is recommended in a supervised clinical environment or at home under professional guidance.
Adherence is difficult to maintain in long-term programs. To this end, physicians must be in contact with other health professionals (i.e., occupational therapists, nurses, dietitians, psychologists, and social workers) in order to support and motivate patients by helping them to cope with the disease in a positive way. Health professionals must convince RA patients that becoming more physically active is the best choice they can make to take care of themselves and their joints.
Although there is a lack of studies designed to evaluate the role of PA during RA flare, the most extended attitude among physicians is to recommend a reduction in the duration and intensity of exercises, specifically avoiding the resistance exercise in these periods of high disease activity.
A study in 2013 involving an online survey of more than 7000 smartphone owners in the U.S. showed that 79% of people 18–44-years-old have their smartphones with them 22hours a day.71 The newest generation of smartphones now on the market features built-in accelerometer sensors. This technological advance, in tandem with the increased and continuous social connectedness exhibited by our society, can provide a convenient and inexpensive method for accurately evaluating PA in today's population, including RA patients. Although these devices have not yet been validated in RA patients, the implementation of such tools will enable clinicians to better gauge the actual PA undertaken by patients. Since there appears to be a relationship between levels of PA, disease activity5 and response to treatment7 in RA patients, the use of smartphones to evaluate PA might help physicians, not only in establishing recommendations about cardiovascular risk, but also in making therapeutic decisions in these patients.
Concluding remarksAerobic and resistance exercises, 2–3 times per week for 30–60min not only provide general health gains, but have also been shown to confer disease-specific advantages such as reduced pain, improved muscle function, and delayed onset of disability in RA patients. Consequently, a periodic assessment of PA constitutes a useful tool for adjusting the exercise habits of RA patients in order to reduce their high cardiovascular risks and improve locomotion function and health status in general. Since, disease activity negatively impacts the PA capacity of RA patients, the assessment of PA with objective methods (e.g., mobile devices built-in accelerometry) may be helpful to evaluate the overall response to treatment and disease activity in RA patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis work was supported by a grant from the Spanish Ministry of Health (Fondo de Investigaciones Sanitarias) (FIS PI12/02499) and cofinanced by the European Regional Development Fund to F.D.G and by REUNINVES (Asociación para la Ayuda a la Investigación en Reumatología del Hospital Universitario de Canarias).
Conflicts of interestThe authors have declared no conflicts of interest.
The authors would like to thank Mercedes Guerra from the Spanish Rheumatology Society for the systematic search of the literature, and the members of the Department of Rheumatology of Hospital Universitario de Canarias for their continuous support. Authors also are indebted with Prof. Jose Antonio L. Calbet, Department of Physical Education of University of Las Palmas de Gran Canaria for the critical reading of the manuscript.