Osteoarthritis is the most common joint disorder worldwide. The predominant symptom, pain, is usually treated with acetaminophen or oral non-steroidal anti-inflammatory drugs, although they are associated with a significant risk of side effects. Topical capsaicin may represent an effective and safe alternative.
The aim of this review is to examine the evidence for the efficacy and safety profile of topical capsaicin in the management of pain caused by osteoarthritis. Databases were searched for articles published between 2004 and 2016, in Portuguese, English or Spanish, using the search terms “capsaicin” and “osteoarthritis”. When compared to placebo, it was found that topical capsaicin has a good safety profile and efficacy in reducing osteoarthritis pain of the hand, knee, hip or shoulder. However, the studies have significant limitations, the most important the difficulty of blinding. It is attributed to this review the strength of recommendation B.
La osteoartritis es la enfermedad articular más común mundialmente. El síntoma predominante, el dolor, se trata generalmente con paracetamol oral o antiinflamatorios no esteroideos, a pesar de que están asociados con un riesgo significativo de efectos secundarios. Capsaicina tópica puede representar una alternativa eficaz y segura.
El objetivo de esta revisión es examinar la evidencia disponible acerca de la eficacia y del perfil de seguridad de la capsaicina en el tratamiento del dolor. Se realizaron búsquedas en bases de datos de artículos publicados entre 2004 y 2016, en portugués, inglés o español, utilizando los términos «capsaicina» y «osteoartritis». En comparación con el placebo, la capsaicina tiene un buen perfil de seguridad y eficacia en la reducción del dolor de la osteoartritis de la mano, la rodilla, la cadera o el hombro. Sin embargo, los estudios tienen limitaciones significativas, principalmente la dificultad de cegamiento. Se atribuye a esta revisión una fuerza de recomendación B.
Osteoarthritis (OA) is the most common joint disorder worldwide.1 It is estimated that approximately 18% of women and 10% of men above 60 years old have symptomatic OA and that more than 50% of people over the age of 65 have radiological evidence of OA.2–4 Ageing populations are expected to make OA the fourth leading cause of disability by the year 2020.4
The management of OA ranges from non-pharmacologic interventions and drugs to surgical approach.5–8 With no current cure for OA, treatment is directed towards reducing pain and stiffness, improving joint mobility and quality-of-life and preventing progression of disease.5,9,10 Non-pharmacologic interventions are the primary approach to the management of OA.10 Physical exercise, weight loss, physiotherapy and patient education are some of the proposed interventions.6,10 Pharmacologic treatment should be considered when pain or functional status does not respond to non-pharmacologic interventions.6,10 Acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) are the most used drugs.6,10 However, high doses of acetaminophen are associated with liver toxicity and oral NSAIDs increase the risk of gastrointestinal and renal adverse effects.6,10,11
Topical agents, such as NSAIDs and capsaicin are valuable choices when systemic side effects of some drugs are not acceptable.6,11,12 Capsaicin is the neurotoxin of chilli peppers and it is the compound that makes them taste “hot”.13 It binds selectively to the vaniloid compound receptor (TRPV1) of type C afferent fibres and increases P substance in synaptic cleft.13,14 While first applications of capsaicin are associated with a burning sensation over the applied surface, after continued use, persistent desensitization and analgesia occurs both due to P substance neural depletion and reversible and selective destruction of primary afferent fibres.13–15 The selective neuronal destruction assumes greater importance in OA due to the abundance of nociceptive fibres on joint cartilage.16 The main indications of capsaicin are the treatment of pain from post-herpetic neuralgia, diabetic neuropathy, rheumatoid arthritis and OA.12,13 Capsaicin presents a good safety profile. Local skin irritation and burning sensation, the two most important side-effects, are commonly identified in about 40% of patients.13 Besides its potential benefit over other drugs, capsaicin still faces great reluctance in medical community due to doubts about its therapeutic efficacy.
The purpose of this article was to review the evidence regarding the efficacy and safety profile of topical capsaicin in the treatment of pain from OA.
MethodsSearch strategyThe following electronic databases were searched: National Guideline Clearinghouse, Canadian Medical Association Practice Guidelines Infobase, Evidence-based Medicine Guidelines, National Institute for Health and Care Excellence, Royal College of Physicians, The Royal Australian College of General Practitioners, The Cochrane Library, DARE, Bandolier, Medline, TRIP database and Index of Portuguese Medical Journals. The following keywords were applied as search terms: “capsaicin” and “osteoarthritis”.
Selection criteriaGuidelines, meta-analysis, systematic reviews and randomized controlled trials (RCTs), published between January 2004 and January 2016, in Portuguese, English or Spanish, were searched. We included articles whose population have been diagnosed with OA, not undergoing arthroplasty. Any form of topical capsaicin (gel, cream, ointment, solution), used alone and compared with a control (placebo or no treatment at all) was included. The measured outcome was the reduction of pain. Secondary outcome included any adverse reaction.
Exclusion criteria were: disagreement with the goal of review; duplicate publication; opinion manuscripts and consensus guidelines; classical review papers and summaries of websites; clinical trials included in recent systematic reviews; and systematic reviews with the same total RCTs as the latest review articles.
Study selectionTwo investigators independently assessed the titles of the articles found and excluded duplicates and those clearly irrelevant. Abstracts of the selected articles were examined independently by the two reviewers who applied the selection criteria. If the information in the abstracts was not enough, full papers were analyzed to make a decision. Where disagreements of selections arose, these were discussed until consensus was reached.
Data extractionData were extracted from each eligible study by a single reviewer and checked by the second reviewer. The data items extracted were: number of trials included; number of persons recruited to the trials; type of intervention and control; length of follow-up; evaluated endpoint; results achieved in efficacy and safety; final recommendations.
Evaluation of evidenceTo evaluate the level of evidence and the strength of recommendation, the Strength of Recommendation Taxonomy (SORT) of the American Academy of Family Physicians was used.17 When the classification was based on other grading scales, we described its meaning.
ResultsA total of 120 studies were found, and, from these, 114 were excluded and 6 fulfilled the inclusion criteria: three systematic reviews and three guidelines.
Excluded studies were mainly duplicates, studies on diseases other than OA, study designs other than guidelines, meta-analysis, systematic reviews and RCTs, studies of other drugs or of complementary medicines such as acupuncture, studies whose control was active, systematic reviews with the same total RCTs as the latest review articles and studies on animals. Fig. 1 describes the process of identification of relevant studies. All studies included are summarized in Tables 1 and 2.
Systematic reviews.
| Reference | Included studies | Results/conclusions | Level of evidence (SORT)17 |
|---|---|---|---|
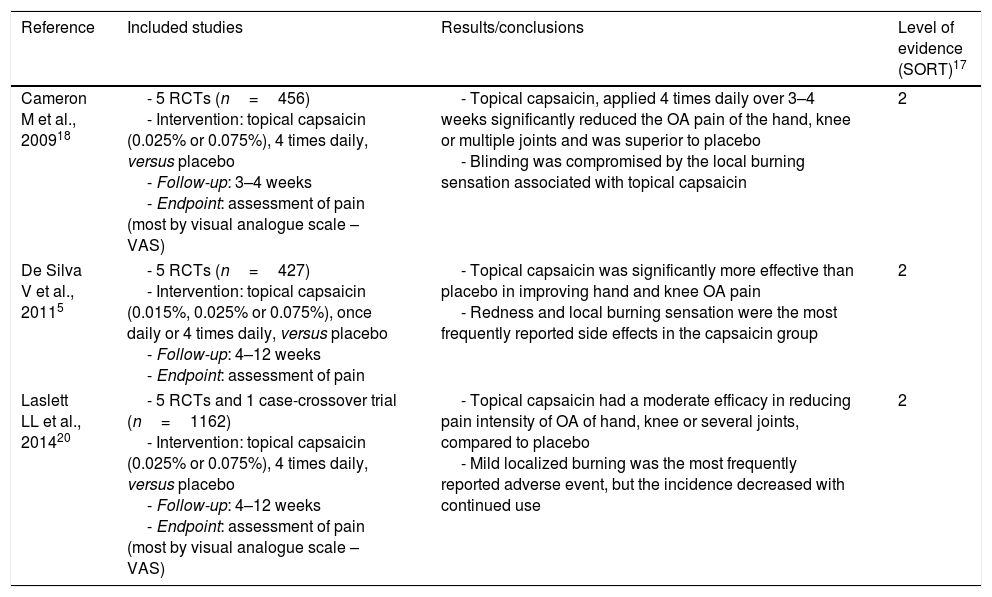
| Cameron M et al., 200918 | - 5 RCTs (n=456) - Intervention: topical capsaicin (0.025% or 0.075%), 4 times daily, versus placebo - Follow-up: 3–4 weeks - Endpoint: assessment of pain (most by visual analogue scale – VAS) | - Topical capsaicin, applied 4 times daily over 3–4 weeks significantly reduced the OA pain of the hand, knee or multiple joints and was superior to placebo - Blinding was compromised by the local burning sensation associated with topical capsaicin | 2 |
| De Silva V et al., 20115 | - 5 RCTs (n=427) - Intervention: topical capsaicin (0.015%, 0.025% or 0.075%), once daily or 4 times daily, versus placebo - Follow-up: 4–12 weeks - Endpoint: assessment of pain | - Topical capsaicin was significantly more effective than placebo in improving hand and knee OA pain - Redness and local burning sensation were the most frequently reported side effects in the capsaicin group | 2 |
| Laslett LL et al., 201420 | - 5 RCTs and 1 case-crossover trial (n=1162) - Intervention: topical capsaicin (0.025% or 0.075%), 4 times daily, versus placebo - Follow-up: 4–12 weeks - Endpoint: assessment of pain (most by visual analogue scale – VAS) | - Topical capsaicin had a moderate efficacy in reducing pain intensity of OA of hand, knee or several joints, compared to placebo - Mild localized burning was the most frequently reported adverse event, but the incidence decreased with continued use | 2 |
OA: osteoarthritis; RCTs: randomized controlled trials.
Guidelines.
| Reference | Recommendations |
|---|---|
| Royal College of Physicians, 20082 | Topical capsaicin should be considered as an adjunct to core treatment (education, exercise and weight loss) for knee and hand OA – strength of recommendation B (limited-quality patient-oriented evidence). |
| Royal Australian College of General Practitioners, 20093 | Topical capsaicin is recommended, alone or in combination with other drugs, for the short-term treatment of mild-moderate persistent symptoms of hip and knee OA, and for acute flares of symptoms – grade of recommendation D (body of evidence is weak and recommendation must be applied with caution) |
| American College of Occupational and Environmental Medicine, 201121 | Topical capsaicin is recommended for chronic hand OA or acute flares of OA – strength of recommendation C (the intervention is recommended, although there is limited evidence regarding the benefits of this intervention) |
OA: osteoarthritis.
The systematic review of Cameron et al.18 (Table 1) included thirty-five RCTs that examined the effects of herbal medicinal products in patients diagnosed with OA according to the American College of Rheumatolgy (ACR) criteria. Five RCTs (n=456) compared the analgesic effect of topical capsaicin (0.025% or 0.075%, four times daily) in OA of the hand, knee or multiple joints (hip, knee, shoulder and hand) with placebo. All studies assessed pain (four of them by visual analogue scale – VAS), with similar and consistent results among themselves after three to four weeks of treatment. After three weeks the mean difference (MD) was −18.50% in terms of VAS percentage changes, with a confidence interval (CI) reported at 95% of −40.95 to 3.95. After four weeks, the results were similar (MD −13.90%, CI −32.39 to 4.59). Absolute VAS pain scores after three to four weeks were in favour of capsaicin as well. Thus, topical capsaicin, applied four times daily over three to four weeks reduced the OA pain and was superior to placebo.
Although the placebo cream appear indistinguishable from the capsaicin, placebo validity and blinding was compromised by the local burning sensation associated with topical capsaicin. In one study, this side effect occurred in 44% of participants treated with capsaicin and in only one treated with placebo.
Despite adequate sample size and consistent results for all studies, the level of evidence attributed to this review was two (limited-quality patient-oriented evidence),17 since the allocation concealment was unclear in each of the studies and the blinding was problematic.
In the systematic review of De Silva et al.5 fifty six studies were included to evaluate the evidence regarding complementary and alternative medicine in the treatment of OA (Table 1). Five RCTs (n=427) tested the efficacy of capsaicin gel (0.015%, 0.025% or 0.075%, once daily or four times daily) in the treatment of hand or knee OA compared to placebo. A median Jadad score19 of four was attributed by the authors. The sample sizes ranged from fourteen to two hundred patients and the duration of the studies between four and twelve weeks. In all RCTs it was found that topical capsaicin was significantly more effective than placebo in improving OA pain. After four weeks of treatment, there was a 33% reduction in pain intensity compared with 20% on placebo, whereas in the twelve-week study the reductions were 53 and 27%, respectively. Redness and local burning sensation were the most frequently reported side effects in the capsaicin group (44–46% of patients in two studies).
The main limitations of this study were the small number of trials, the small number of patients in some RCTs and the possibility of publication bias (submission of studies with unfavourable results less likely). The double-blinding method was not assessed in this study. Comparing with the systematic review of Cameron et al.,18 this review included only one new RCT.
Although there is consistent evidence that capsaicin gel is effective in the management of OA, the primary data is of low quality. As such, the level of evidence two was attributed to this review.17
The systematic review of Laslett et al.20 included five RCTs, and one case-crossover trial (n=1162) in which the analgesic effect of topical capsaicin (0.025% or 0.075%, four times daily) was compared with placebo, in the OA of the hand (one study), knee (three studies) or several joints (hand, knee, hip or shoulder) (two studies) (Table 1). Selection criteria varied between trials but patients typically have at least moderate pain and radiological and/or clinical evidence of OA. The sample sizes ranged from fourteen to 695 hundred patients. Most studies applied VAS to assess pain after four to twelve weeks of treatment. It was found that topical capsaicin had a moderate efficacy in reducing pain intensity after four to twelve weeks of treatment, compared to placebo. Indeed, over four weeks of treatment the change in VAS pain score was moderate (standardized mean difference 0.44; 95% CI 0.25–0.62). These results were consistent across trials, suggesting no differences between different doses of capsaicin and between different application sites. It was also reported that continuing use of capsaicin beyond four weeks was beneficial. One study, in a long-term open label extension, reported that differences between groups increase over time, even up to 20 weeks. However, four weeks is probably sufficient to support a therapeutic trial.
Mild localized burning was the most frequently reported adverse event (35–100% of patients affected, with a risk ratio of 4.22, 95% CI 3.25–5.48). It was also reported a greater mean application discomfort score at baseline in the capsaicin group (2.41; 95% CI 1.52–3.30) compared to placebo (0.90; 95% CI 0.21–1.59) (p<0.001). However, burning decreased with continued use. As such, topical capsaicin was considered safe, with no reports of systemic toxicity. Besides, the burning sensation was not associated with clinical response.
The main limitations mentioned in this review were the difficulty of treatment blinding due to the burning sensation associated with capsaicin, the short duration of RCTs and the limited data of capsaicin efficacy in patients with OA at other sites than hand or knee (specially non-superficial joints such as the hip).
Overall, this study suggests that capsaicin should be used for superficial joints like the hand or knee over twelve weeks and possibly longer. The level of evidence two was attributed to this review.17
GuidelinesThe guideline from the Royal College of Physicians2 (Table 2) developed its recommendations using studies with high or very high levels of evidence. Four RCTs were found on topical capsaicin given four times daily in OA patients, compared with placebo. These trials evaluated the impact of treatment on pain and morning stiffness, functional capacity and quality of life, with samples of variable size (59–200 patients) and a short study duration (four to twelve weeks). They also differed in terms of osteoarthritis site: knee and shoulder osteoarthritis were evaluated in a RCT; knee osteoarthritis in the second RCT; knee, hip, shoulder and hand osteoarthritis in the third RCT; and hand osteoarthritis in the fourth RCT. It was found that topical capsaicin was superior to placebo in reducing pain intensity (assessed by VAS) and articular tenderness, the difference being statistically significant in most of the variables.
One RCT reported more adverse events in the capsaicin group (69% of patients) compared to placebo (30% of patients). However, no serious side effects have been reported with the use of topical capsaicin.
Thus, based on the evidence, this guideline recommended that topical capsaicin should be considered as an adjunct to core treatment (education, exercise and weight loss) for knee and hand OA with a strength of recommendation B (limited-quality patient-oriented evidence).
The guideline from the Royal Australian College of General Practitioners3 (Table 2) developed recommendations on the non-surgical management of hip and knee OA in primary health care. A single RCT of low quality was found, which included two hundred patients with hip (n=33), knee (n=66), shoulder or hand OA. Topical capsaicin (0.025%, four times daily) was more effective than placebo in improving pain (measured by VAS) after six weeks of treatment.
Its use was associated with local adverse events such as transient local burning and erythema, having higher baseline discomfort scores (averaged over the first 5 days) than other groups. However, this diminishes with continued use. No serious side effects have been reported with the use of topical capsaicin. The authors recommend that the application of capsaicin cream should be with a glove to prevent inadvertent spread to eyes and other mucous membranes.
The main limitation mentioned in this guideline was the small number of participants with OA of the hip and knee in each group, hindering analysis of differences between the groups.
Based on this evidence, the use of topical capsaicin was recommended, alone or in combination with other drugs, for the short-term treatment of mild-moderate persistent symptoms of hip and knee OA, and for acute flares of symptoms. The grade of recommendation D (body of evidence is weak and recommendation must be applied with caution) was attributed.
The guideline from the American College of Occupational and Environmental Medicine21 (Table 2) also issued recommendations supported on clinical evidence and oriented for primary health care. The use of topical capsaicin was recommended for chronic hand OA or acute flares of OA, with a strength of recommendation C (the intervention is recommended, although there is limited evidence regarding the benefits of this intervention).
DiscussionThe available evidence supports the effectiveness of topical capsaicin in reducing OA pain, compared to placebo. As showed by the 2011 comparative efficacy review of Chou et al. topical capsaicin was superior to placebo for 50% pain reduction22 and according to the 2004 systematic review of Mason et al. the number needed to treat at four weeks with capsaicin 0.025% was 8.1.13 The 2014 OARSI consensus guidelines regarding the non-surgical management of knee osteoarthritis formally recommend capsaicin for the treatment of knee-only OA patients without relevant co-morbidities such as cardiovascular risk factors, renal failure, gastrointestinal bleeding, depression or physical impairment limiting activity, including obesity.23 It is uncertain that individuals with multi-joint OA or with relevant co-morbidities benefit from capsaicin.23 On the other hand the 2012 ACR recommendations on OA conditionally recommend topical capsaicin for the initial management of hand OA; however it should not be used for the initial management of knee OA.24
In addition to its analgesic efficacy, topical capsaicin has a good safety profile. The most common adverse effect is localized burning sensation which typically presents as a mild to moderate intensity symptom and usually decreases with continued use due to persistent desensitization and P substance neural depletion.13–15 According to Mason et al. one in three patients treated with capsaicin will experience a local adverse effect and one in ten patients treated with capsaicin will withdraw treatment due to its side effects.13 Since its potential for systemic toxicity and drug interactions is reduced, topical capsaicin may be considered an option in polymedicated patients. In the study of Fraenkel et al.25 published in 2004 asking the preferences of older patients in the choice of OA treatment (non-steroidal anti-inflammatory drugs, opioids, capsaicin, glucosamine and chondroitin sulphate), it was found that patients might be willing to accept less effective treatments in favour of a lower probability of adverse effects, highlighting the importance of safe therapeutic options, such as capsaicin, in this age group.
Unfortunately, the available RCTs have a short duration (three to twelve weeks) and samples of reduced size. The main limitation reported in these trials was the difficulty of creating double blinding conditions, due to local burning sensation that may occur with topical capsaicin application. Thus it is attributed to this review the strength of recommendation (SORT) B (recommendation based on inconsistent or limited-quality patient-oriented evidence).17
Despite the effectiveness and the good safety profile of topical capsaicin, further studies are needed with larger samples and longer periods of follow-up.
Some authors have argued that capsaicin leads to a selective destruction of primary afferent fibres, which contributes to analgesia.13 This neuronal destruction assumes greater importance in OA due to the abundance of nociceptive fibres on joint cartilage.16 Nolano et al. have showed significant loss of epidermal nerve fibres within a few days of capsaicin 0.075% use, although once it is discontinued, reinnervation takes place (over six weeks after three weeks of treatment) with almost full return of sensation.26 As far as we know, it is uncertain whether chronic topical capsaicin may disrupt the process of nerve regeneration and its effect in patients with ongoing nerve disease, so studies in these areas are also needed.
The response predictors must also be determined and its effectiveness and safety should be compared with other topical and oral analgesics such as non-steroidal anti-inflammatory drugs. To identify which patients may benefit most from capsaicin treatment, the analgesic efficacy according to the location of the OA should be determined as well.
It is important to bear in mind that although capsaicin might be less effective, it may play an important role whenever even a small reduction of pain is beneficial.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe authors declare that there is no funding.
Conflict of interestsThe authors declare no conflict of interest.