Recent evidence shows that COVID-19 infection does not have a worse prognosis in patients with immune-mediated inflammatory diseases (IMID), although they develop a worse response to vaccination.
ObjectiveTo compare the incidence of COVID-19 and clinical features in patients with IMID between the first and sixth waves.
MethodProspective observational study of two cohorts of IMID patients diagnosed with COVID-19. First cohort March to May 2020, and second cohort December/2021 to February/2022.
Sociodemographic and clinical variables were collected and, in the second cohort, COVID-19 vaccination status. Statistical analysis established differences in characteristics and clinical course between the two cohorts.
ResultsIn total, 1627 patients were followed up, of whom 77 (4.60%) contracted COVID-19 during the first wave and 184 in the sixth wave (11.3%). In the sixth wave, there were fewer hospitalisations, intensive care unit admissions, and deaths than in the first wave (p=.000) and 180 patients (97.8%) had at least one dose of vaccine.
ConclusionEarly detection and vaccination have prevented the occurrence of serious complications.
Las últimas evidencias revelan que la infección por COVID-19 no tienen peor pronóstico en los pacientes con enfermedades inflamatorias inmunomediadas (EIMI), aunque desarrollan menor respuesta a la vacunación.
ObjetivoComparar la incidencia de COVID-19 y características clínicas en pacientes con EIMI entre la primera y sexta olas.
MétodoEstudio observacional prospectivo de 2 cohortes de pacientes con EIMI diagnosticados de COVID-19. Primera cohorte: marzo-mayo de 2020; segunda cohorte: diciembre/2021 a febrero/2022.
Se recogieron variables sociodemográficas y clínicas, y en la segunda cohorte el estado de vacunación contra la COVID-19. El análisis estadístico estableció las diferencias de las características y la evolución clínica entre ambas cohortes.
ResultadosDe un total de 1.627 pacientes en seguimiento, contrajeron COVID-19 durante la primera ola 77 (4,60%) y 184 en la sexta (11,3%). En la sexta hubo menos hospitalizaciones, ingresos en cuidados intensivos y fallecimientos que en la primera (p=0,000) y 180 pacientes (97,8%) tenían al menos una dosis de vacuna.
ConclusiónLa detección precoz y la vacunación han evitado la aparición de complicaciones graves.
SARS-CoV-2 disease was first identified in China in late 2019. Its mechanism of infection via droplet, aerosol, and contact makes it very transmissible and hence its rapid spread to the rest of the world.1,2 Risk factors for a poorer COVID-19 outcome include advanced age (>65 years), chronic diseases and comorbidities, immunosuppression, being under immunosuppressive/immunomodulatory treatment such as patients with immune-mediated inflammatory diseases (IMIDs).3,4 Monitoring for early detection of SARS-CoV-2 infection was recommended in the latter, and in the event of COVID-19 infection, suspension of treatment advised on an individual basis.5–7 The new mRNA vaccines against SARS-CoV-2 have shown high efficacy in the general population and are one of the main preventive strategies in patients receiving immunosuppressive/immunomodulatory therapy. However, a lack of response to vaccination has been detected IMID patients, but there is evidence that, in those who do not respond to the first dose of vaccine, booster doses increase efficacy by up to 47%.8
In the first wave, nurses undertook telematic follow-up and treatment monitoring. However, in the sixth wave, face-to-face care prevailed over telematic care, which mainly covered vaccination-related queries. Therefore, the aim of our study was to compare the incidence of COVID-19 in IMID patients between the first and sixth waves of the pandemic, and to describe the clinical characteristics of patients treated in a centre specialising in these diseases.
Material and methodsWe conducted a prospective observational study of 2 cohorts of patients under follow-up/treatment in an IMID centre of a high-complexity hospital with a confirmed diagnosis of COVID-19. The first cohort was from March 12 to May 29, 2020, and the second from December 1, 2021, to February 28, 2022.
Participants were patients on follow-up/treatment with biologics or targeted small molecule drugs in an IMID centre linked to a high complexity hospital. Inclusion criteria were patients who acquired COVID-19 infection during the study period, which was confirmed by PCR or antigen testing.
Socio-demographic variables were collected, age and sex, and clinical variables: IMID, specialty, treatment and route of administration, symptoms, need for hospitalisation for COVID-19, and admission to intensive care units (ICU). Vaccination against COVID-19, and in those patients who were vaccinated, the number of doses administered were also collected as variables in the second cohort.
A descriptive analysis was performed presenting quantitative variables with mean (X¯) and standard deviation (SD) or median and interquartile range for variables with asymmetric distribution, and qualitative variables with frequencies (Fr) and percentages (%). The Mann–Whitney and Fisher exact tests were used to verify the association between variables. In all tests p-values <.05 were considered statistically significant. SPSS® v.24 was used for data analysis.
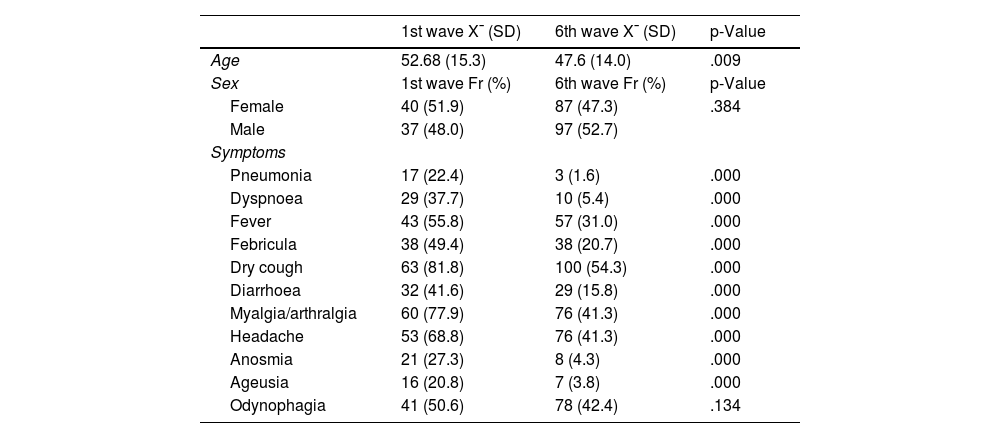
ResultsOf the 1627 patients with IMID in follow-up, 77 (4.60%) contracted COVID-19 during the first wave and 184 in the sixth wave (11.3%). In the first wave, 40 women (51.9%) had COVID-19 and 87 (47.3%) in the sixth wave, with no significant differences found between the two waves in terms of sex (p=.384). The mean age in the first wave was 52.7 years (SD: 15.3), significantly higher than in the sixth wave (p=.009), where the mean age was 47.6 years (SD: 14.0). The socio-demographic variables of the first and sixth waves are shown in Table 1.
Sociodemographic variables and symptoms in the 1st, and 6th waves.
| 1st wave X¯ (SD) | 6th wave X¯ (SD) | p-Value | |
|---|---|---|---|
| Age | 52.68 (15.3) | 47.6 (14.0) | .009 |
| Sex | 1st wave Fr (%) | 6th wave Fr (%) | p-Value |
| Female | 40 (51.9) | 87 (47.3) | .384 |
| Male | 37 (48.0) | 97 (52.7) | |
| Symptoms | |||
| Pneumonia | 17 (22.4) | 3 (1.6) | .000 |
| Dyspnoea | 29 (37.7) | 10 (5.4) | .000 |
| Fever | 43 (55.8) | 57 (31.0) | .000 |
| Febricula | 38 (49.4) | 38 (20.7) | .000 |
| Dry cough | 63 (81.8) | 100 (54.3) | .000 |
| Diarrhoea | 32 (41.6) | 29 (15.8) | .000 |
| Myalgia/arthralgia | 60 (77.9) | 76 (41.3) | .000 |
| Headache | 53 (68.8) | 76 (41.3) | .000 |
| Anosmia | 21 (27.3) | 8 (4.3) | .000 |
| Ageusia | 16 (20.8) | 7 (3.8) | .000 |
| Odynophagia | 41 (50.6) | 78 (42.4) | .134 |
In terms of specialty, there were significant differences (p=.023), with a higher incidence of COVID-19 in rheumatology patients in the first wave (33 patients, 41.8%), whereas in the sixth wave, the specialty with the highest number of infected patients was gastroenterology with 93 patients (50.5%). The incidence of symptoms in both waves is shown in Table 1. In terms of the diseases of the patients in both periods, most of the patients who contracted COVID-19 had Crohn’s disease (CD), in the first wave 22 (27.2%) patients vs. 62 (33.7%) in the sixth wave, with no statistically significant differences (.055).
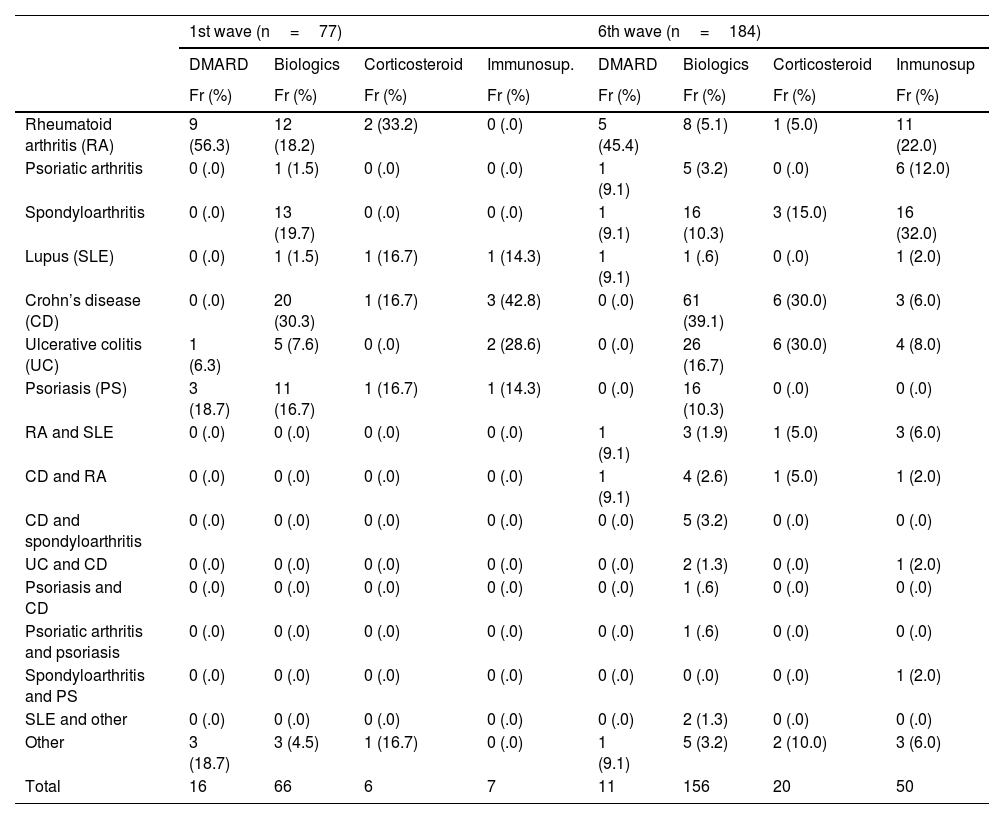
Table 2 shows the description of type of immune-mediated disease and treatment. In the first wave 16 (20.8%) patients were being treated with DMARDs or immunomodulators, 66 (85.7%) with biologic therapies, 6 (7.8%) with corticosteroids, and 7 (9.1%) with immunosuppressants, and the patients with rheumatological disease were on treatment with DMARDs 12 (75%), biologics 30 (45.5%), corticosteroids 4 (66.8%), and immunosuppressants one (14.3%). In the sixth wave, 11 (6.0%) were on DMARDs, 158 (85.9%) on biologics, 20 (10.9%) on corticosteroids, and 40 (21.7%) on immunosuppressive treatment, most of the patients with rheumatological disease were on DMARDs, 10 (90.9%), and immunosuppressants, 40 (80%), and the patients with digestive disease on biologics, 89 (57.1%), and corticosteroids, 12 (60%).
Immune-mediated disease vs. type of treatment.
| 1st wave (n=77) | 6th wave (n=184) | |||||||
|---|---|---|---|---|---|---|---|---|
| DMARD | Biologics | Corticosteroid | Immunosup. | DMARD | Biologics | Corticosteroid | Inmunosup | |
| Fr (%) | Fr (%) | Fr (%) | Fr (%) | Fr (%) | Fr (%) | Fr (%) | Fr (%) | |
| Rheumatoid arthritis (RA) | 9 (56.3) | 12 (18.2) | 2 (33.2) | 0 (.0) | 5 (45.4) | 8 (5.1) | 1 (5.0) | 11 (22.0) |
| Psoriatic arthritis | 0 (.0) | 1 (1.5) | 0 (.0) | 0 (.0) | 1 (9.1) | 5 (3.2) | 0 (.0) | 6 (12.0) |
| Spondyloarthritis | 0 (.0) | 13 (19.7) | 0 (.0) | 0 (.0) | 1 (9.1) | 16 (10.3) | 3 (15.0) | 16 (32.0) |
| Lupus (SLE) | 0 (.0) | 1 (1.5) | 1 (16.7) | 1 (14.3) | 1 (9.1) | 1 (.6) | 0 (.0) | 1 (2.0) |
| Crohn’s disease (CD) | 0 (.0) | 20 (30.3) | 1 (16.7) | 3 (42.8) | 0 (.0) | 61 (39.1) | 6 (30.0) | 3 (6.0) |
| Ulcerative colitis (UC) | 1 (6.3) | 5 (7.6) | 0 (.0) | 2 (28.6) | 0 (.0) | 26 (16.7) | 6 (30.0) | 4 (8.0) |
| Psoriasis (PS) | 3 (18.7) | 11 (16.7) | 1 (16.7) | 1 (14.3) | 0 (.0) | 16 (10.3) | 0 (.0) | 0 (.0) |
| RA and SLE | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 1 (9.1) | 3 (1.9) | 1 (5.0) | 3 (6.0) |
| CD and RA | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 1 (9.1) | 4 (2.6) | 1 (5.0) | 1 (2.0) |
| CD and spondyloarthritis | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 5 (3.2) | 0 (.0) | 0 (.0) |
| UC and CD | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 2 (1.3) | 0 (.0) | 1 (2.0) |
| Psoriasis and CD | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 1 (.6) | 0 (.0) | 0 (.0) |
| Psoriatic arthritis and psoriasis | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 1 (.6) | 0 (.0) | 0 (.0) |
| Spondyloarthritis and PS | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 1 (2.0) |
| SLE and other | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 0 (.0) | 2 (1.3) | 0 (.0) | 0 (.0) |
| Other | 3 (18.7) | 3 (4.5) | 1 (16.7) | 0 (.0) | 1 (9.1) | 5 (3.2) | 2 (10.0) | 3 (6.0) |
| Total | 16 | 66 | 6 | 7 | 11 | 156 | 20 | 50 |
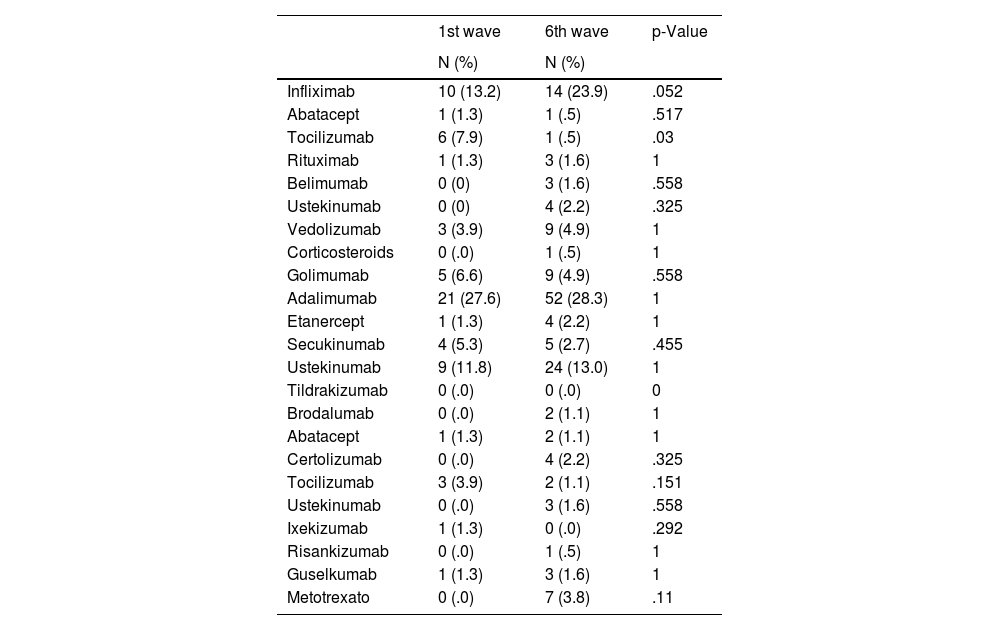
No differences were found between the two cohorts in terms of intravenous (p=.057), subcutaneous (p=.389), and oral (p=.250) treatment administration routes. The intravenous and subcutaneous treatments that the patients were receiving are described in Table 3.
Intravenous and subcutaneous treatments.
| 1st wave | 6th wave | p-Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Infliximab | 10 (13.2) | 14 (23.9) | .052 |
| Abatacept | 1 (1.3) | 1 (.5) | .517 |
| Tocilizumab | 6 (7.9) | 1 (.5) | .03 |
| Rituximab | 1 (1.3) | 3 (1.6) | 1 |
| Belimumab | 0 (0) | 3 (1.6) | .558 |
| Ustekinumab | 0 (0) | 4 (2.2) | .325 |
| Vedolizumab | 3 (3.9) | 9 (4.9) | 1 |
| Corticosteroids | 0 (.0) | 1 (.5) | 1 |
| Golimumab | 5 (6.6) | 9 (4.9) | .558 |
| Adalimumab | 21 (27.6) | 52 (28.3) | 1 |
| Etanercept | 1 (1.3) | 4 (2.2) | 1 |
| Secukinumab | 4 (5.3) | 5 (2.7) | .455 |
| Ustekinumab | 9 (11.8) | 24 (13.0) | 1 |
| Tildrakizumab | 0 (.0) | 0 (.0) | 0 |
| Brodalumab | 0 (.0) | 2 (1.1) | 1 |
| Abatacept | 1 (1.3) | 2 (1.1) | 1 |
| Certolizumab | 0 (.0) | 4 (2.2) | .325 |
| Tocilizumab | 3 (3.9) | 2 (1.1) | .151 |
| Ustekinumab | 0 (.0) | 3 (1.6) | .558 |
| Ixekizumab | 1 (1.3) | 0 (.0) | .292 |
| Risankizumab | 0 (.0) | 1 (.5) | 1 |
| Guselkumab | 1 (1.3) | 3 (1.6) | 1 |
| Metotrexato | 0 (.0) | 7 (3.8) | .11 |
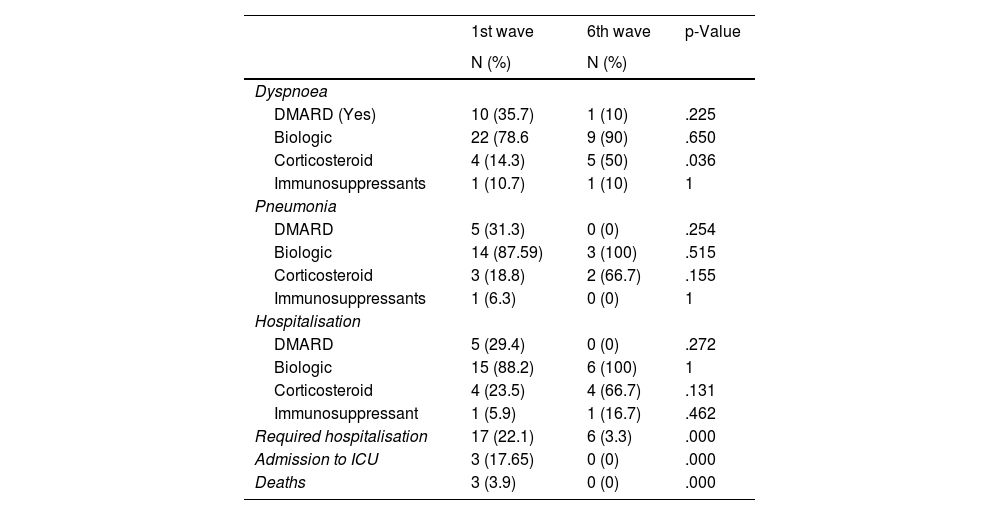
In the first wave, 17 (22.1%) of the total number of infected patients were admitted and in the sixth wave, only 6 patients (3.3%) were hospitalised, 4 from gastroenterology and 2 from rheumatology. Of the 23 patients requiring admission, 12 (52.2%) were rheumatology, 8 (34.8%) gastroenterology, and 3 (13.0%) dermatology, and 5 (21.7%) were being treated with adalimumab, rituximab 3 (13.1%), ustekinumab 3 (13.1%), infliximab 2 (8.7%), golimumab 2 (8.7%), tocilizumab 3 (13.1%), abatacept 2 (8.7%), secukinumab 2 (8.7%), and etanercept one (4.3%).
In the first wave, three patients died, one with rheumatoid arthritis treated with abatacept, one with spondyloarthritis treated with certolizumab, and one with Crohn’s disease treated with adalimumab. Severe symptoms and treatment, as well as hospitalisations, intensive care unit (ICU) admissions, and deaths due to COVID-19 are shown in Table 4.
Severe symptoms and treatment, hospitalisations, admissions to intensive care unit (ICU) and deaths from COVID-19.
| 1st wave | 6th wave | p-Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Dyspnoea | |||
| DMARD (Yes) | 10 (35.7) | 1 (10) | .225 |
| Biologic | 22 (78.6 | 9 (90) | .650 |
| Corticosteroid | 4 (14.3) | 5 (50) | .036 |
| Immunosuppressants | 1 (10.7) | 1 (10) | 1 |
| Pneumonia | |||
| DMARD | 5 (31.3) | 0 (0) | .254 |
| Biologic | 14 (87.59) | 3 (100) | .515 |
| Corticosteroid | 3 (18.8) | 2 (66.7) | .155 |
| Immunosuppressants | 1 (6.3) | 0 (0) | 1 |
| Hospitalisation | |||
| DMARD | 5 (29.4) | 0 (0) | .272 |
| Biologic | 15 (88.2) | 6 (100) | 1 |
| Corticosteroid | 4 (23.5) | 4 (66.7) | .131 |
| Immunosuppressant | 1 (5.9) | 1 (16.7) | .462 |
| Required hospitalisation | 17 (22.1) | 6 (3.3) | .000 |
| Admission to ICU | 3 (17.65) | 0 (0) | .000 |
| Deaths | 3 (3.9) | 0 (0) | .000 |
ICU: intensive care unit.
In terms of vaccination against COVID-19, in wave 6 180 patients (97.8%) had received at least one dose of vaccine, of which 104 patients (58.1%) had received 3 doses, 66 patients (36.9%) had received 2 doses, and 9 patients (5.0%) had received 1 dose. In wave 6, 184 patients contracted COVID-19, of whom 27 (14.7%) had previously contracted the disease and 13 patients (48.1%) had an immuno-compromised rheumatological disease, with no significant differences in relation to the other specialties (p=.890).
DiscussionSince the beginning of the SARS-CoV-2 pandemic, IMID patients have been considered a vulnerable population due to the immune-mediated nature of the disease, and the use of immunomodulatory/immunosuppressive drugs that may contribute to increased risk of infection.3,4 To date, the literature suggests that the risk of SARS-CoV-2 infection in IMID patients is no higher than in the general population, but that the increased risk is due to the associated comorbidities.9–11
Information on how infection affected a cohort of patients with immune-mediated disease is of particular interest given the characteristics of the diseases and treatments to control inflammatory activity, which were not known to modify the course of COVID-19 during the first wave, and the influence they have had on the sixth wave. The saturation of the healthcare system during the first wave may have resulted in patients with IMID being underdiagnosed, as diagnostic tests were only performed on those who required hospital care. In our study, COVID-19 infection during the sixth wave affected twice as many patients with IMID as in the first wave, while more severe symptoms of infection such as pneumonia, dyspnoea, and fever were significantly less. Furthermore, the patients in this study had mild symptoms of the infection, no patient required admission to the ICU, and no deaths were recorded. Therefore, the severity of SARS-CoV-2 infection in the sixth wave can be considered to be lower than in the first wave, which would show the efficacy of the vaccines in preventing severe disease. Therefore, to maintain these health outcomes, and given that certain immunosuppressive therapies would influence attenuated response to the SARS-CoV-2 vaccine, it would be advisable to give these patients vaccine booster dose.12,13
These results are particularly relevant because a significant number of IMIDs share genetic architecture with severe manifestations of COVID-19. The literature describes extensively the results of risk assessment of infections in rheumatic diseases, according to treatment and disease stage.14–18 Patients with rheumatic diseases are also more susceptible to infections that may be related to immunological changes associated with their disease and/or the immunosuppressive effects of the treatments used. The data provided corroborate and extend those already published.
In our study, the adults who died were treated with biologic disease-modifying antirheumatic drugs (biologic DMARDs), the other drugs were only related to hospital admission and pneumonia, and this result coincides with data from international registries.19–25
In conclusion, multidisciplinary and coordinated care has guaranteed continuity of care. Nursing consultations have been key in promoting adherence to treatment and avoiding treatment interruption in IMID patients. Moreover, nurses are in an optimal position to answer any questions about vaccines and to support confidence in vaccination programmes. Follow-up and monitoring of IMID patients during the first wave has prevented a higher number of complications occurring during the pandemic. It would be interesting for future studies to assess symptomatology, admissions, and mortality in IMID patients receiving a vaccine booster dose.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the study subjects, Dr C.M. González Fernandez, and Beatriz Villarrubia Martin for their contribution to data collection for this study, Mª Jesús Fernández Lizcano for administrative support, and the Nursing Research Support Unit for their methodological contributions.