To determine the effectiveness and the incidence of severe adverse events in a cohort of Costa Rican patients with Rheumatoid Arthritis (RA) treated with intravenous (IV) tocilizumab (TCZ).
Patients and methodsA retrospective analysis was carried out in 45 patients that were unresponsive to disease-modifying antirheumatic drugs (DMARDs). The study included patients who received IV TCZ every 4 weeks (4 mg/kg) along with methotrexate or leflunomide. Effectiveness was measured through the incidence of clinical remission according to a disease activity score – erythrocyte sedimentation rate (DAS28-ESR) less than 2.6. Safety was assessed by the incidence rate of serious adverse events. An univariate and multivariate logistic regression analysis was performed to assess the association of potential variables with the probability of achieving remission during the first 3 months of TCZ therapy.
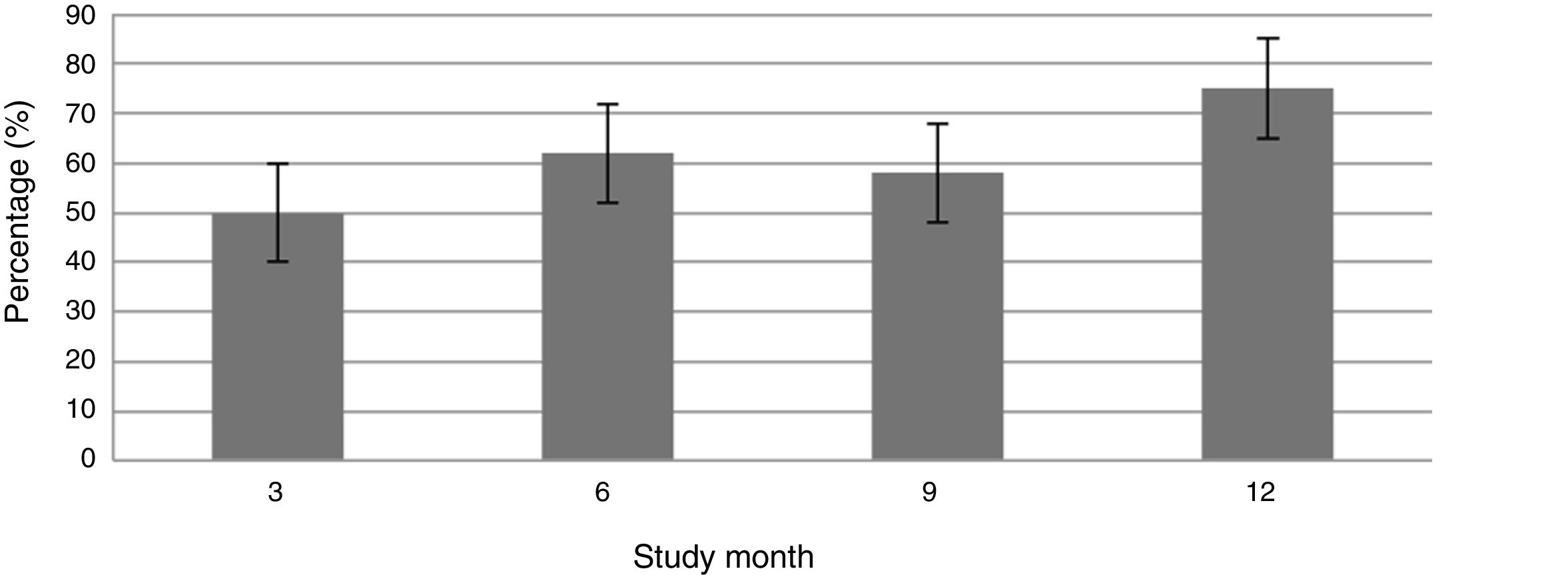
ResultsDuring the 3rd month of TCZ therapy, a total of 22 patients (48.9%; 95% Confidence Interval (CI) 34.3%–63.5%) achieved remission. The cumulative incidence of patients with remission at month 12 was 75.0% (n = 34) (95% CI: 62.3%–87.6%). A total of 18 patients (40%; 95% CI: 25.7%–54.3%) were switched to a 8 mg/kg dose due to the absence of remission. The incidence rate of serious adverse events was .98 per 100 patients/year, all of them due to infectious diseases with no fatal events reported. Only basal DAS28-ESR was associated with the probability of achieving remission at month 3.
ConclusionsIV TCZ (4 mg/kg) is an effective and safe treatment for RA patients in a clinical setting in Costa Rica.
Determinar la efectividad y la incidencia de eventos adversos graves del tocilizumab (TCZ) en una cohorte de pacientes costarricenses con artritis reumatoide (AR).
Pacientes y métodosSe realizó un análisis retrospectivo de 45 pacientes con AR, refractarios al uso previo de fármacos modificadores de la enfermedad reumática (FAME), que utilizaron TCZ a una dosis inicial de 4 mg/kg intravenoso (IV) cada 4 semanas en asociación con metotrexato o leflunomida. La medida de efectividad fue la incidencia de remisión clínica, determinada cada 3 meses y definida por un puntaje de actividad de la enfermedad en 28 articulaciones con velocidad de sedimentación globular (DAS28-VSG) menor de 2,6. La seguridad del fármaco se evaluó mediante la tasa de incidencia de eventos adversos severos. Se realizó un modelo de regresión logística uni- y multivariado para determinar las variables asociadas con la probabilidad de remisión a los 3 meses de iniciado el tratamiento.
ResultadosA los 3 meses de tratamiento un total de 22 pacientes (48,9%; intervalo de Confianza [IC] del 95%: 34,3–63,5%) alcanzaron remisión, en tanto que a los 12 meses de terapia con TCZ el valor aumentó a 34 pacientes (75%; IC 95%: 62,3–87,6%). Un total de 18 pacientes (40%; IC 95%: 25,7–54,3%) requirieron aumento de dosis del TCZ de 4 a 8 mg/kg ante la ausencia de remisión. La tasa de incidencia de eventos adversos severos fue de 0,98 por 100 pacientes/año, correspondiendo todos ellos a cuadros infecciosos que resolvieron sin ningún desenlace fatal. Solo el DAS28-VSG inicial se asoció de forma independiente con la probabilidad de remisión a los 3 meses.
ConclusionesEl uso de TCZ IV a una dosis inicial de 4 mg/kg en pacientes costarricenses con AR es efectivo y seguro en la práctica clínica.
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that predominantly affects the joints, causing damage and leading to functional impairment in patients, in some cases irreversible.1 Treatment of RA includes low-dose prednisone and non-steroidal anti-inflammatory drugs (NSAIDs), in conjunction with disease-modifying antirheumatic drugs (DMARDs), which have been shown to improve physical function and slow joint damage.1 Although the incorporation of biologic and synthetic therapies against tumour necrosis factor, B cells or T cells has resulted in higher clinical response rates, 30%–40% of patients fail to achieve and maintain a major clinical response according to the American College of Rheumatology (ACR) criteria, or a good response according to the criteria defined by the European League Against Rheumatism (EULAR).2,3 These limitations have led to the development of new drugs such as tocilizumab (TCZ). This drug is a humanised monoclonal antibody that inhibits the activity of interleukin-6 (IL-6) by preventing this cytokine binding to its receptor, blocking the classical activation, trans-signalling, and trans-presentation pathways.4 TCZ has been shown in several clinical trials5–7 to be safe and effective in reducing acute phase protein concentrations, inflammatory symptoms and progression of joint damage when used as monotherapy.8
TCZ has been shown in several clinical trials5–11 to be a safe and effective drug in the treatment of RA, reducing acute-phase protein concentrations, inflammatory symptoms, and progression of joint damage in patients who have been exposed10 or not exposed11 to prior treatment with biologic DMARDs. Furthermore, the efficacy of TCZ has been proven in both subcutaneous and intravenous (IV) administration9 and in various monotherapy8 or combined treatment regimens.7 TCZ is currently approved for the treatment of RA that has responded inadequately to prior use of biologic or synthetic DMARDs.12 Despite the demonstrated efficacy and safety of TCZ in the abovementioned clinical trials, most of these trials did not include a significant proportion of patients of Latin American origin. Recent studies have highlighted ethnic differences in the progression and response to treatment of RA patients, with individuals of Hispanic origin reported to have higher rates of disease activity and lower rates of clinical remission.13,14 This study, therefore, sought to evaluate the effectiveness of IV TCZ in Costa Rican RA patients refractory to prior use of synthetic or biologic DMARDs, in a context of usual clinical practice and dose optimisation using 4 mg/kg every 4 weeks as the initial dose.
Patients and methodsWe undertook a retrospective analytical study of medical records of the Hospital Rafael Ángel Calderón Guardia, San José, Costa Rica (Caja Costarricense de Seguro Social). The analysis included the entire population of RA patients treated since the drug became available in Costa Rica (January 2013) until December 2016. Subjects over 18 years of age with a diagnosis of RA according to the ACR/EULAR 2010 criteria, who received TCZ treatment for at least one year during the study period, were included. The indication for TCZ treatment was not having responded to treatment with DMARDs (methotrexate, leflunomide, sulfasalazine or hydroxychloroquine, in combined regimens and at the maximum dose described for each drug), defined by a 28-joint disease activity score with erythrocyte sedimentation rate (DAS28-ESR) greater than 5.1.
Patients with rheumatological or inflammatory diseases other than RA, and patients with a follow-up of less than 12 months were excluded. Variables of interest were collected from the clinical records and included age, sex, smoking, presence of comorbidities, age at diagnosis and duration of RA, time between RA diagnosis and initiation of TCZ, and use of DMARDs. The dose of TCZ (IV) used was 4 mg/kg every 4 weeks, which was used in combination with methotrexate and/or leflunomide at the discretion of the treating physician. In case of documented failure to respond during the follow-up period, defined as a DAS28 score greater than 2.6, the TCZ dose was modified to 8 mg/kg every 4 weeks IV. Patients were assessed by the rheumatologist at least every 3 months. The effectiveness of TCZ was determined by EULAR criteria according to the change in DAS28-ESR score, assessed every 3 months. Patients were classified according to the response obtained with TCZ into remission (DAS28-ESR < 2.6), low-disease activity (2.6 < DAS28-ESR < 3.2), moderate disease activity (3.2 < DAS28-ESR < 5.1) and severe disease activity (>5.1). Drug safety was assessed by the incidence of severe adverse events occurring during the entire follow-up of the patients analysed. An adverse event was defined as severe when it resulted in hospitalisation of the patient, use of IV antibiotic treatment or death.
The study was approved by the Scientific Ethical Committee of the Caja Costarricense de Seguro Social and adhered to best clinical practice.
Statistical analysisContinuous variables are expressed as means and standard deviation. Categorical variables are expressed as absolute frequencies and percentages. The 95% confidence interval (95% CI) was calculated for each effectiveness and safety variable. The DAS28-ESR value at study entry and at week 52 was compared using the Student’s t-test for paired data, previously checking the statistical assumptions of normality and homoscedasticity. A univariate and multivariate logistic regression model was performed to identify variables associated with remission (according to a DAS28-ESR score <2.6) at 3 months as a binary dependent variable. The analysis was performed on all patients who completed the expected follow-up period (per-protocol analysis). A P-value of less than .05 was considered statistically significant. The analysis was performed using SPSS 21.0 for Mac (Chicago, USA).
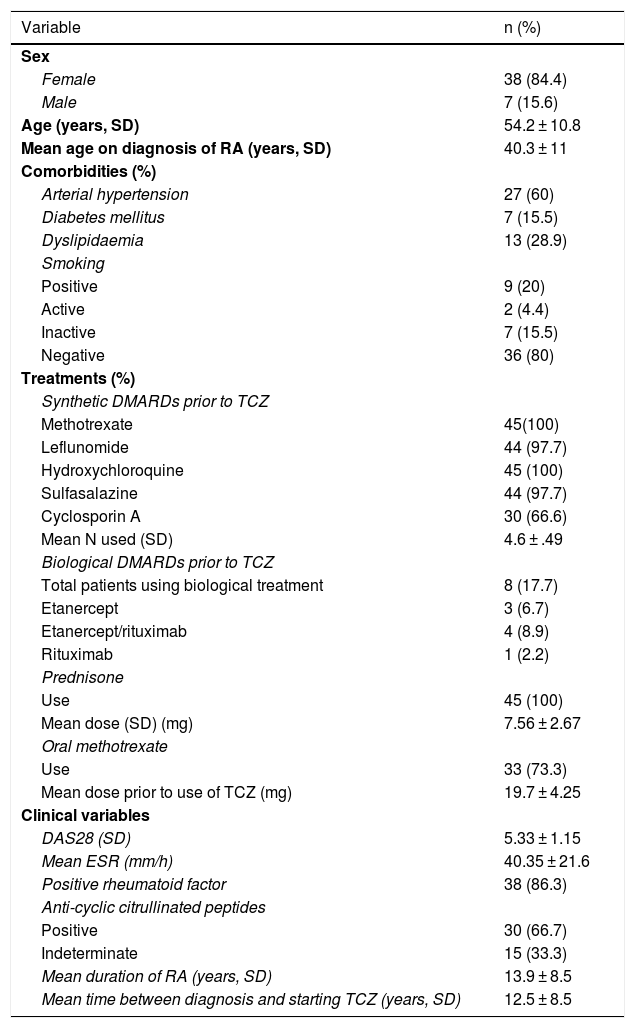
ResultsPatient characteristicsDuring the study period, 45 patients taking IV TCZ were identified who met the inclusion criteria. The general characteristics of the included patients are summarised in Table 1. Most of the patients were female and rheumatoid factor and anti-cyclic citrullinated peptide positive.
Clinical characteristics of the patients included.
| Variable | n (%) |
|---|---|
| Sex | |
| Female | 38 (84.4) |
| Male | 7 (15.6) |
| Age (years, SD) | 54.2 ± 10.8 |
| Mean age on diagnosis of RA (years, SD) | 40.3 ± 11 |
| Comorbidities (%) | |
| Arterial hypertension | 27 (60) |
| Diabetes mellitus | 7 (15.5) |
| Dyslipidaemia | 13 (28.9) |
| Smoking | |
| Positive | 9 (20) |
| Active | 2 (4.4) |
| Inactive | 7 (15.5) |
| Negative | 36 (80) |
| Treatments (%) | |
| Synthetic DMARDs prior to TCZ | |
| Methotrexate | 45(100) |
| Leflunomide | 44 (97.7) |
| Hydroxychloroquine | 45 (100) |
| Sulfasalazine | 44 (97.7) |
| Cyclosporin A | 30 (66.6) |
| Mean N used (SD) | 4.6 ± .49 |
| Biological DMARDs prior to TCZ | |
| Total patients using biological treatment | 8 (17.7) |
| Etanercept | 3 (6.7) |
| Etanercept/rituximab | 4 (8.9) |
| Rituximab | 1 (2.2) |
| Prednisone | |
| Use | 45 (100) |
| Mean dose (SD) (mg) | 7.56 ± 2.67 |
| Oral methotrexate | |
| Use | 33 (73.3) |
| Mean dose prior to use of TCZ (mg) | 19.7 ± 4.25 |
| Clinical variables | |
| DAS28 (SD) | 5.33 ± 1.15 |
| Mean ESR (mm/h) | 40.35 ± 21.6 |
| Positive rheumatoid factor | 38 (86.3) |
| Anti-cyclic citrullinated peptides | |
| Positive | 30 (66.7) |
| Indeterminate | 15 (33.3) |
| Mean duration of RA (years, SD) | 13.9 ± 8.5 |
| Mean time between diagnosis and starting TCZ (years, SD) | 12.5 ± 8.5 |
DAS28: 28-joint disease activity score; DMARDs: disease-modifying antirheumatic drugs; ESR: erythrocyte sedimentation rate; RA: rheumatoid arthritis; SD: standard deviation; TCZ: tocilizumab.
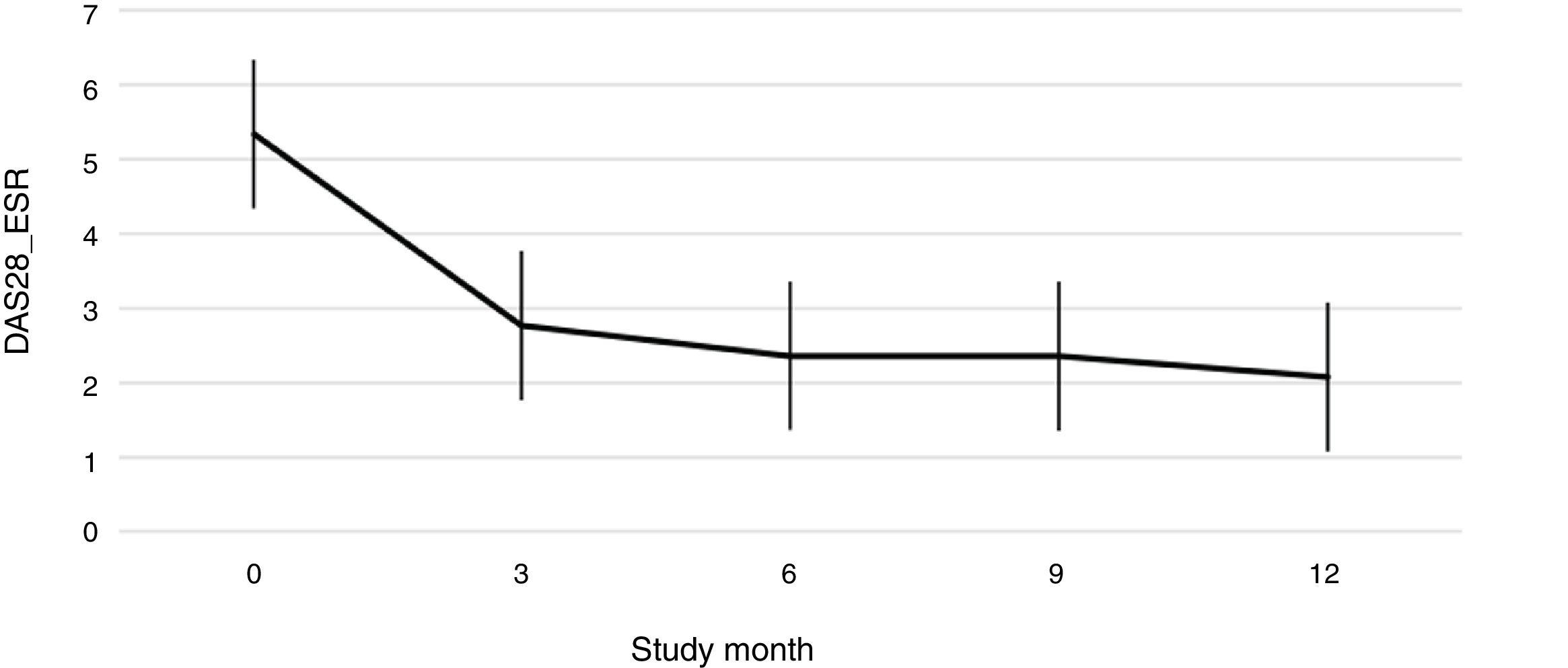
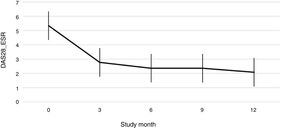
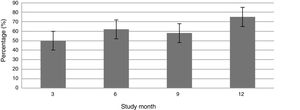
Fig. 1 shows the course of the DAS28-ESR score over the 52 weeks of clinical follow-up. According to the DAS28-ESR score, at 3 months after starting treatment, a total of 22 patients (48.9%; 95% CI: 34.3%–63.5%) achieved remission. This score gradually increased at the 6- and 12-month follow-up where the proportion of patients with remission was 62% (95% CI: 47.8%–76.2%) (n = 28) and 75% (95% CI: 62.3%–87.6%) (n = 34), respectively (Fig. 2). A statistically significant difference was detected between the mean basal DAS28-ESR score and that obtained at 52 weeks (5.33 vs. 2.18; p < .001). The percentage of remission at 3 months was not significantly different between the subgroup of patients who had received prior biologic treatment and those naive to biologic therapy (71.4% vs. 45.5%; respectively; p = .20).
According to EULAR response criteria, by the third month of treatment, 22 patients (48.9%) had a good response, 18 patients (40%) a moderate response and only 5 patients (11.1%) did not respond to therapy. A total of 18 patients (40%; 95% CI 25.7%–54.3%) required an increase in drug dose to 8 mg/kg, which occurred at month 6.5 ± 4.48 from starting TCZ. There was persistent treatment failure in only 2 patients after increasing the dose.
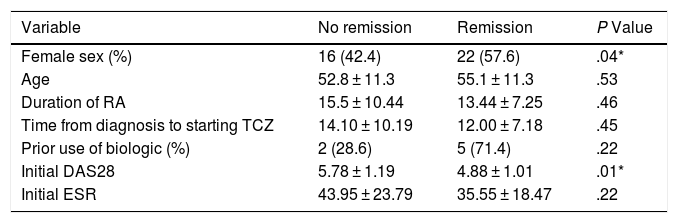
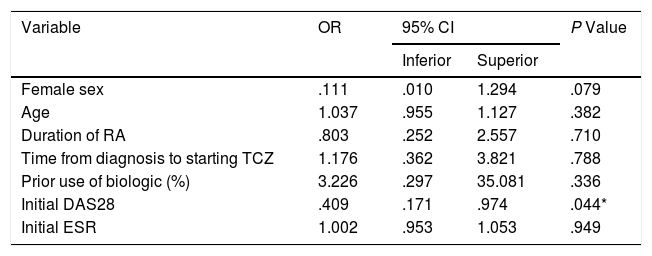
The univariate and multivariate analysis of potential determinants of remission at the third month of treatment (according to DAS28-ESR score <2.6) is shown in Tables 2 and 3. While female sex was associated with a higher probability of remission in the univariate analysis, only the basal DAS28-ESR was independently associated with the probability of remission at 3 months after multivariate analysis adjusted for the other confounding variables.
Univariate analysis of potential predictors of remission (DAS28 < 2.6) at 3 months.
| Variable | No remission | Remission | P Value |
|---|---|---|---|
| Female sex (%) | 16 (42.4) | 22 (57.6) | .04* |
| Age | 52.8 ± 11.3 | 55.1 ± 11.3 | .53 |
| Duration of RA | 15.5 ± 10.44 | 13.44 ± 7.25 | .46 |
| Time from diagnosis to starting TCZ | 14.10 ± 10.19 | 12.00 ± 7.18 | .45 |
| Prior use of biologic (%) | 2 (28.6) | 5 (71.4) | .22 |
| Initial DAS28 | 5.78 ± 1.19 | 4.88 ± 1.01 | .01* |
| Initial ESR | 43.95 ± 23.79 | 35.55 ± 18.47 | .22 |
DAS28: 28-joint disease activity score; ESR: erythrocyte sedimentation rate; TCZ: tocilizumab.
Multivariate analysis of potential predictors of remission (DAS28 < 2.6) at 3 months.
| Variable | OR | 95% CI | P Value | |
|---|---|---|---|---|
| Inferior | Superior | |||
| Female sex | .111 | .010 | 1.294 | .079 |
| Age | 1.037 | .955 | 1.127 | .382 |
| Duration of RA | .803 | .252 | 2.557 | .710 |
| Time from diagnosis to starting TCZ | 1.176 | .362 | 3.821 | .788 |
| Prior use of biologic (%) | 3.226 | .297 | 35.081 | .336 |
| Initial DAS28 | .409 | .171 | .974 | .044* |
| Initial ESR | 1.002 | .953 | 1.053 | .949 |
CI: confidence interval; DAS28: 28-joint disease activity score; ESR: erythrocyte sedimentation rate; OR: odds ratio; TCZ: tocilizumab.
Over the entire follow-up period (median follow-up: 12 months), 6 severe infectious events affecting 4 patients were counted, for a cumulative incidence of 8.8% (95% CI: .05%–17.1%) and an incidence rate of .98 events per 100 patients/year. To be specific, three events of community-acquired pneumonia, two complicated urinary tract infections and one Fournier’s gangrene were diagnosed. None of these complications had a fatal outcome and all cases were resolved with the use of antibiotic therapy.
DiscussionThe present study describes for the first time the effectiveness and safety results of IV TCZ at an initial dose of 4 mg/kg every 4 weeks in a Costa Rican population. The use of TCZ was associated with a reduction in RA activity parameters by both clinical and analytical variables. Specifically, the cumulative incidence of remission at 6 months was 62%, a much higher percentage than that obtained in the clinical trial published by Smolen et al., which reported a remission rate of 13% at 6 months with the same TCZ dose and administration route as used in this group of patients.7 Although the objective of the above-mentioned clinical trial was remission according to ACR20 and not DAS28-ESR, the differences in remission rates are very wide. These discrepancies may be explained by the different basal characteristics of the patients included, the main differences being ethnicity and the presence of younger patients with higher exposure to DMARDs in the present study.
The remission rate reported at 6 months was also higher than that described in a Canadian observational study, which reported a remission rate of 36.3% when TCZ (8 mg/kg) was used as monotherapy or combined therapy in a group of patients previously treated with a DMARD.15 However, other authors have reported 6-month remission rates that are more in line with those described in this study. More specifically, the remission rates, published for a cohort of Spanish,16 Italian17 and Japanese18 patients, were 75.5%, 52.3% and 47.6%, respectively, although a TCZ dose of 8 mg/kg was used in these scenarios.
Although some pharmacological studies have shown that TCZ pharmacokinetics are not affected by ethnicity or RA inflammatory conditions,19 the variability observed in the pharmacological response to TCZ in different populations suggests a non-uniform distribution of certain predictive variables among the groups analysed, such as age, duration of RA and DAS28-ESR score at start of treatment. Several authors agree in identifying young age and low initial DAS28-ESR score as predictors of response to TCZ. Pers et al. described that younger patients had better response rates to TCZ compared to older individuals.20 Narvaez et al. reported that an elevated DAS28-ESR score at the start of biologic therapy was associated with early remission failure with IV TCZ.21 In line with these reports, our findings identified basal DAS28-ESR score as the only independent variable associated with remission failure. It is clinically useful to identify this prognostic variable as it could select a group of patients in whom starting with a dose of 4 mg/kg is not the most recommended approach. Other recently described predictors are high basal IL-6 concentrations and low levels of soluble IL-6 receptor, which could identify a subgroup of patients who will benefit particularly from this therapy.22
This study reports the experience of a group of patients of a different ethnicity than those studied in the clinical trials that demonstrated the efficacy and safety of IV TCZ.18 This finding is of relevance as recent epidemiological studies have shown that the Hispanic population is characterised by high rates of RA activity and lower clinical response rates in similar settings, related to the low socioeconomic status and difficult access to health services of this ethnic group, compared to other populations in developed countries.23
However, the present study demonstrates that despite these socio-cultural factors, the use of TCZ gives similar or superior responses of effectiveness than those described in other populations in industrialised countries.
Few studies have published the clinical effectiveness of an initial dose of 4 mg/kg IV TCZ. Although some clinical trials have reported that this dose could be lower than 8 mg/kg every 4 weeks,7,24 data from the present analysis show that this approach may be effective when used as a starting dose in patients with previous failure of synthetic DMARDs. Furthermore, other clinical trials have not shown such differences in the efficacy of TCZ according to the dose used.6 However, the findings of this observational study need to be confirmed prospectively and supported by scientific evidence from clinical trials comparing both doses of TCZ in search of an optimal regimen of maximum cost-effectiveness, especially in populations with limited access to financial resources. In addition, longer follow-up of this cohort of patients will be particularly useful clinically to understand the long-term effects of the TCZ dosage used.
In terms of safety, similar behaviour was found to that described in other pharmacovigilance studies. For example, Morel et al.25 described a French cohort of nearly 1500 patients in which most of the serious infectious complications occurring RA patients exposed to TCZ were in the lower airway and soft tissues (35% and 32% of all documented infectious events), with an incidence rate of 4.7/100 patients/year.
The findings of this study should be interpreted with caution due to the retrospective and uncontrolled design of the study, which may have resulted in selection bias. In addition, the sample size and the single-centre nature of the analysis diminish the external validity of the findings. Another limitation of this study was that no other drug response variables were determined such as functionality and quality of life scales, which were not routinely used in the clinical practice of the hospital studied. The omission of these variables may have left out of the analysis other factors that have been linked to the effectiveness of TCZ, such as psychosocial variables, depression, and fatigue.26
However, despite the above limitations, the results obtained are encouraging in that they show the effectiveness of an optimised dose in a usual clinical setting. Furthermore, even though we used a per-protocol analysis, no patients were lost to follow-up, and therefore this type of analysis did not bring information bias to the study results.
In conclusion, the present study demonstrates the effectiveness and safety of IV TCZ (4 mg/kg every 4 weeks) in a particular group of Costa Ricans and suggests the usefulness of an optimised dosing regimen that could mean a reduction in the costs associated with the treatment of RA patients.
FundingThis research received no specific support from public sector agencies, the commercial sector, or non-profit organisations.
Conflict of interestsA Ramos-Esquivel has participated on Advisory Boards for Roche, Bayer, Stein and Astrazeneca.
M. Cordero-Alfaro has no conflict of interests to declare.
C. León-Céspedes has no conflict of interests to declare.
Please cite this article as: Cordero-Alfaro M, León-Céspedes C, Ramos-Esquivel A. Efectividad del tocilizumab intravenoso en la práctica clínica usual de una cohorte de pacientes costarricenses con artritis reumatoide. Reumatol Clin. 2021;17:329–334.