To describe a multicentre case series of new onset or worsening of psoriasis in patients treated with biological drugs.
Material and methodsDescriptive study. We reviewed the clinical history of patients with chronic inflammatory disease (CID) treated with biological drugs, who developed new onset or worsening of psoriasis during the follow-up period.
ResultsTwenty-six cases of paradoxical psoriasis (PP) were recorded. Ninety-three percent of the patients were treated with anti-TNFα and adalimumab was responsible for 50% of the cases. Only 5 patients had a personal history of psoriasis. The biological drug was discontinued in 13 patients. Lesion recurrence was more frequent when another anti-TNFα was reintroduced.
ConclusionsThe PP is a reversible adverse effect that can be observed in patients exposed to biological drugs, mainly anti-TNFα.
Describir una serie multicéntrica de casos de inducción o empeoramiento de psoriasis en pacientes tratados con fármacos biológicos.
Material y métodosEstudio descriptivo. Se revisó la historia clínica de pacientes con enfermedad inflamatoria crónica (EIC) en tratamiento con fármacos biológicos, y que presentaron durante el período de seguimiento, psoriasis de nueva aparición o empeoramiento de la misma.
ResultadosSe registraron 26 casos de psoriasis paradójica (PP). El 93% de los pacientes estaban en tratamiento con un anti-TNFα, siendo el adalimumab el responsable del 50% de los casos. Solo 5 pacientes presentaban antecedentes personales de psoriasis. En 13 pacientes fue necesario retirar el fármaco biológico y la recidiva de las lesiones fue más frecuente en los pacientes en los que se reintrodujo otro anti-TNFα.
ConclusionesLa PP es un efecto adverso reversible que se puede observar en pacientes expuestos a fármacos biológicos, principalmente a anti-TNFα.
The use of biological drugs in patients with chronic inflammatory disease (CID) has increased in recent years. Safety studies of these drugs have focused mainly on the increased risk of infections, the development of neoplasms and demyelinating diseases, local reaction at the injection site and the immunogenicity they can generate through the production of antibodies. However, it has been shown that these drugs can have different effects at skin level, including the onset of psoriatic lesions or the worsening of pre-existing lesions, a phenomenon known as paradoxical psoriasis (PP).1
The aim of our study was to describe a multi-centre series of cases of induced or worsened psoriasis in patients treated with biological drugs.
Material and methodsDescriptive study. We reviewed the clinical history of patients with CID undergoing treatment with anti-TNFα or other biological drugs, who presented with new or worsening psoriasis. The patients came from 3 hospitals: Hospital General Universitario de Valencia, Hospital Universitario Doctor Peset and Hospital Universitario de Bellvitge, and were registered from January 2008 to May 2019. Demographic (sex and age) and clinical variables were collected (inflammatory disease, current biological treatment and time of exposure to the drug, personal and family history of psoriasis, the morphology of the lesions and treatment used for them). Cases where the biological drug was discontinued were also recorded, as well as the rate of subsequent relapses.
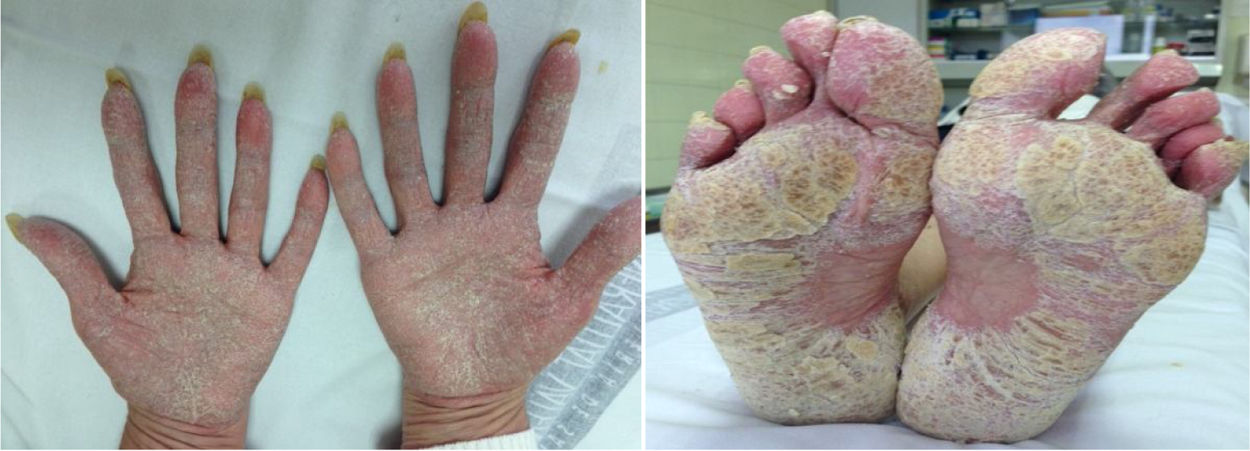
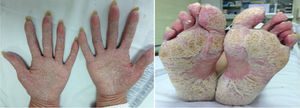
ResultsA total of 26 patients were collected. Of these, 50% were female and the mean age was 46 (SD: ±15) years. With respect to baseline inflammatory disease, 12 patients presented spondyloarthropathy (46%), 7 Crohn's disease (CD) (27%), 5 rheumatoid arthritis (RA) (19%), 2 psoriasis (8%), one recurrent polychondritis (4%) and one chronic juvenile polyarthritis (4%). Two patients were diagnosed with more than one disease: psoriatic arthritis-associated CD and RA-associated CD. Thirteen patients were treated with adalimumab (50%), 5 with infliximab (19%), 3 with golimumab (12%), 2 with etanercept (8%), one with certolizumab (4%), one with ustekinumab (4%) and one with abatacept (4%). The average time of exposure to the biological drug until the appearance of skin lesions was 57 (SD: ±60) weeks. Five patients had psoriasis associated with their underlying inflammatory disease (19%) and four had a family history of psoriasis (15%). Twenty-five cases of psoriasis and one case of lichenoid pityriasis (histologically confirmed) were recorded. Skin biopsy was performed in 5 patients, confirming the presence of histological changes consistent with psoriasis in 4 of them. Lesion morphology included: 13 patients with palmoplantar pustulosis (50%) (Fig. 1), 7 with plaque psoriasis (27%), 5 with scalp psoriasis (19%), 5 with psoriasiform reaction (19%), 4 with guttate psoriasis (15%) and one with inverted psoriasis (4%); 7 patients experienced more than one type of lesion (27%). Topical treatment was instituted in all cases, and methotrexate was required in 7 patients. Treatment was maintained in 50% of patients. Of the 13 patients in whom treatment was discontinued, another anti-TNFα drug was started in 4 patients (2 adalimumab, 1 golimumab, 1 certolizumab), but skin lesions reappeared in 3 of them. In 6 patients the anti-TNFα was replaced by a non-anti-TNFα biological drug (3 ustekinumab, one rituximab, one secukinumab, one ixekizumab), with the lesions reappearing only in the patient treated with secukinumab. Two patients remain without systemic treatment at present, and the remaining patient died of non-study causes. In the patient with lichenoid pityriasis the biological treatment (etanercept) was maintained, and his lesions have progressed well with topical treatment.
DiscussionThe term PP refers to the development of de novo psoriasis or exacerbation of pre-existing lesions, with a therapeutic agent typically used to treat these lesions.2 This phenomenon was initially described in patients treated with anti-TNFα, but as new biological agents have been introduced, cases have been reported with other non-anti-TNFα, biological drugs.3 Throughout the text, we will refer at all times to anti-TNFα-induced PP, otherwise we will indicate it specifically.
According to the available data, anti-TNFα-induced PP has a low incidence (1.04–3.0/1000 persons/year), and the prevalence varies according to the different studies between .6% and 5.3%.4 Although there are cases described in virtually all CIDs, most are patients with CD5 or RA.6 However, in our series most paradoxical reactions are observed in patients with spondyloarthropathy. The time relationship between anti-TNFα and the onset of psoriasis supports a causal relationship. Although there is mixed data on this, the mean latency time from starting the drug to the onset of lesions is 10 months,4 somewhat less than that observed in our patients.
Palmoplantar pustulosis and plaque psoriasis, as in most series, is the main morphology of the lesions we observed in our study.6 Skin biopsy can help confirm the diagnosis of psoriasis and differentiate it from other entities.7 In fact, in our series we observed a case of histologically confirmed lichenoid pityriasis. It has been described that up to 15% of patients may present with more than one type of lesion,6 and consistent with our data, most patients do not have a family or personal history of psoriasis, and if they had psoriasis it appears in unusual locations.8
Cases of PP have been described with all anti-TNFαs and in all diseases where they are indicated, and therefore we can consider this to be a class effect. We agree with other studies that the highest incidence of this phenomenon occurs in patients treated with adalimumab or infliximab.9 As initially indicated, cases have also been described with non-anti-TNFα drugs such as rituximab,10 abatacept,11 tocilizumab12 and ustekinumab.13 The latter, although used for the treatment of PP, has also been associated with relapses of pustular psoriasis.
One of the hypotheses postulated for the development of PP is the correlation between TNFα and interferon 1 (IFN-1). The latter is synthesised by dermal plasmacytoid dendritic cells and is central to the development of psoriasis. Under normal conditions, TNFα inhibits the synthesis of IFNα,-1 by dendritic cells. After administration of anti-TNFα, the imbalance may lead to local production of IFN-1α, developing a psoriasis flare-up.4 Another theory argues that the IL-23/Thelper17 (Th17) axis plays a key role in the pathogenesis of psoriasis. IL-23 is a pro-inflammatory cytokine that drives the efficacious response of Thelper1 (Th1) and Th17, both of which have been implicated in the pathogenesis of CIDs, including psoriasis. Genome studies have revealed a specific association between polymorphisms in the IL-23 receptor gene and increased susceptibility to CD and psoriasis. Thus, inhibition of IL-12/23 with ustekinumab has been effective in treating CD in patients who develop anti-TNFα-induced psoriasis.5,14
In most cases, discontinuation of the biological drug is usually sufficient to resolve the lesions. However, it can be accompanied by an exacerbation of CID, and therefore in most cases it is difficult to discontinue it. A patient with suspected PP should first be referred to a dermatologist for diagnosis and histological confirmation if necessary. If the psoriasis affects <5% of body surface, topical treatment is indicated (corticosteroids, keratolytics and vitamin D analogues), if there is no improvement, the next level would be to combine oral methotrexate or ultraviolet phototherapy. By contrast, if the body surface involvement is >5% or there is palmoplantar pustulosis, topical treatment and phototherapy are recommended, and if there is no response systemic treatment with methotrexate, retinoids and/or cyclosporine should be considered. In case of inefficacy, biological therapy should be discontinued and the change of therapeutic target to an anti-IL17 or anti-IL 12/23 should be considered; the change to another anti-TNFα is controversial, since retreatment with a second anti-TNFα has been associated with recurrence of psoriasis in 48%–85% of cases. If there is still no improvement, experts propose optimising combination therapy or considering new drugs such as anti-IL-2315 biological drugs.15
ConclusionsPP is a reversible adverse effect that can be seen in patients exposed to biological drugs, mainly anti-TNF. It is necessary to know about this phenomenon and to establish adequate treatment, sometimes requiring discontinuation of the biological drug and/or change of therapeutic target.
FundingThe authors of this publication declare that they have received no fees from any public or private agency.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors would like to thank all the services involved in this study, as without their collaboration patient recruitment would not have been possible. Special thanks to Dr Paredes Arquiola, Dr Santos Alarcón, Dr Notario Rosa, Dr Ybañez García, Dr Valls Pascual, Dr Aguilar Zamora and Dr Orenes Vera. Thanks also to Ana Sendra for her collaboration in the analysis and interpretation of results.
Please cite this article as: Montolio Chiva L, Martínez Ferrer À, Mateu Puchades A, Campos Fernández C, Narváez Garcia J, Alegre Sancho JJ. Psoriasis inducida por terapia biológica. Reumatol Clin. 2021;17:437–439.