It is not clear whether patients with some degree of immunosuppression have worse outcomes in SARS-CoV-2 infection, compared to healthy people.
ObjectiveTo carry out a narrative review of the information available on infection by SARS-CoV-2 in immunosuppressed patients, especially patients with cancer, transplanted, neurological diseases, primary and secondary immunodeficiencies.
ResultsPatients with cancer and recent cancer treatment (chemotherapy or surgery) and SARS-CoV-2 infection have a higher risk of worse outcomes. In transplant patients (renal, cardiac and hepatic), with neurological pathologies (multiple sclerosis (MS), neuromyelitis optica (NMODS), myasthenia gravis (MG)), primary immunodeficiencies and infection with human immunodeficiency virus (HIV) in association with immunosuppressants, studies have shown no tendency for worse outcomes.
ConclusionGiven the little evidence we have so far, the behaviour of SARS-CoV-2 infection in immunosuppressed patients is unclear, but current studies have not shown worse outcomes, except for patients with cancer.
No es claro si los pacientes con algún grado de inmunosupresión, tienen peores desenlaces en la infección por SARS-CoV-2, en comparación con la población sana.
ObjetivoRealizar una revisión narrativa de la información disponible sobre infección por SARS-CoV-2 en pacientes inmunosuprimidos, especialmente pacientes con cáncer, trasplantados, patologías neurológicas, inmunodeficiencias primarias y secundarias.
ResultadosLos pacientes con cáncer y tratamiento reciente del mismo (quimioterapia o cirugía) e infección por SARS-CoV-2 tienen mayor riesgo de peores desenlaces. En los pacientes trasplantados (renal, cardiaco y hepático), con patologías neurológicas (esclerosis múltiple (EM), neuromielitis óptica (NMODS), miastenia gravis (MG)), inmunodeficiencias primarias e infección de virus de inmunodeficiencia humana (VIH) en asociación con uso de inmunosupresores, los estudios no han mostrado tendencia a peores desenlaces.
ConclusiónDada la poca evidencia con que contamos hasta el momento no es claro el comportamiento de la infección por SARS-CoV-2 en pacientes con inmunosupresión, pero los estudios actuales no han mostrado peores desenlaces en este tipo de pacientes a excepción de los pacientes con cáncer.
In December 2019, a group of five patients with severe pneumonia of unknown origin were reported to have had contact with a seafood market in the city of Wuhan, Hubei Province, China, as an epidemiological link. The Chinese Centre for Disease Control and Prevention (China CDC), deployed a rapid response for the epidemiological and aetiological investigation of cases and identified a new coronavirus with the ability to cause severe lung disease that can rapidly progress to death in affected patients.1,2
Given its rapid progression and the poor knowledge of the infection, how it behaves in patients with multiple comorbidities is not clear, especially patients with some degree of immunosuppression, due either to their underlying disease or to the use of immunosuppressants to manage it. In this review, we will focus on describing the literature on SARS-COV-2 infection in patients with some degree of immunosuppression, other than rheumatological diseases, among these, cancer patients, transplant recipients, primary immunodeficiency, and HIV patients.
SARS-COV-2 infectionInitially the virus was termed the new coronavirus (2019-nCoV) and variations of same. SARS-CoV-2 is the name currently used for the virus, which shares genetic similarities with the SARS-CoV virus. COVID-19 (coronavirus disease 2019) is the name of the disease generated by SARS-CoV-2 infection.3
EpidemiologyFrom December 18th to 29th, samples of bronchoalveolar lavage fluid were collected from patients hospitalized for severe pneumonia in the city of Wuhan, the epicentre of the pandemic, and the new coronavirus was isolated. Results for viruses such as severe acute respiratory syndrome (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), influenza, avian influenza, and other common respiratory pathogens were negative.1,3
On January 24, a case series of 41 confirmed SARS-CoV-2 patients treated at a Wuhan hospital was released. Most were male (73%), with a median age of 49 years, and less than half had comorbidities (32%) such as diabetes mellitus (20%), hypertension (15%), and cardiovascular disease (15%). Of these patients, 66% had a history of exposure to the seafood market.4
In another case series of 138 hospitalized patients in Wuhan, China, 41% of the infected patients were presumed to have been infected by nosocomial transmission, 26% of the patients required ICU hospitalization, and mortality was about 4.3%.5
The infection rapidly escalated and was declared a public health emergency by the World Health Organization (WHO) on January 30, 2020.
As of today, June 27, 214 affected countries have been reported, and the number of confirmed cases worldwide is close to ten million (9,770,954), with a total of 493,898 deaths and 5,391,416 cases that have recovered. The country with the most confirmed cases is currently the United States, followed by Brazil and Russia. China, the first country affected, has a declining case curve and is now in twentieth place worldwide.6 In the Americas, there are 4,933,972 confirmed cases with 241,931 deaths; the United States and Brazil together account for 75% of all cases and 75% of all deaths currently reported in the region.7
Infection in children is less frequent and most reported cases are among family members and, to a lesser extent, from close contact with infected patients.8
Immunopathogenesis and transmissionThe coronaviruses (CoV) are a group of viruses discovered in 1960 that have a single strand of RNA (∼26−32 kb in length) that codes for structural, envelope, membrane and nucleotide proteins, as well as for non-structural proteins.9
They belong to the Coronaviridae family which in turn is part of a larger family, the Nidovirals. The Coronaviridae family is divided into two subfamilies: Orthocoronavirinae and Torovirinae. The former is classified into four genera: alphacoronavirus, betacoronavirus, gammacoronavirus and deltacoronavirus. They are zoonotic viruses, bats have been acknowledged as natural hosts, but six types have been recognized as having the ability to infect humans: two alpha-coronaviruses (229E and NL63) and four betacoronaviruses (OC43, HKU1, SARS-CoV and MERS-CoV).1,9,10
In 2003, there was an outbreak of SARS-CoV that caused 794 deaths worldwide. In 2012, MERS-CoV was discovered in Middle Eastern countries with a fatality rate of 35.5%.1
SARS-CoV-2 is a betacoronavirus, subgenus Sarbecovirus and from the subfamily Orthocoronavirinae with an envelope composed of a lipid bilayer derived from the host membrane. The genome encodes for spike glycoprotein (S), small envelope protein (E), membrane protein (M), and nucleocapsid protein (N). It also encodes accessory proteins that interfere with the host's immune response.11
Its name is due to its similarity to a crown, given the spherical morphology of the virus and the projections on its surface that correspond to the S protein, which is glycosylated and mediates the viral entry into the host cells. The M protein gives the shape to the viral particle and together with the E protein directs the assembly of the virus and its maturation. The N protein participates in the packaging of the viral RNA during assembly. Haemagglutinin is one of the accessory proteins, which binds to sialic acid in host glycoproteins, improving entry into the cell.10
Like SARS-CoV, SARS-CoV-2 uses the receptor for the angiotensin 2 converting enzyme (ACE2) as a means of entry into the cell where it binds by means of the S protein, however, unlike the other viruses, SARS-CoV-2 binding is much stronger since this protein undergoes a residue substitution in its C-terminal domain that increases affinity for the receptor.11,12 The S protein has two subunits, S1 which determines the cell tropism and S2 which mediates the fusion of the virion to the membrane so that it can enter the cell where it rapidly translates two polyproteins that form the replication/transcription complex into a double-membrane vesicle; the virion contained in these vesicles fuses with the plasma membrane to be released later.11 The viral genome found in cytoplasm acts as pathogen-associated molecular patterns (PAMPS) and are recognized by the molecular pattern recognition receptors (PRRs) that are toll-like receptors (TLR 3, TLR7, TLR8 and TLR9). The RIG-I receptor (retinoic-acid inducible gene-I), the cytosolic receptor MDA-5 (melanoma differentiation-associated gene 5) and cGAS (nucleotidyltransferase cyclic GMP-AMP synthase) recognize viral RNA and recruit adaptive molecules that trigger a response cascade leading to the activation of the nuclear transcription factor-κβ and interferon regulatory factor 3 (IRF3), producing interferon α and β and pro-inflammatory cytokines.11
Different elevated cytokines have been found in patients with COVID-19: IL-1, IL2, IL-4, IL-7, IL-10, IL-12, IL-13, IL-17, macrophage colony-stimulating factor (MCSF), MCP-1, hepatocyte growth factor (HGF), IFN-γ and TNF-α. This supports the fact that lung damage is secondary to a cytokine storm induced by the inflammatory response, resulting in the person entering a critical condition.11,13
The transmission dynamics are not yet fully known. The intermediate host between the natural reservoir and humans is unknown. However, it has been possible to confirm person-to-person transmission, which contributes to the rapid spread of the disease, and this is confirmed by the data found in the case series, which show that as of January 1, 55% of cases were linked to the seafood market in Wuhan (China); however, of the cases reported after this date, only 8.6% had this link.14
To date, person-to-person transmission has been considered to occur via respiratory droplets produced by coughing or sneezing. However, the presence of the virus has been detected in other fluids such as blood, faeces, and saliva.15,16
Initially, it was believed that spread was by people with clinical manifestations. However, it has been shown that asymptomatic carriers also transmit, and some people have even been recognized as “super spreaders”, infecting many people, including health workers.3
Vertical transmission of the virus in pregnancy has not been proven, however it is not known whether there is a risk during delivery through the vaginal canal.17
Clinical manifestationsClinical manifestations can range from being asymptomatic to acute respiratory distress syndrome and multiorgan dysfunction. The incubation period is estimated at 5.2 days; however, this can vary.18
In the first case series of Zhu et al. and Ren et al. of patients treated at a hospital in Hubei, the predominant symptoms were dry cough, dyspnoea, and fever. Lung opacities consistent with a pneumonic process, worsening rapidly (two to four days) and requiring invasive mechanical ventilation, are recorded.1,2
In general, the most common clinical manifestations are fever (although not present in all cases), cough, odynophagia, fatigue, and myalgia. Other less frequent symptoms are sputum production, headache, haemoptysis, and diarrhoea. Cases of keratoconjunctivitis and fulminant myocarditis have also been described.4,5,14
In early stages of the disease, chest x-ray can be normal, but as the disease progresses, bilateral ground glass opacity or consolidation can be found in more than 89% of patients. Chest CT is much more sensitive than radiography. These findings can be found in asymptomatic patients.13,19 It has been reported that up to 17% of patients will have no radiological changes.20
As for laboratory findings, lymphopenia was common, found in more than 80% of patients, with less frequent evidence of thrombocytopenia and leukopenia. A large proportion presented with elevated C-reactive protein, and in fewer cases, elevated alanine aminotransferase, aspartate aminotransferase, creatine kinase, and D-dimer were found. Disturbances are pronounced in patients with severe disease.20
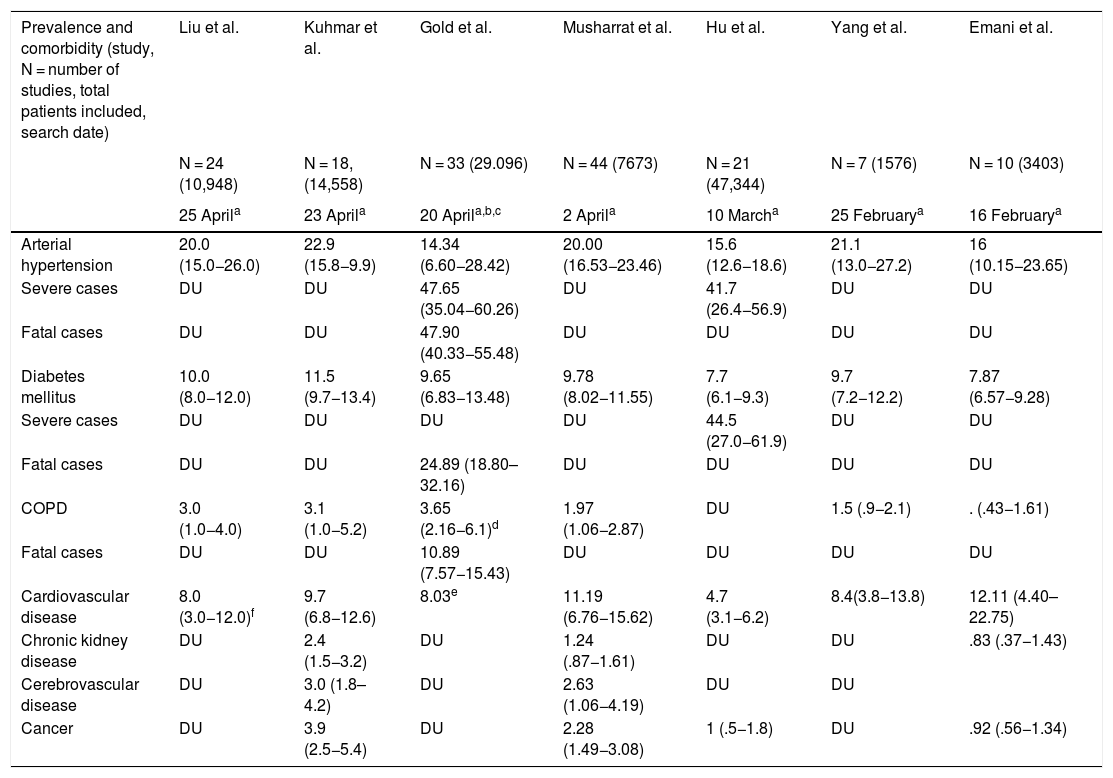
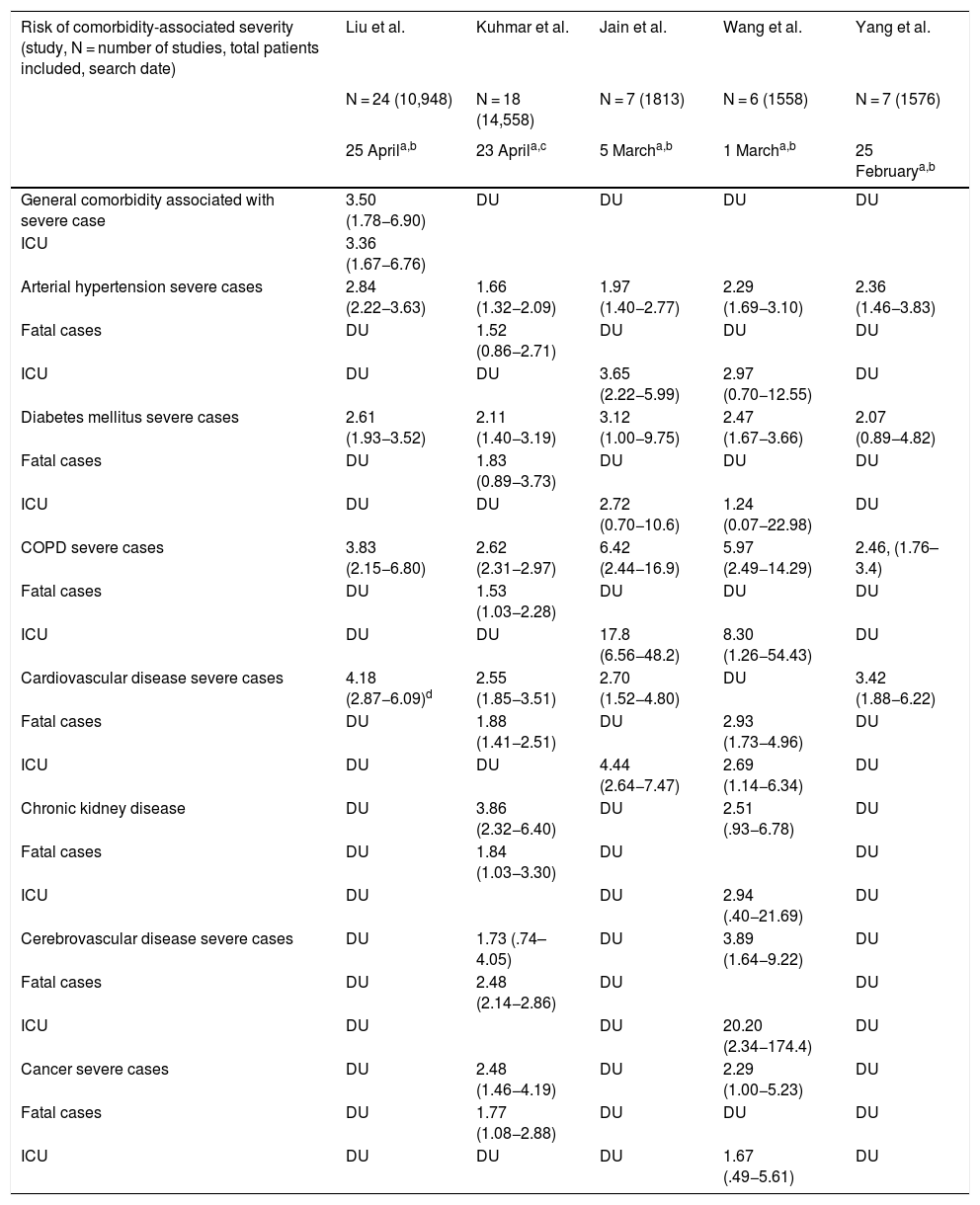
Poor prognostic factors, mortalityIn the different case series and epidemiological reports published since the appearance of SARS-CoV-2, comorbidities have been highlighted as risk factors associated with severity. It has also been established that patients admitted to the Intensive Care Unit have more comorbidities than those in general hospitalization. The most prevalent underlying diseases are high blood pressure, diabetes mellitus, cardiovascular and cerebrovascular disease.21,22 The results of nine meta-analyses in patients with COVID-19 published to date are summarised in Tables 1 and 2. Table 1 presents the prevalence of comorbidities in patients with COVID-19, most of them hospitalized, and analysed through seven meta-analyses. In turn, Table 2 shows the risk associated with severity, death, or fatality due to the presence of comorbidities, through seven meta-analyses.
Prevalence of comorbidities in published meta-analyses of patients with COVID-19.
| Prevalence and comorbidity (study, N = number of studies, total patients included, search date) | Liu et al. | Kuhmar et al. | Gold et al. | Musharrat et al. | Hu et al. | Yang et al. | Emani et al. |
|---|---|---|---|---|---|---|---|
| N = 24 (10,948) | N = 18, (14,558) | N = 33 (29.096) | N = 44 (7673) | N = 21 (47,344) | N = 7 (1576) | N = 10 (3403) | |
| 25 Aprila | 23 Aprila | 20 Aprila,b,c | 2 Aprila | 10 Marcha | 25 Februarya | 16 Februarya | |
| Arterial hypertension | 20.0 (15.0−26.0) | 22.9 (15.8−9.9) | 14.34 (6.60−28.42) | 20.00 (16.53−23.46) | 15.6 (12.6−18.6) | 21.1 (13.0−27.2) | 16 (10.15−23.65) |
| Severe cases | DU | DU | 47.65 (35.04−60.26) | DU | 41.7 (26.4−56.9) | DU | DU |
| Fatal cases | DU | DU | 47.90 (40.33−55.48) | DU | DU | DU | DU |
| Diabetes mellitus | 10.0 (8.0−12.0) | 11.5 (9.7−13.4) | 9.65 (6.83−13.48) | 9.78 (8.02−11.55) | 7.7 (6.1−9.3) | 9.7 (7.2−12.2) | 7.87 (6.57−9.28) |
| Severe cases | DU | DU | DU | DU | 44.5 (27.0−61.9) | DU | DU |
| Fatal cases | DU | DU | 24.89 (18.80–32.16) | DU | DU | DU | DU |
| COPD | 3.0 (1.0−4.0) | 3.1 (1.0−5.2) | 3.65 (2.16−6.1)d | 1.97 (1.06−2.87) | DU | 1.5 (.9−2.1) | . (.43−1.61) |
| Fatal cases | DU | DU | 10.89 (7.57−15.43) | DU | DU | DU | DU |
| Cardiovascular disease | 8.0 (3.0−12.0)f | 9.7 (6.8−12.6) | 8.03e | 11.19 (6.76−15.62) | 4.7 (3.1−6.2) | 8.4(3.8−13.8) | 12.11 (4.40–22.75) |
| Chronic kidney disease | DU | 2.4 (1.5−3.2) | DU | 1.24 (.87−1.61) | DU | DU | .83 (.37−1.43) |
| Cerebrovascular disease | DU | 3.0 (1.8–4.2) | DU | 2.63 (1.06−4.19) | DU | DU | |
| Cancer | DU | 3.9 (2.5−5.4) | DU | 2.28 (1.49−3.08) | 1 (.5−1.8) | DU | .92 (.56−1.34) |
COPD: Chronic Obstructive Pulmonary Disease; DU: Data unavailable.
Risk of severity (severe case, fatal case, or requirement for Intensive Care Unit) associated with comorbidities.
| Risk of comorbidity-associated severity (study, N = number of studies, total patients included, search date) | Liu et al. | Kuhmar et al. | Jain et al. | Wang et al. | Yang et al. |
|---|---|---|---|---|---|
| N = 24 (10,948) | N = 18 (14,558) | N = 7 (1813) | N = 6 (1558) | N = 7 (1576) | |
| 25 Aprila,b | 23 Aprila,c | 5 Marcha,b | 1 Marcha,b | 25 Februarya,b | |
| General comorbidity associated with severe case | 3.50 (1.78−6.90) | DU | DU | DU | DU |
| ICU | 3.36 (1.67−6.76) | ||||
| Arterial hypertension severe cases | 2.84 (2.22−3.63) | 1.66 (1.32−2.09) | 1.97 (1.40−2.77) | 2.29 (1.69−3.10) | 2.36 (1.46−3.83) |
| Fatal cases | DU | 1.52 (0.86−2.71) | DU | DU | DU |
| ICU | DU | DU | 3.65 (2.22−5.99) | 2.97 (0.70−12.55) | DU |
| Diabetes mellitus severe cases | 2.61 (1.93−3.52) | 2.11 (1.40−3.19) | 3.12 (1.00−9.75) | 2.47 (1.67−3.66) | 2.07 (0.89−4.82) |
| Fatal cases | DU | 1.83 (0.89−3.73) | DU | DU | DU |
| ICU | DU | DU | 2.72 (0.70−10.6) | 1.24 (0.07−22.98) | DU |
| COPD severe cases | 3.83 (2.15−6.80) | 2.62 (2.31−2.97) | 6.42 (2.44−16.9) | 5.97 (2.49−14.29) | 2.46, (1.76–3.4) |
| Fatal cases | DU | 1.53 (1.03−2.28) | DU | DU | DU |
| ICU | DU | DU | 17.8 (6.56−48.2) | 8.30 (1.26−54.43) | DU |
| Cardiovascular disease severe cases | 4.18 (2.87−6.09)d | 2.55 (1.85−3.51) | 2.70 (1.52−4.80) | DU | 3.42 (1.88−6.22) |
| Fatal cases | DU | 1.88 (1.41−2.51) | DU | 2.93 (1.73−4.96) | DU |
| ICU | DU | DU | 4.44 (2.64−7.47) | 2.69 (1.14−6.34) | DU |
| Chronic kidney disease | DU | 3.86 (2.32−6.40) | DU | 2.51 (.93−6.78) | DU |
| Fatal cases | DU | 1.84 (1.03−3.30) | DU | DU | |
| ICU | DU | DU | 2.94 (.40−21.69) | DU | |
| Cerebrovascular disease severe cases | DU | 1.73 (.74–4.05) | DU | 3.89 (1.64−9.22) | DU |
| Fatal cases | DU | 2.48 (2.14−2.86) | DU | DU | |
| ICU | DU | DU | 20.20 (2.34−174.4) | DU | |
| Cancer severe cases | DU | 2.48 (1.46−4.19) | DU | 2.29 (1.00−5.23) | DU |
| Fatal cases | DU | 1.77 (1.08−2.88) | DU | DU | DU |
| ICU | DU | DU | DU | 1.67 (.49−5.61) | DU |
COPD: Chronic Obstructive Pulmonary Disease; DU: Data unavailable; ICU: Intensive Care Unit.
The meta-analysis by Wang et al. is noteworthy, which identifies arterial hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), cardiovascular and cerebrovascular disease as factors that negatively impact mortality. Specifically, COPD increases by 5.9 times23 the risk of progression and deterioration of patients with COVID-19. These data are supported by the results of another meta-analysis by Zhao et al. where the severity of COVID-19 is four times higher in COPD patients; they also assessed the impact of active smoking, which increases the risk of severe COVID-19 two-fold.24
It has been widely demonstrated that patients suffering from diabetes mellitus are admitted more frequently to the Intensive Care Unit and have a higher mortality, as shown in Table 2. The results of a primary study by Roncon et al. are highlighted (OR 3.21, 95% CI 1.82–5.64; p < .0001, I2 = 16%).25 These patients tend to present a more severe pneumonic process, with greater inflammatory response and tissue damage, which makes them more prone to cytokine storm leading to rapid deterioration, which is why patients with this history should be strictly monitored.26
Cardiovascular disease is a risk factor per se increased by COVID-19 infection that generates or aggravates myocardial damage, but when associated with myocardial injury the results are usually fatal for patients.27,28
Among other comorbidities, chronic kidney disease is associated with in-hospital mortality, as are cancer and cerebrovascular disease, demonstrated through two meta-analyses that included over fifteen thousand patients (Table 2); studies suggest that superficial fungal infections and psoriasis confer vulnerability to COVID-19; a body mass index (BMI) > 40 kg/m2 is an independent risk factor for complications from the infection; and there are discouraging results regarding underlying neurological disease and SARS-CoV-2.29–32
In general, the presence of comorbidities should imply strict follow-up of patients to detect early complications; however, more attention should be paid to certain comorbidities where strong associations have been found with COVID-19 infection and its severe outcomes.
Overall mortality is .5% to 4%, but it changes to 5% to 15% among patients requiring hospitalization and increases to 62% in critically ill patients. Studies have shown two peaks, at 14 and 22 days. Among the causes of death, respiratory failure prevails, followed by shock due to myocardial dysfunction and finally, the combination of the two.33
DiagnosisIt is important to maintain a high degree of diagnostic suspicion in patients with fever or respiratory symptoms who have travelled to affected areas or have had close contact with suspected or confirmed cases 14 days before the onset of symptoms. In this scenario, confirmation by molecular testing is required. Real-time reverse transcription polymerase chain reaction is performed on specimens collected from the lower or upper respiratory tract if the former cannot be obtained.34
There are several assays that are performed on serum or plasma for the detection of both viral proteins and antibodies. The most widely used are to detect immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies, which are produced in the second week of infection.35
TreatmentThere are several therapeutic goals and they are directed at different levels: inhibition of virus entry into the cell, inhibition of fusion of the viral envelope to the membrane, transcription inhibition, inhibition of viral proteins and blockade of IL-6 signalling to prevent the cytokine storm.35
At first, chloroquine (CLC) and hydroxychloroquine (HCQ) were shown to block SARS-CoV-2 in vitro, with better results for the latter and therefore its use was indicated for the management of COVID-19 infection.36 Promising results have been shown in case series; however, the studies have limitations such as the sample size used.
However, preliminary results were recently published from the RECOVERY study,37 a randomized clinical trial to evaluate potential drugs for management of the infection in the United Kingdom, in which they concluded no beneficial effect of the use of antimalarials in hospitalized patients, and therefore they stopped including patients for this treatment arm and the recommendation that it should not be used has been extended worldwide. Additional results have recently been published in several articles on the efficacy and safety of the antimalarials chloroquine and hydroxychloroquine for the treatment of different phases of SARS-CoV-2 infection. However, the data are controversial, some not demonstrating efficacy or reporting a high number of adverse events, primarily associated with cardiac arrhythmias.38 It is important to note that a number of criticisms and concerns have been raised regarding the accuracy of the data from these studies and they have therefore been withdrawn.39 To date there are more than 200 clinical trials underway with HCQ, and 80 with CLQ,35 several of which are in prophylactic use in healthcare workers and others post-exposure.36,40
Lopinavir/ritonavir has studies in SARS and MERS, the data published for COVID-19 are reports and retrospective studies with which the effect cannot be established with certainty. There is a clinical trial of 199 patients with COVID-19 with no difference in mortality, hospital discharge or recovery. However, despite this, in some centres it is still being used at doses of 400 mg/100 mg twice a day for 14 days.35
Ribavirin, like lopinavir/ritonavir, has activity against other coronaviruses and was considered to be a possible treatment for SARS-CoV-2; however, studies carried out for SARS show limited, high-dose activity leading to a high rate of haematological and hepatic adverse events, therefore its use is now limited.35,41
Other antivirals such as oseltamivir were used in the first cases in Hubei, China, because it was suspected to be seasonal influenza; they are now not indicated for use in SARS-CoV-2.35 Remdesivir is a nucleoside analogue that showed in vitro activity against SARS-CoV-2, used later in a 53-patient cohort in Canada, the United States, Europe, and Japan, achieving a satisfactory response in 36 patients.42 Based on preliminary studies, some drug regulatory agencies (United States and Japan),43 conducted emergency approvals for use in hospitalized patients. Results from ongoing studies are expected to evaluate efficacy and safety.
Currently umifenovir or arbidol is under study, an antiviral that aims to inhibit the interaction between protein S and ACE2.35
The use of corticosteroids is limited in SARS-CoV-2 infection to scenarios of chronic obstructive pulmonary disease exacerbation and refractory shock, taking into account previous studies in influenza pneumonia where they were associated with increased mortality.35 Recently, preliminary results from the RECOVERY study44 showed that the use of dexamethasone reduced mortality in one-third of critically ill patients who were on mechanical ventilation, while reduction was one-fifth in those receiving non-invasive oxygen. Definitive results are expected from this and the more than 10 clinical trials currently underway to define the particular subgroups that would benefit from this treatment.
Monoclonal antibodies against IL-6 are another therapy studied, in phases of adult respiratory distress syndrome (ARDS), with promising results in small case series.35
Convalescent plasma is another therapy used as salvage in SARS and MERS. At the beginning of the pandemic, a case series of five critical patients from China, who were given convalescent plasma, showed improvement in their clinical status. More recently, several case series and preliminary trial results have demonstrated clinical benefits and decreased mortality with its use,45 particularly in hospitalized patients with moderate to severe involvement; however, results from more than 200 clinical trials46 are awaited to clarify the characteristics of the plasma, the donors, and the specific individuals who could benefit.47
Despite the abovementioned therapies, there is still no specific treatment and therefore the recommendations are symptomatic management in mild cases, supportive therapy in cases of critical illness and management of ventilation in cases of ARDS.35
Immunosuppression and SARS-CoV-2Given the suddenness of the pandemic, and its rapid spread, little is known about SARS-COV-2 infection and certain types of condition or disease, this is the case for people with some type of immunosuppression (either primary, associated to underlying or pharmacological diseases), which given the physiopathogenesis of SARS-COV-2 infection known so far, would raise two hypotheses: it could be a possible benefit, since this state of immunosuppression could avoid that uncontrolled immune response or “cytokine storm”, but on the other hand, it is equally clear from previous studies that immunosuppressant use or status is associated with increased risk of infection.
In epidemics such as the abovementioned SARS-CoV, immunosuppressed patients, especially transplant recipients, did not have worse outcomes than the general population.48,49 Similar findings were presented in the MERS epidemic, being male, advanced age and comorbidities such as diabetes mellitus, obesity, pulmonary pathology and renal disease being found as risk factors, and immunosuppression status not being associated as a factor of poor prognosis.50
To date, the Centres for Disease Control and Prevention (CDC) and other international agencies51 have included as poor prognostic factors patients with some degree of immunosuppression, including people with a history of cancer treatment, smokers, transplant recipients, people with immunodeficiencies, poorly controlled HIV or AIDS and people with prolonged use of steroids or immunosuppressive drugs, all based on previous studies that associate such diseases with respiratory infections, especially of viral aetiology.52
Current evidence of conditions associated with immunosuppression and SARS-CoV-2 infectionCancerOn March 21, Liang et al.,53 published a study collecting data from 575 hospitals in China, up until January 31, 2020, on patients with SARS-COV-2, comparing those with a history of cancer and those without. They collected 1519 patients, 18 (1%) with a history of cancer. The most frequent neoplasm was lung (five cases [28%]), and of the total cancer patients, four (25%) had undergone chemotherapy or surgery within the previous month, and the rest were cancer survivors, with strict follow-up. In terms of sociodemographic characteristics, the cancer patients were older, had a greater history of exposure to cigarettes, presented more polypnoea and had more severe pulmonary tomographic manifestations.
In the analysis of outcomes, they showed that patients with a history of cancer and SARS-CoV-2 infection were at greater risk of serious events (defined as the percentage of patients admitted to the Intensive Care Unit requiring invasive ventilation or death) compared to patients without cancer (seven (39%) of 18 patients vs. 124 (8%) of 1572 patients; p = .0003). In addition, patients who underwent chemotherapy or surgery in the last month had a higher risk (three (75%) out of four patients) of clinically severe events than those who had not undergone chemotherapy or surgery (six (43%) out of 14 patients). These data were confirmed by logistic regression (odds ratio (OR) 5.34; 95% CI 1.80–16.18; p = .0026) after adjusting for other risk factors such as age and smoking history. In addition, the patients with cancer deteriorated more rapidly than those without cancer (median time to severe events 13 days (CI 6–15 vs. 43 days, 20-unreached; p < .0001). Furthermore, Desai et al.,54 recently published a meta-analysis, in which they included 11 studies, finding a prevalence of cancer in patients with COVID-19 of 2% (CI 2.0%–3.0%; I2 = 83.2%). We should clarify that some authors consider that the current evidence is insufficient in this field, however, the number of research results has been increasing, showing similar results.55,56
Taking into account the results mentioned, it can be stated that cancer and its recent treatment are bad prognostic factors for SARS-CoV-2 infection. Therefore, special recommendations should be considered for these patients, such as postponing adjuvant chemotherapy or elective surgery in people with "stable" cancer, especially in endemic areas, adopting stricter personal protection measures for cancer patients or cancer survivors, and considering stricter surveillance or treatment when cancer patients are infected with SARS-CoV-2.57 In general, decisions should be made on a “patient-to-patient” basis.55,58
TransplantWe highlight the study of a case series,59 two heart and kidney transplants and one liver (paediatric population). The heart transplant patients were confirmed by PCR to be infected, one of them was 51 years old, came with immunosuppression with tacrolimus 2 mg per day and mycophenolate 1 g per day, and attended consultation for fever, fatigue and liquid stools, with characteristic findings of SARS-CoV-2 infection on chest tomography. He presented criteria of severe pneumonia, immunosuppression was discontinued, and he was managed with immunoglobulin (IVIg) 10 g/day and methylprednisolone 80 mg/day and made adequate medical progress. The second patient, 43 years old, in immunosuppression with tacrolimus 3.5 mg a day and mycophenolate 1 g a day, attended with fever and fatigue, had lymphopenia, did not require hospitalization, nor discontinuation of immunosuppression, and was managed with ceftriaxone and ganciclovir, with an adequate outcome.
Their adequate clinical course suggests that in patients with this type of transplant the disease has a similar presentation to non-transplanted patients.59 It should be noted that in a series of seven cases60 (two liver, three kidney, one lung and one heart) an initial attenuated inflammatory response was evident, suggesting that although patients with transplant immunosuppression may have higher susceptibility to SARS-CoV-2 infection, their clinical course could be similar to that of immunocompetent patients.
With regard to the renal transplantation patients, until the time of the report, a 50-year-old patient remained in ICU managed with lopinavir/ritonavir, requiring suspension of immunosuppression who came under management with tracrolimus, everolimus and prednisolone at intermediate doses. He was admitted for fever and vomiting that progressed to respiratory symptoms, and had thrombocytopenia, lymphopenia, and elevated D-dimer as factors of poor prognosis. The second, a 52-year-old, under immunosuppression with tacrolimus, mycophenolate, and prednisolone, consulted for fatigue, abdominal pain, dyspnoea, fever, and dry cough, presented lymphopenia and imaging findings typical of SARS-CoV-2 infection. He was managed with IVIg (5 g, then 10 g/day × 11 days), methylprednisolone 40 mg/day and interferon α (5 million/U day), in addition to suspension of immunosuppression, and responded adequately to treatment. With regard to this type of transplant, the atypical presentation of the first case is noteworthy, and the adequate response of the second that could be associated with the use of multiple therapies, without it being possible conclude whether renal transplantation is associated or not with a worse prognosis.61 Gandolfini et al.,62 publish two cases of renal transplant and COVID-19, a 75-year-old male and a 52-year-old female patient under management with tacrolimus, corticoids and mycophenolate, who developed severe pneumonia; in addition to suspending immunosuppressants, management with hydroxychloroquine, lopinavir/ritonavir and colchicine was started, due to the unavailability of tocilizumab. The administration of colchicine achieved an impact in decreasing IL-6 serum levels, thanks to its interfering with inflammasome assembly which leads to the production of IL-1b and other interleukins such as IL-6.
In Italy, the paediatric liver transplant group of Hospital Papa Giovanni xxiii Bergamo followed up 700 liver transplants (two in the last three months), associated with autoimmune liver diseases (100 patients), three additionally in chemotherapy (for hepatoblastoma). Of the total number of transplant recipients, three were confirmed to be infected with SARS-CoV2, and all remained asymptomatic without requiring hospitalization or suspension of immunosuppression. Additionally, Qin et al.,63 report the case of a patient with hepatocellular carcinoma who underwent liver transplantation and suffered an undetected SARS-CoV-2 infection in the perioperative period; immunosuppression was initiated with tacrolimus and glucocorticoids; however, persistence of fever led to confirmation of SARS-CoV-2 infection; management with oseltamivir and immunoglobulin was initiated, and despite a prolonged convalescence, they did not present multiorgan failure, thus immunosuppression was maintained. The importance of SARS CoV-2 detection is highlighted for organ receptors and donors to reduce the transmission and risk of severe infection or rejection due to adjustments in immunosuppression.
Given the above, it is not clear whether transplantation and use of immunosuppressants in this context is a risk or severity factor for SARS-CoV-2 infection. Likewise, in the event of SARS-CoV-2 infection, the adjustment or suspension of immunosuppressors should be assessed, and we should always seek to protect graft function with the administration of glucocorticoid doses and support measures, among others.64
Neurological diseases and immunosuppressionNeurology is a continuously growing specialty. Many diseases have a component that compromises autoimmune aggression to a greater or lesser extent and therefore go on to require immunosuppressant or immunomodulatory management.
Within the multiple entities, two diseases have become relevant in recent times, multiple sclerosis and optical neuromyelitis, due on the one hand to their physiopathological mechanism that involves neurodegeneration and inflammation by excessive activity of the immune system derived from antigenic epitopes and proinflammatory molecules, and on the other hand the use of therapies that trigger regulation of immune cells, affecting in some cases innate and adaptive immunity in most cases.
If we consider that the response mechanisms to viral infections are based on inhibition of the infection by type I interferons and the death of the infected cells by NK lymphocytes (innate immunity), the generation of antibodies that block the union and entry of the virus into the cells, and the elimination of cells infected by cytotoxic T cells (adaptive immunity), the different drugs currently used could to a greater or lesser extent alter the immune response to SARS-CoV-2 infection, and this is why there are now different considerations when initiating or continuing therapies.65
Multiple sclerosis (MS)People with MS are at higher risk of admission to the Intensive Care Unit due to infections, and higher mortality at one year after admission than the general population. The use of disease-modifying therapies implies a higher risk of infections, however, to date there is no data to indicate that patients with MS are at higher risk of SARS-COV-2 infection, or more severe infection. It is even possible that such disease-modifying therapies and their immunosuppressive effect may play a protective role during 19-COVID infection by preventing or dampening hyperimmune activity that, in some cases, could lead to clinical deterioration; there is even a report of a patient with primary progressive multiple sclerosis receiving treatment with ocrelizumab and becoming infected with SARS-COV-2, in the context of lymphopenia and hypogammaglobulinema expected for this type of treatment, without generating major clinical complications, this hypothesis is obviously limited for now only to academic deductions and limited information.66,67 In recent results of the multicentre registry COVISEP,68 which includes information from 347 patients with MS and COVID-19, it was demonstrated that age, obesity and highest score in the Expanded Disability Status Scale, were independent risk factors for severity of COVID-19.
It is suggested that people with MS and related disorders receiving immunotherapy continue to receive the therapy during mild viral infections. In those with documented mild SARS-CoV-2 infection, it may be reasonable to continue treatment. Neurologists should have a lower threshold for suspending treatment in people taking therapies with greater immunosuppressant effects.69
Consideration should be given to suspending treatment in those who are hospitalized with severe or complicated SARS-CoV-2 infection. Treatment may be restarted after four weeks or when symptoms have completely resolved, considering the risk of rebound of MS activity with S1P modulators and natalizumab. Neurologists should alert intensive care physicians to the importance of fever management in people with MS.70
In people with MS and disease-modifying treatment, the decision to start, continue, temporarily suspend, or defer doses should be individualized,71 taking into account factors such as disease activity and the possibility of disease progression, as well as considerations of the mechanism of action of the drugs and their ability to deplete lymphocytes. Recommendations from experts suggest not suspending first-line drugs (interferons, glatiramer acetate, teriflunomide, or dimethyl fumarate) and considering deferring therapies such as cladribidine and alemtuzumab based on their ability to deplete lymphocyte counts rapidly and aggressively.72,73
Neuromyelitis optica (NMOSD)A survey of patients with NMOSD or MS from China, from 10 centres, did not find an increased risk of infection by COVID-19, suggesting as a possibility the role of self-care and protective measures taken by patients and their healthcare team, regardless of their condition and immunosuppression drug.74
Relapses in patients with NMOSD can be devastating and patients should be encouraged to continue therapies for the prevention of attacks, including corticosteroids, azathioprine, mycophenolate mofetil, rituximab, tocilizumab and eculizumab. If there is a clinical need to discontinue or delay treatment in patients with NMOSD, corticosteroids may be used in moderate doses to prevent relapses in the short-term, it is important to consider individualized therapy and comorbidities when deciding on management of this condition during the Covid-19 pandemic.69
Myasthenia Gravis/ Lambert-Eaton myasthenic syndrome (MG/LEMS)Because most patients with myasthenia gravis (MG) are on immunosuppressant or immunomodulatory therapies and may also have muscle weakness and ventilatory failure, there is a theoretical concern that they may be at increased risk for infection or experience severe manifestations of SARS-CoV-2 infection. In a series of five cases75 with MG hospitalized for COVID-19 infection, a variable clinical course was demonstrated, with three requiring mechanical ventilation and one presenting MG crises, and although it is difficult to assess the latter due to intubation and sedation in two of the cases, none had a fatal outcome. Two additional cases have been reported, one developing crises due to myasthenia76 and the other with chronic refractory MG,77 with good outcome, without complications or worsening of their baseline condition.
There are numerous recommendations circulating that attempt to provide clarity and guidance. However, the differences between the recommendations have created confusion, because decision- making varies in different countries, and due to the lack of databases with an adequate number of patients.
Patients with MG/LEMS should continue their treatment and are advised not to discontinue any existing medications; there is no scientific evidence to suggest that symptomatic therapies such as pyridostigmine increase the risk of infection and they should not be discontinued unless there are other clinical reasons to do so, given the risk of increased disease activity and/or MG exacerbation or crisis.1 With regard to certain therapies (immunoglobulins, plasmapheresis) there is no information pointing to increased risk of infection, however, the use of immunoglobulin should be based on the individual need of the patient and indiscriminate use should be avoided. In general, these therapies should be reserved for patients with acute exacerbation and if required as maintenance therapy on an exceptional basis, additional precautions should be taken.
In patients with severe SARS-CoV-2 infection, temporary suspension of immunosuppression may need to be considered.
It is important to note that decisions to intensify or change treatment should be individualized based on the relative severity of the SARS-CoV-2 infection.78
Primary immunodeficienciesThere is very little data regarding the impact of SARS-CoV-2 infection on primary immunodeficiencies (PI), which is why several international organizations such as the European Society for Immunodeficiency (ESID),79 the Reference centre for hereditary immunodeficiencies (Le Centre de Référence Déficits Immunitaires Héréditaires, CEREDIH)80 and the International Patient Organization for Primary Immunodeficiencies (IPOP)81 are collecting data through a survey of physicians in order to gather information and provide them better care.
Both the IDF (Immune Deficiency Foundation)82 and the ASCIA (Australasian Society of Clinical Immunology and Allergy)83 and other agencies84 have considered their patients’ increased risk of severe respiratory infections or of experiencing a more severe disease course, however, they recognize that it cannot be said whether people with primary immunodeficiencies are at higher or lower risk of severe SARS-CoV-2 infection. ASCIA and IPOPI promote measures to prevent the spread of the virus, social isolation, and call for early consultation with medical services when infection is suspected, and recommend maintaining continuity of medication, especially in those receiving immunoglobulin.81,83
Virtual resources with patient and medical community education are available in the IDF. These experts on the subject have theorized the possible effects of SARS-CoV-2 in different populations,82 for example, in the group of T lymphocyte (TL) immunodeficiency (combined immunodeficiency, DiGeorge syndrome, among others) measures of isolation and protection must be maximised, since the action of these defence cells is necessary for the control of the virus; in B-lymphocyte deficiency (agammaglobulinaemia, common variable immunodeficiency) the risk of infection is not thought to be higher than in the community, except in patients with structural involvement at the lung level, and in the phagocytosis deficiency immunodeficiency group (neutropenia and chronic granulomatous disease) although neutrophils are not as important in controlling the virus, the possibility of co-infection needs to be considered, while the chronic granulomatous disease group is not thought to be at increased risk of infection or severe manifestation.85
Other recommendations according to the IPOPI Joint Statement on the Current Coronavirus Pandemic, are the use of PCR tests for diagnosis, since for some forms of PI there is no production of antibodies, and therefore tests based on immunoglobulins are not effective. In this same publication, as of April 5, 2020, 15 SARS-CoV-2 cases in different types of PI, exhibiting typical symptoms (fever, cough, and upper respiratory symptoms), 13 of which were under 45 years of age; seven required hospitalization (two developed adult respiratory distress syndrome) and all were under 45 years of age. In the most recent update, based on collected (unpublished) data, there does not appear to be an increased risk of SARS-CoV-2 infection, especially in its severe form, however, given the still limited information and risk for these patients, isolation and infection prevention measures should be maintained as much as possible.81
Another important aspect for these patients is the impact during the SARS-CoV-2 pandemic on their health-related quality of life (HRQoL), requiring strict isolation and a remote care programme. In an Italian cohort of 158 patients with PI due to a BL defect, two scales were evaluated, one specific to health-related quality of life, CVIDQoL (Common Variable Immune Deficiency Quality of Life), and another generic scale to assess anxiety and depression, GHQ-12 (12-item General Health Questionnaire); finding that the remote care programme does not affect HRQoL, however, in the group of patients at risk of anxiety/depression there is impaired quality of life, emphasizing the importance of individualizing each patient and psychosocial support.86
Human immunodeficiency virus (HIV)Blanco et al.87 published a case series of the evidence regarding SARS-CoV-2 infection in patients with HIV immunosuppression. Beginning with the experience reported from Wuhan, China, where a single case of a SARS-CoV-2 patient co-infected with HIV has been documented, despite there being 37.9 million people with HIV worldwide, according to the Joint United Nations Programme on HIV/AIDS.88
Based on the experience of a single centre in Spain of 543 patients with SARS-CoV-2 infection admitted to the Clinic de Barcelona, five have a history of HIV. They attended a consultation in the context of sepsis of pulmonary aetiology requiring invasive ventilatory support (in two of them). During the stay at this institution infection was documented by nasopharyngeal sample associated with amplification of the betacoronavirus E gene and the specific SARS-CoV-2 RdRp gene by PCR, comorbidities such as hypothyroidism and asthma were also identified in these patients. All came under antiretroviral treatment, with CD4 cell count (>400 cell/mm3 in four of the patients). Prior classification according to the patient's clinical status as mild, moderate or severe, under the precept that protease inhibitors could have activity against coronavirus protease, cobicistat + darunavir was considered appropriate in two of them, starting ritonavir + lopinavir in the rest combined with hydroxychloroquine, azithromycin, corticosteroids, interferon β-1b and even tocilizumab, according to duration and progression. The survival of all is documented up to the time of publication and the conclusion is that patients in advanced stages of the disease should be guaranteed differential diagnosis by opportunistic pulmonary agents, inferring that they may have a poorer outcome, as well as an ominous prognosis.89,90 In a recent study of incidence and severity91 of COVID-19 involving 60 HIV clinics in Spain (77,590 patients), 236 patients were diagnosed with COVID-19, 151 were hospitalized, 15 required intensive therapy and 20 died. It was found that patients receiving tenofovir disoproxil fumarate (TDF)-emtricitabine (FTC) reverse transcriptase inhibitors had a lower risk of developing COVID-19 and of hospitalization compared to groups receiving other treatments.
There are isolated cases92 in which patients coinfected with HIV and COVID-19 in management with lopinavir and ritonavir had a favourable clinical response, considering that this reaction can have two effects: inhibition of SARS-CoV-2 replication, as well as inhibition of HIV replication, allowing a slight activation of the immune system capable of responding to SARS-CoV-2 without the progression of the patient to hyperinflammatory status, even highlighting that lymphopenia would not be considered a marker of poor prognosis but a protective immune effect, being considered an disturbance of the overactive response of the immune system, avoiding serious clinical manifestations.92 Other hypotheses raised as a favourable clinical response dependent on the patient's immune status, coinfection or history of opportunistic infections, mainly at the pulmonary level, the risk or benefit in relation to the use of glucocorticoids and even the benefit of introducing tocilizumab early were not omitted.93
In patients with HIV and SARS-CoV-2 infection it can be concluded that the immune response, prognosis and outcome are highly variable and / or subjective according to the antiretroviral treatment in place, duration in relation to diagnosis and viral suppression, because HIV patients without treatment, newly diagnosed or with no viral suppression may have a compromised immune system (mediated by a low CD4 count), even being vulnerable not only to the worst outcomes due to SARV-CoV-2, but alleged coinfection by other agents in pulmonary opportunists. Patients with adherence to antiretroviral treatment, who have achieved viral suppression and do not have low CD4 count, will be affected by SARS-CoV-2 with the same chances as immunocompetent patients who develop mild manifestations, regardless of change in antiretroviral treatment or adjustment with ritonavir and lopinavir, however, it is important to emphasize the divergences in approach, management and choice versus antiretroviral treatment documented to date. For this type of patients, as well as those mentioned previously, recommendations have already been published on their management with COVID-19.94
Pharmacological immunosuppressionThe degree of pharmacological immunosuppression is assessed according to the patient's immunological risk, the type of protocol used, the type of target at which the drug or group of drugs is directed, and the type of disease for which it is indicated (e.g., neoplasms, transplants, immunological, etc.). Although in all cases it is not easy to directly assess the degree of immunosuppression, some biomarkers have been developed that reflect the individual's response to immunosuppressants, which can range from general tests such as liver function enzymes, to the use of specific genotypes, including cell counts of specific lymphocyte populations, cytokines, leukocyte markers and target enzymes, among others.95 In other cases, the degree is assessed according to the number of immunosuppressant drugs, dose and time employed, being low when a drug is used in low or moderate doses for a short time, and increasing to a higher degree when two or more immunosuppressants are used in combination, regardless of the dose.96
There are many drugs used in different medical specialties that are associated with a certain degree of immunosuppression and that render patients vulnerable to one or another infectious process. However, in the field of SARS-CoV-2 infection, the data are scarce and unknown.
As mentioned throughout the review, comorbidities are the most important risk factors compared to drugs used. The literature search predominantly reveals information about possible drug interactions that can occur with the treatment of COVID-19, which can clearly also generate damage and worsen the clinical picture. To date there are no data on specific drugs that are associated to a greater or lesser degree with infection by the new coronavirus.97
There is a warning about possible drug interactions between immunosuppressive drugs and those under investigation for the treatment of COVID-19, generating alerts and guidelines for the development of this complex task by clinicians. Remdesivir, an antiviral with promising results in the treatment of COVID-19 as mentioned, has no data so far on possible drug interactions with immunosuppressive drugs, unlike chloroquine/hydroxychloroquine and lopinavir/ritonavir, which although their use is declining, were initially an active part of treatment, suggesting major interactions with calcineurin inhibitors, mTOR (mammalian target of rapamycin inhibitors) and corticosteroids, especially lopinavir/ritonavir.97
There are recommendations about discontinuing mycophenolate in critically ill transplant recipients,98 which would also apply to COVID-19, bearing in mind that during the H1N1 pandemic it was documented that this drug decreased the serological response in transplant recipients.97 However, given the scarcity of data, this decision must be tailored to each patient in this special subgroup.
In reference to calcineurin inhibitors (tacrolimus, cyclosporine), a dose minimization scheme is proposed, with the possibility of increasing the interval for its administration, suggesting safety in these regimens, particularly in individuals with kidney transplantation and COVID-19.99
Multiple recommendations and guidelines have been generated around the use of immunosuppressors or cytostatics in the oncology field,100 such as cyclophosphamide, doxorubicin, cytarabine, vinblastine, as well as immunotherapy and the use of biological drugs in the context of cancer, according to the type of neoplasm in the context of risk or presence of COVID-19 infection, suggesting in general a decrease in dose, but always balancing individual cases according to the type of neoplasm, stage and immunosuppressive scheme proposed.58
The recommendation is to avoid high doses of corticosteroids since they could, as observed in patients with MERS-CoV, prolong viral replication in patients with COVID-19.97 As mentioned above, their use would be reserved for specific subgroups of critically ill patients
Other drugs, not immunosuppressants, but associated with the physiopathogenesis of the disease, such as angiotensin-converting enzyme inhibitors or renin-angiotensin-aldosterone system inhibitors, have not been shown to increase the risk of SARS-CoV-2 infection, and conversely, their withdrawal could be harmful.101
ConclusionConsidering the evidence available to date on SARS-CoV-2 infection outcomes in patients with immunosuppression (either due to their disease or the use of immunosuppressants) its behaviour is not clear in this type of individuals. We can highlight that patients with cancer and recent treatment of cancer (chemotherapy or surgery) have a higher risk of worse outcomes, with faster deterioration than those without cancer, an increased risk of severity and mortality having been shown through two meta-analyses. With regard to transplant patients (kidney, heart and liver), patients with neurological disease associated with the use of immunosuppression (MS, NMODS, MG), primary immunodeficiencies and HIV, studies have not shown a tendency to poorer outcomes than patients without these diseases or drugs and have SARS-CoV-2 infection, similar to that found in rheumatological diseases.102 This could perhaps be explained in that the severity of SARS-CoV-2 infection has been associated with an aberrant inflammatory response (cytokine storm). For the time being and as more information is obtained, and based on the aforementioned literature and recommendations of societies, it is suggested that immunosuppressant therapy be continued, starting new therapies should be avoided as much as is possible (especially in endemic areas) and in case of infection, depending on its severity, the risk/benefit should be evaluated of suspending it during the period of infection. It should be noted that the presence of comorbidities, such as high blood pressure, diabetes mellitus and COPD, increases the risk of severity, intensive care requirements and mortality.
FundingNo specific grants were received for this research from funding agencies in the public, commercial or non-profit sectors
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Cajamarca-Baron J, Guavita-Navarro D, Buitrago-Bohorquez J, Gallego-Cardona L, Navas A, Cubides H, et al. SARS-CoV-2 (COVID-19) en pacientes con algún grado de inmunosupresión. Reumatol Clin. 2021;17:408–419.