Traditionally, the health-related quality of life (HRQoL) of patients with systemic lupus erythematosus (SLE) has been assessed using instruments that neglect the specific characteristics of the disease. This study determines the validity of the Lupus Quality of Life (LupusQoL) questionnaire as a psychometrically stable instrument to measure the HRQoL of patients with SLE in Venezuela and establishes the cutoff points of the questionnaire for the Venezuelan population.
Patients and methodsA cross-sectional study was conducted that included patients with SLE from April to July 2018. Patients completed the LupusQoL and the “Generalitat de Catalunya” (GENCAT) scale; sociodemographic data, activity index (SLEDAI) and accumulated damage (SLICC), were obtained. Reliability was evaluated by internal consistency and the convergent validity of the LupusQoL was determined with the GENCAT scale.
ResultsOf the 100 patients, 93% were women, the mean age was 42 years old (SD: 13) and the mean duration of the disease was 11 years (SD: 9); the mean of SLEDAI and SLICC was 3 and 1, respectively. The cutoff point that defined a “better” or “worse” HRQoL for LupusQoL was 64.55 points. A moderate convergence was found after grouping, according to the cutoff points, of the LupusQoL with the GENCAT scale (Cohen’s kappa coefficient = .556; p = .000).
ConclusionsThe LupusQoL is a valid psychometrically stable instrument to measure the HRQoL of patients with SLE in Venezuela. Cutoff points were established to stratify the HRQoL in the Venezuelan population with LES, being useful to complement a comprehensive evaluation.
Tradicionalmente, la calidad de vida relacionada con la salud (CVRS) de los pacientes con lupus eritematoso sistémico (LES) ha sido evaluada utilizando instrumentos que desatienden las características específicas de la enfermedad. Este estudio determina la validez del cuestionario Lupus Quality of Life (LupusQoL) como instrumento psicométricamente estable para medir la CVRS de los pacientes con LES en Venezuela, y establece los puntos de corte del cuestionario para la población venezolana.
Pacientes y métodosSe realizó un estudio de corte transversal que incluyó pacientes con LES desde abril hasta julio de 2018. Los pacientes completaron el LupusQoL y la escala Generalitat de Catalunya (GENCAT); se obtuvieron los datos sociodemográficos, índices de actividad (SLEDAI) y daño acumulado (SLICC). Se evaluó la fiabilidad mediante consistencia interna y se determinó la validez convergente del LupusQoL con la escala GENCAT.
ResultadosDe los 100 pacientes, el 93% eran mujeres, la media de edad fue de 42 años (DE: 13) y la media de duración de la enfermedad fue de 11 años (DE: 9); la media de SLEDAI y SLICC fue de 3 y 1, respectivamente. El punto de corte que definió una «mejor» o «peor» CVRS para el LupusQoL fue 64,55 puntos. Se encontró una convergencia moderada posterior a la agrupación, según los puntos de corte, del LupusQoL con la escala GENCAT (coeficiente kappa de Cohen = 0,556; p = 0,000).
ConclusionesEl LupusQoL es válido como instrumento psicométricamente estable para medir la CVRS de los pacientes con LES en Venezuela. Se establecieron los puntos de corte que permiten estratificar la CVRS de los pacientes venezolanos con LES, siendo de utilidad para complementar una evaluación integral.
Systemic lupus erythematosus (SLE) is chronic, autoimmune disease of unknown aetiology with variable clinical presentation and severity. It presents with a relapsing and remitting pattern of progression which is characterised by periods of remission and activity1. Life expectancy of patients with SLE has improved due to early diagnosis and better therapeutic strategies. However, the use of drugs which are not toxicity-free, together with the actual symptoms of the disease, significantly impact the quality of life of these patients2. Health-related quality of life (HRQoL) must be considered both in the right treatment and control of the disease as well as in patients’ evolution with SLE3.
Different studies report that there is poorer HRQoL in patients with SLE compared with healthy people and similar HRQoL—or at times worse—compared with other chronic patients4–7. According to Testa8, measuring HRQoL provides a description of a health condition or status, pointing out changes on how the patient functions, providing a prognosis or establishing reference guidelines. The HRQoL of patients with SLE has been assessed using two types of tools9: generic questionnaires, such as the Medical Outcome Survey Short Form 36 (MOSSF-36) and the European Quality of Life Questionnaire-5 Dimensions (EQ-5D), which lack specific domains for SLE; and specific questionnaires such as the Systemic Lupus Erythematosus Quality of Life Questionnaire (SLEQOL), the Systemic Lupus Erythematosus Symptom Checklist (SSC), the Systemic Lupus Erythematosus Quality of Life Scale (L-QoL) and the Lupus Quality of Life (LupusQoL).
The LupusQoL was validated using several cross-sectional and longitudinal studies in patients with SLE2,10–19. However, there are no published data to determine the validity of the questionnaire nor cut-off values to stratify HRQoL in Venezuelan patients with SLE. As a result and given the importance of measuring quality of life in these patients, the aims of this study were to determine the validity of the LupusQoL as a psychometrically stable tool for measuring HRQoL in Venezuelan patients with SLE and establishing cut-off points of the questionnaire for the Venezuelan population. We chose this specific tool because its items are based on the perception of the HRQoL of the patients themselves who have SLE. A score is given by domains with the higher sensitivity, specificity, and response capability (sensitivity to change) than the generic questionnaires and their development and validation prove they have stable psychometric properties10,20. Standardisation of this technique will allow researchers and healthcare providers to objectively analyse the HRQoL of Venezuelan patients with SLE and to use this information to improve the quality of medical care for these patients. In addition to this, this method may be adopted by other countries of the Americas with similar patient populations.
Materials and methodsPatients and study designA cross-sectional study was conducted that included patients with SLE who were attached to the External Consultation centre of the Rheumatology Unit of the “Ruíz y Páez” University Hospital Complex and the Centro Clínico Universitario de Oriente in Ciudad Bolívar, Venezuela, between April and July 2018, with at least 4 classification criteria from the 1982 American College of Rheumatology21. Patients with additional diagnoses of other different autoimmune diseases to SLE were excluded, save those patients with a diagnosis of anti-phospholipid syndrome.
Ethical aspects and informed consentThe study was conducted in keeping with the ethical principles for medical research in human beings from the Declaration of Helsinki22, with the corresponding informed consent signed by all patients.
ToolsThe LupusQoL10 questionnaire, consolidated with a valid, reliable HRQoL from the patient and specific to the disease for adults with a diagnosis of SLE, contains eight domains and a total of 34 items which are responded to using a five-point Likert scale. It gives a score by domains which ranges from 0 (worse HRQoL) to 100 (best HRQoL), which may be obtained if the following formula is followed: the responses by domain are added up and divided by the total number of items of this domain. The resulting score is divided by 4 and then multiplied by 100.The version translated into Spanish of the LupusQoL was used; usage licence was requested through the online system of RWS Life Sciences, Inc.
The “Generalitat de Catalunya”23 (GENCAT) scale form — an objective tool designed in keeping with advances made on the multidimensional general quality of life model proposed by Schalock in 2002 and Verdugo in 2003 was used. It contains eight dimensions and a total of 69 items which are responded to using a four-option scale of frequency. The GENCAT scale is a valid and reliable tool23 which has been used in national24,25 and international studies26,27 to measure the quality of life in patients with SLE.
The accumulated disease activity and damage were calculated through the Systemic Lupus Erythematosus Disease Activity Index28 (SLEDAI) and Systemic Lupus International Collaborating Clinics29 (SLICC), respectively. The SLEDAI assesses disease activity in the last 10 days and includes 24 items which determine specific symptoms in 9 organs or systems, with a maximum score of 10528. The SLICC assesses irreversible damage of the disease during the last 6 months and includes 42 items to measure involvement of the 12 domains with a maximum score of 46 points29. Both models have been proven to be valid and reliable for assessing activity (SLEDAI) and accumulated damage (SLICC) in patients with SLE28,29.
ProcedurePatients were approached during their routine rheumatology consultation. Those who agreed to participate in the study received an envelope with an informed consent form, a sociodemographic data form, a copy of the LupusQoL and a copy of the GENCAT scale for them to complete in the medical centre or take home to complete and deliver within a week. The patients who could not read or write received help to complete the documents, with by the authors in the medical centre or their family members when they were at home.
The SLEDAI and SLICC indices of the medical records for the previous 6 months were later obtained.
Statistical analysisReliability was determined by internal consistency using Cronbach’s alpha coefficient. Cut-off points were generated for the LupusQoL and the GENCAT scale from an analysis of latent classes which highlighted one or more non observed (latent) classes with respect to a variable. By applying an analysis of the Bayesian information criterion values, we confirmed that two classes were optimum for both questionnaires. Means and marginal probabilities were then studied for the latent classes, grouping each patient into one of the two classes. From these, using Receiver Operating Characteristics curves (ROC), the cut-off points for the LupusQoL were determined (for each domain and the total) and for the GENCAT scale. These cut-off points led to stratifying the HRQoL of the patients into “better” or “worse”. Convergent and discriminating validity was analysed using Cohen’s kappa coefficient and the Spearman correlation coefficient, respectively. Finally, the predictive validity was analysed using COR curves with SLEDAI and SLICC values grouped into: <4 or ≥4 points and 0 or ≥1 point, respectively; these cut-off points were taken because they had been satisfactorily used in previous validation studies of LupusQoL11–16,19. The STATA version 16 and the SPSS version 25 were used for statistical analysis. Statistical significance was set at p < .05.
ResultsSociodemographic characteristicsA total of 100 patients with SLE completed the questionnaires; 93% were women, with mean age at 42 years (SD: 13) and mean disease duration of 11 years (SD: 9). SLEDAI and SLICC means were 3 and 1, respectively. The other sociodemographic characteristics are contained in Table 1.
Sociodemographic characteristics of Venezuelan patients with SLE.
| n = 100 | |
|---|---|
| Female sex, n (%) | 93 (93) |
| Mean age, years (SD) | 42 (13) |
| Level of education, n (%) | |
| Illiterate | 4 (4) |
| Primary level | 13 (13) |
| Secondary level | 40 (40) |
| Further education level | 43 (43) |
| Mean study time, years (SD) | 12 (4) |
| Mean disease duration, years (SD) | 11 (9) |
| SLEDAI, mean (SD) | 3 (4,7) |
| SLICC, mean (SD) | 1 (1) |
SD: standard deviation.
Internal consistency was .96, which proves LupusQoL’s high reliability.
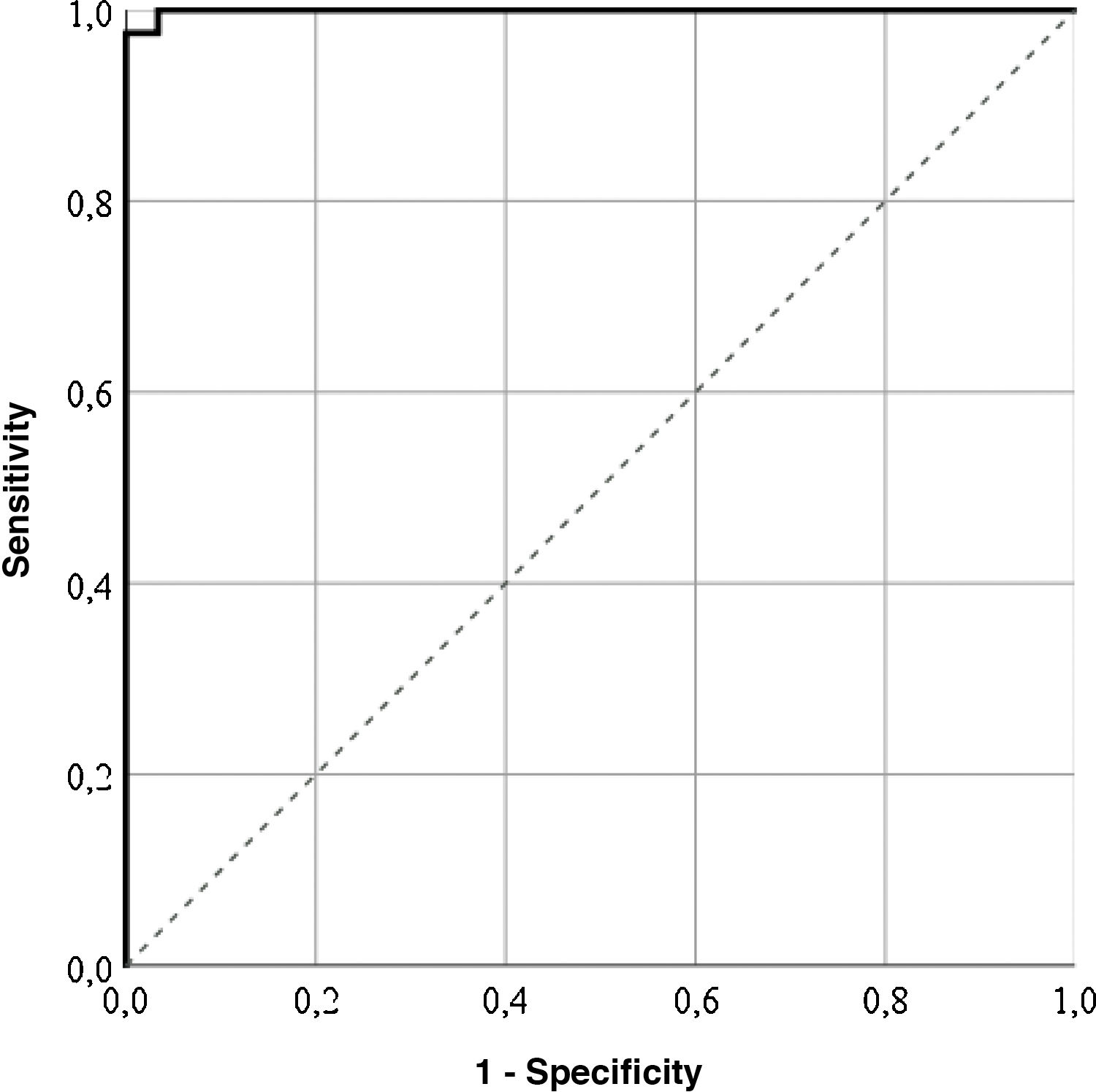
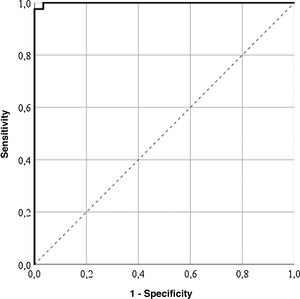
Cut-off pointsThe highest correctly classified patient percentage (99%) was obtained with a value of 64.55 points in the LupusQoL (area below the curve = .99; sensitivity: 100%; specificity: 97.56%), suggesting that the patients with 64.55 points or more presented with a good HRQOL (Fig. 1). Furthermore, cut-off points for each LupusQoL domain (Table 2) domain were obtained.
Cut-off points of the LupusQoL in Venezuelan patients with SLE.
| Domains | Scoring of the cut-off points | Area under the curve | p | Correctly classified patients (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| Physical health | 56.25 | .993 | 0 | 97 | 100 | 89.6 |
| Pain | 58.33 | 1 | 0 | 100 | 100 | 100 |
| Planning | 66.66 | 1 | 0 | 100 | 100 | 100 |
| Sexual relations | 65.2 | 1 | 0 | 100 | 100 | 100 |
| Burden for others | 58.33 | 1 | 0 | 100 | 100 | 100 |
| Emotional health | 54.16 | .99 | 0 | 99 | 98.65 | 100 |
| Body image | 70 | .99 | 0 | 98 | 97.3 | 100 |
| Fatigue | 56.35 | .99 | 0 | 98 | 100 | 95.24 |
| Total | 64.55 | .99 | 0 | 99 | 100 | 97.56 |
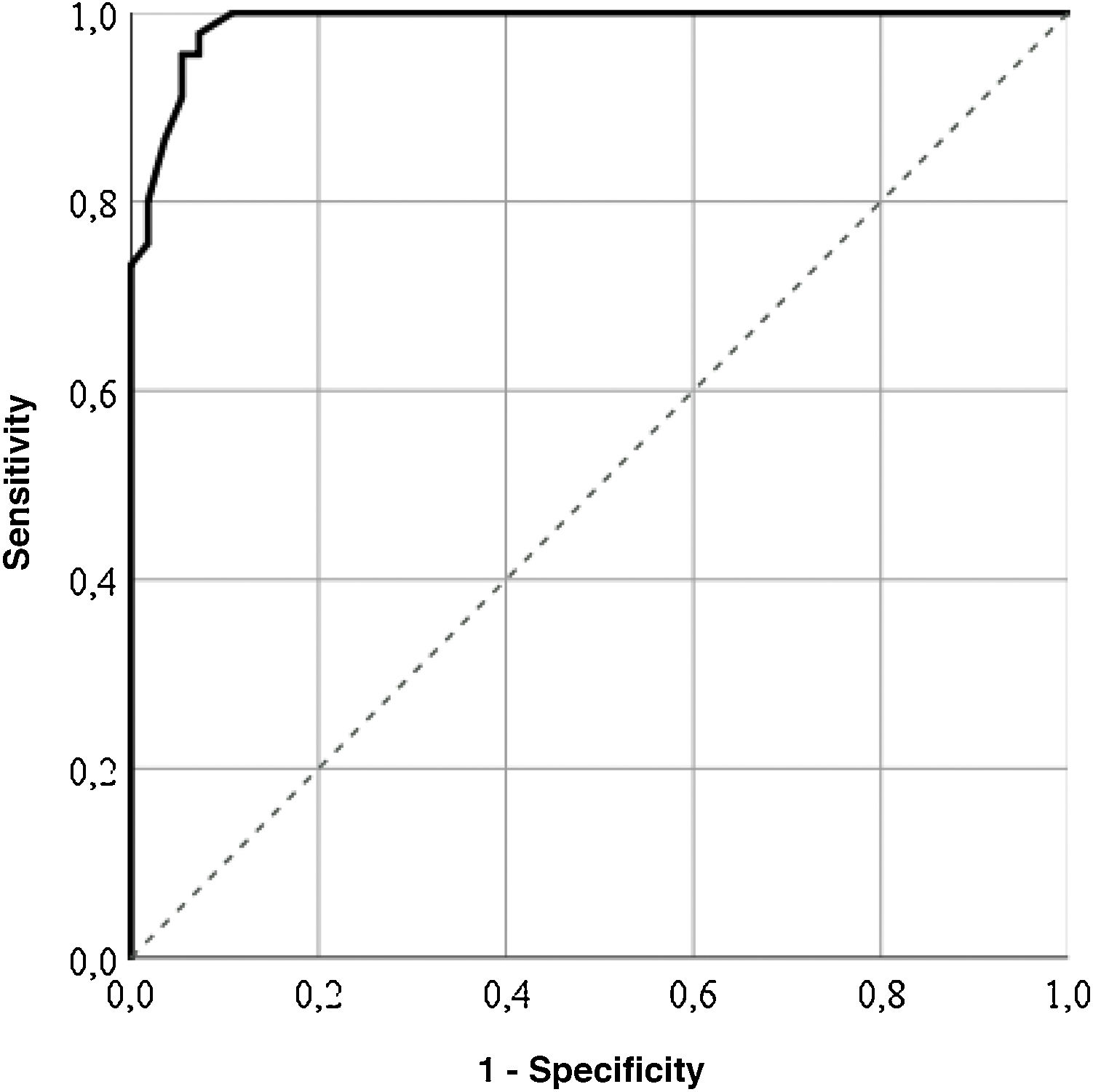
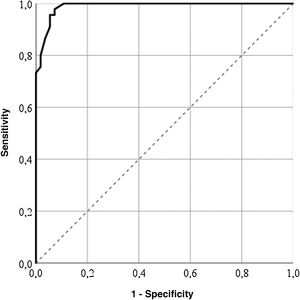
For the GENCAT scale, the cut-off points which correctly classified most patients (95%) was 67 points (area under the curve = .98; sensitivity: 92.73%; specificity: 97.78%), and patients with the same or higher score to this sum therefore presented with a better quality of life (Fig. 2). After grouping the patient in accordance with the LupusQoL cut-off points and the GENCAT scale, a moderate convergent validity which was statistically significant was obtained (Cohen’s kappa coefficient = .556; p = .000).
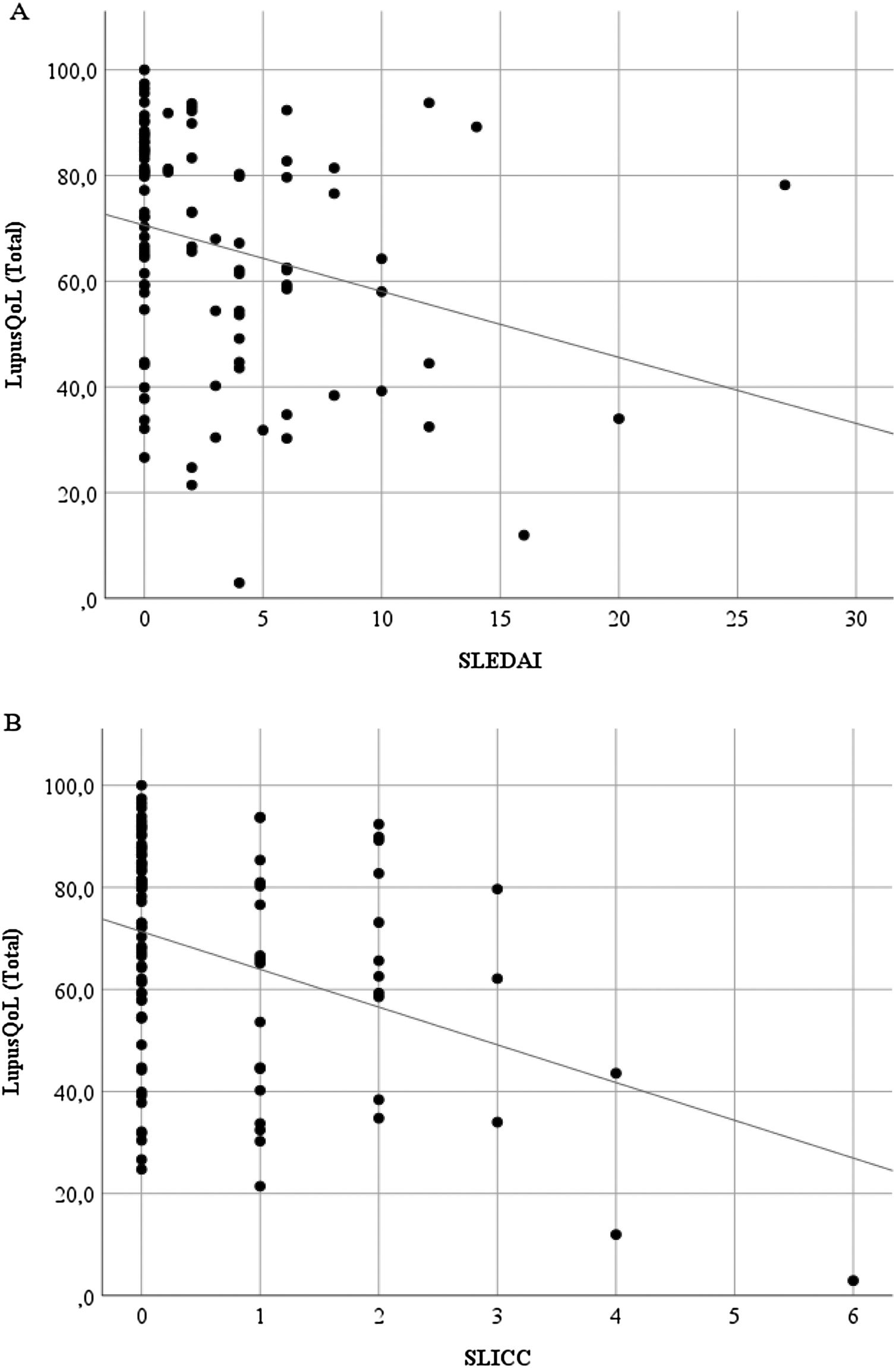
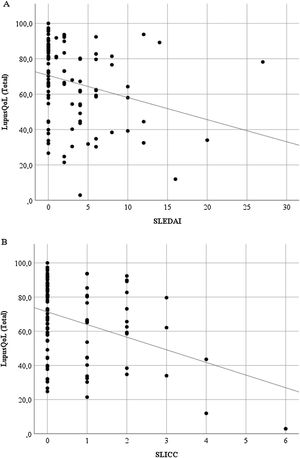
Discriminant validityThe LupusQoL was inversely correlated with the SLEDAI (rho = –3.27; p = .001) (Fig. 3A) and with the SLICC (rho = –.246; p = .014) (Fig. 3B) significantly.
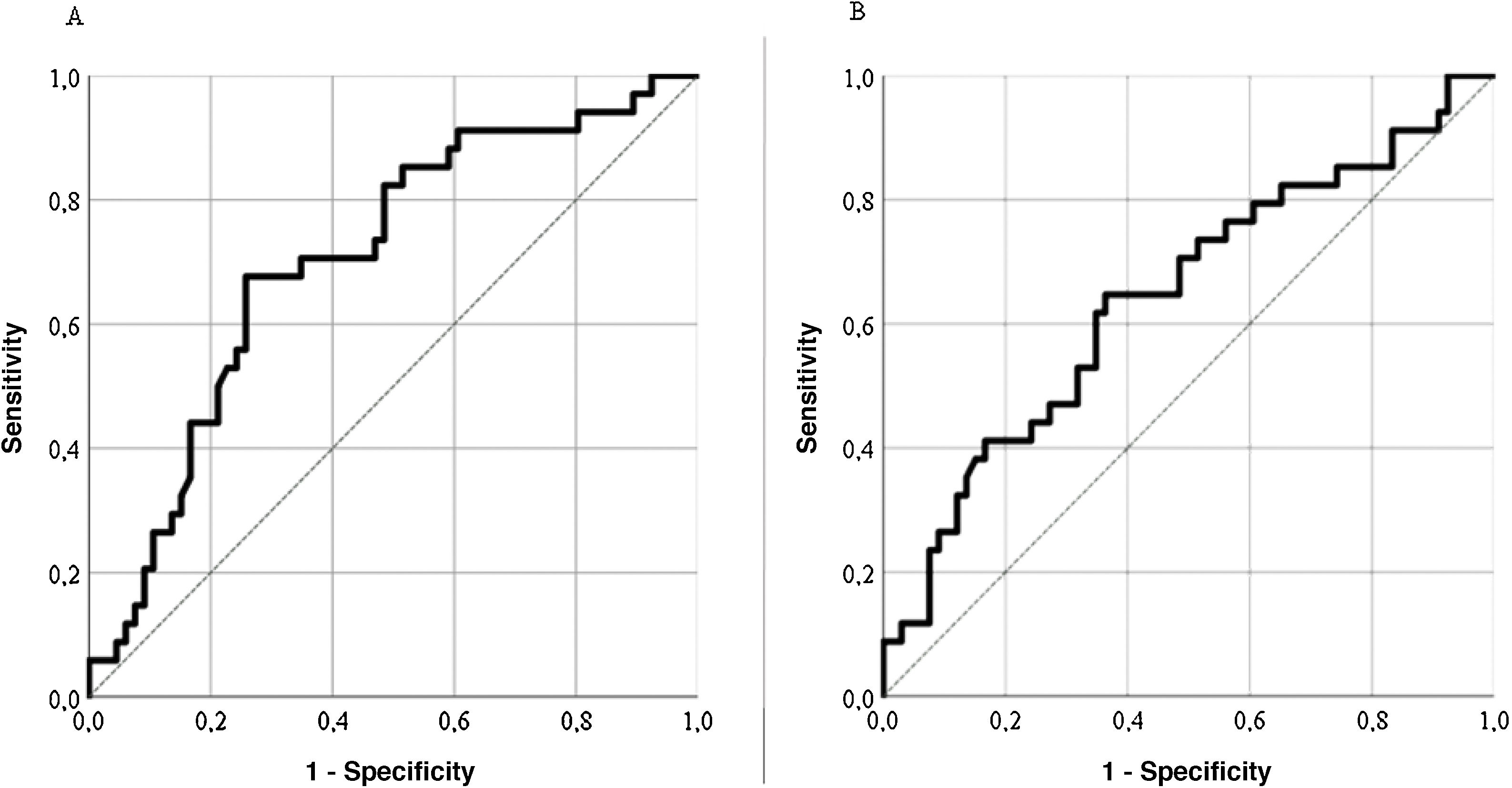
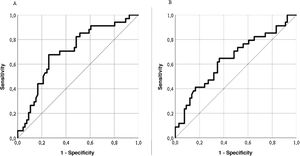
Predictive validityThe LupusQoL discreetly predicted disease activity (SLEDAI) for scores ≥4 (area under the curve = .704; sensitivity: 74.24%; specificity: 67.65%) (Fig. 4A), classifying 72% of patients. The LupusQoL gave a poor prediction of accumulated damage (SLICC) for scores ≥1 (area under the curve = .642; sensitivity: 66.67%; specificity: 52.94%) (Fig. 4B), classifying only 62% of patients.
Average scores for each domain of the LupusQoL were compared in patients with and without disease activity (SLEDAI: <4 or ≥4 points), and also in patients with or without accumulated damage (SLICC: 0 or ≥1 points) (Table 3), with significant differences being found between both groups in all domains, except in “sexual relations” for the SLEDAI and SLICC scales and “burden for others” for the SLICC scale.
Scoring of the LupusQoL according to the disease activity (SLEDAI) and the accumulated damage (SLICC) in Venezuelan patients with SLE.
| Domains | SLEDAI | pa | SLICC | pa | ||
|---|---|---|---|---|---|---|
| <4 | ≥4 | 0 | ≥1 | |||
| n = 66 | n = 34 | n = 66 | n = 34 | |||
| Physical health | 72.1 (24.0) | 58.2 (25.8) | .009 | 73.1 (21.1) | 56.3 (29.4) | .001 |
| Pain | 67.2 (27.2) | 49.8 (25.4) | .003 | 67.0 (25.2) | 50.0 (29.4) | .003 |
| Planning | 78.8 (26.4) | 65.0 (27.1) | .016 | 78.8 (24.6) | 65.0 (30.4) | .016 |
| Sexual relations | 72.3 (29.4) | 65.1 (32.2) | .259 | 71.2 (28.6) | 67.3 (34.0) | .543 |
| Burden for others | 64.5 (30.1) | 46.3 (35.0) | .008 | 61.4 (31.2) | 52.5 (35.5) | .2 |
| Emotional health | 72.3 (24.7) | 53.9 (27.6) | .001 | 71.3 (24.4) | 55.8 (29.2) | .006 |
| Body image | 82.3 (22.8) | 66.2 (23.1) | .001 | 80.2 (23.4) | 70.4 (24.4) | .056 |
| Fatigue | 64.96 (23.43) | 50.92 (26.16) | .008 | 64.96 (22.22) | 50.92 (28.17) | .008 |
| Total | 71.81 (20.42) | 56.91 (22.51) | .001 | 70.99 (19.62) | 58.51 (24.81) | .007 |
During the last decade, assessing the HRQoL of patients with SLE has attracted major interest11. This study determines the validity of a specific tool for measuring the HRQoL of patients with SLE (LupusQoL) and is the first to establish cut-off points that stratify the HRQoL of these patients in Venezuela. The HRQoL of the patients with SLE must consider both the generic domains (physical, emotional and social well-being) and specific domains (pain, sexual relationships, body image, among others) affected by the disease30; this ensures that the results of the domains assessed by a tool will be objective measures of the HRQoL.
In this study, analysis of Cronbach’s alpha coefficient (.96) demonstrated the high reliability of the LupusQoL, in keeping with studies conducted in Latin America11,30, the United States12, Europe2,10,13–15 and Asia16,17. A moderate convergence was also found between the LupusQoL and the GENCAT scale, similar to that reported by several studies2,10–17 which obtained good consistency between the equivalent domains of the LupusQoL and other generic scales. It is well known that the generic questionnaires present a modest convergence with the specific questionnaires, although they measure different parameters of the HRQOL11.
It was also determined that the LupusQoL slightly discriminates the activity of the disease measured by the SLEDAI and the accumulated damage measured by the SLICC in patients with SLE. Current reports on the ability of the LupusQoL to discriminate disease activity and accumulated damage are variables. Several studies show that the LupusQoL does not discriminate between disease activity11,31,32 or accumulated damage11 but most studies present the results for each domain of the questionnaire because there was no equivalence between them and they did not present with significant differences in all domains2,10,12–14,16,17. These discrepancies may be due to many causes, including sociodemographic differences and relationships with the disease among different population groups.
The predictive value of the LupusQoL shows that this questionnaire is useful for predicting disease activity and accumulated damage, since statistically significant differences were found between both groups in all domains, except in “sexual relations” for SLEDAI and SLICC and in “burden for others” for SLICC. This contrasts with what was found by Machado et al.11 who reported that the score of the LupusQoL was inappropriate for predicting disease activity and accumulated damage. Furthermore, no significant differences were reported among scores for each domain of LupusQoL according to the “best” or “worst” HRQoL. Research on these findings needs to be extended in the future for a better analysis.
This study has several limitations: first, 20% of the SLEDAI indices were obtained from a variable point of one to six months between the day of the application of the tools and the most recent indices. Second, the reproducibility of the LupusQoL was not measured through the test-retest.
To conclude, the LupusQoL is valid as a psychometrically stable tool for measuring the HRQoL of Venezuelan patients with SLE. The cut-off points that enable stratification of the HRQoL of the Venezuelan patients with SLE were established and were highly useful for completely assessing the disease progression in these patients and their prognosis.
FinancingThis research study did not receive any specific funding from public sector, commercial sector or not-for-profit entities.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Carrión-Nessi FS, Marcano-Rojas MV, Freitas-DeNobrega DC, Romero Arocha SR, Antuarez-Magallanes AW, Fuentes-Silva YJ. Validación del LupusQoL en Venezuela: una medida específica de la calidad de vida en pacientes con lupus eritematoso sistémico. Reumatol Clin. 2022;18:355–360.