To determine the percentage of Lyme patients with articular manifestations in NW Spain and to know their evolution and response to treatment.
PatientsA retrospective study (2006–2013) was performed using medical histories of confirmed cases of Lyme disease showing articular manifestations. Clinical and laboratory characteristics, together with the treatment and evolution of the patients, were analyzed.
ResultsSeventeen out of 108 LD confirmed patients (15.7%) showed articular manifestations. Regarding those 17 patients, 64.7%, 29.4% and 5.9% presented arthritis, arthralgia and bursitis, respectively. The knee was the most affected joint. Articular manifestations were often associated to neurological, dermatological and cardiac pathologies. Otherwise, most patients were in Stage III. The 11.8% of the cases progressed to a recurrent chronic arthritis despite the administration of an appropriate treatment.
ConclusionsLyme disease patients showing articular manifestations should be included in the diagnosis of articular affections in areas of high risk of hard tick bite, in order to establish a suitable and early treatment and to avoid sequels.
Determinar el porcentaje de pacientes con clínica articular entre los enfermos de Lyme en el NO de España y conocer su evolución y respuesta al tratamiento.
PacientesSe realizó un estudio retrospectivo (2006-2013) revisando las historias clínicas de los enfermos de Lyme con clínica articular. Se analizaron las manifestaciones clínicas, los datos de laboratorio, el tratamiento y la evolución de los enfermos.
ResultadosDiecisiete de 108 pacientes confirmados como enfermos de Lyme (15,7%) presentaban clínica articular. De estos 17, el 64,7% presentó artritis, el 29,4% artralgias y el 5,9% bursitis. La rodilla fue la articulación más afectada. La clínica articular se asoció frecuentemente a manifestaciones neurológicas, dermatológicas o cardíacas. La mayoría de los pacientes estaban en fase iii. El 11,8% evolucionó a artritis crónica recidivante, aunque recibieron tratamiento adecuado.
ConclusionesEn zonas con elevado riesgo de picadura por garrapatas, la presencia de clínica articular debe hacernos sospechar la posibilidad de una enfermedad de Lyme con objeto de establecer de forma precoz un tratamiento adecuado que evite secuelas.
Lyme disease (LD) is a zoonosis of cosmopolitism distribution caused by Gram-negative bacteria, pertaining to the Borrelia burgdorferi (B. burgdorferi) sensu lato. It is transmitted by the tick bite, principally by the Ixodes ricinus in Spain.1
The disease can affect multiple organs and systems and, thus, its clinical spectrum is highly varied. It can involve the skin, heart, nervous system and joints, following a time process in phases. Phase I (early local) includes erythema migrans (EM); phase II (early diffuse), with joint, neurological and cardiac clinical signs; and phase III (late diffuse), with chronic arthritis, acrodermatitis chronica atrophicans and late neuroborreliosis.1,2 However, the clinical findings of each phase can be overlapped, and some patients can show signs of late infection without previous manifestations of early infection.2,3
Musculoskeletal signs, such as arthralgia and myalgia, are observed more often in phase II of the disease, whereas arthritis is more frequent in phase III.3–5 For this reason, after weeks or months, those patients who have not been diagnosed and treated can develop oligoarthritis in large joints3,4 or an intermittent monoarthritis, especially in the knee.6
The diagnostic of LD, except in the case of EM, requires microbiological confirmation (enzyme-linked immunosorbent assay [ELISA] or western blot).1 Moreover, a tick bite must have been possible, and that can be the result of an exhaustive clinical history.3,7
The prognosis of LD is good, whenever an early diagnosis is reached and a proper antibiotic therapy against Borrelia has been initiated. In contrast, a delay in the diagnosis and in the administration of treatment can be associated with a more elevated morbidity.2,4,8
The objective of this study is to determine the percentage of patients with LD whose joint signs, outcome and response to treatment could be identified, based on the data from the case records.
PatientsA retrospective, descriptive, observational study was performed (2006–2013) to examine our knowledge of the joint manifestations of LD. Lucus Augusti Teaching Hospital in Lugo, in the northwestern coastal area of Spain, cover a mean of 223,374 persons. We reviewed 108 case reports of LD, confirmed according to the epidemiology alert of the Centers for Disease Control and Prevention of the United States.9 We selected patients with given joint findings, and collected the data corresponding to their major clinical signs, adjunctive texts, administrative therapy and outcome. Moreover, we took into account the age, sex and habitat (rural or urban) of the patients and whether anyone remembered the tick bite. Rural centers were those with less than 2000 population (n=118 447) and the rest were urban centers (n=104 927). The disease phase (I, II, III) was determined according to the criteria established by the different authors.1,10
ResultsOf the 108 individuals confirmed as LD patients, 15.7% (n=17 patients) had joint signs and all of them had ELISA (IgG and IgM VIDAS® Lyme; bioMérieux, St. Louis, Missouri, United States) and positive IgG western blot to Borrelia species (EUROLINE-WB, Euroimmun AG, Lübeck, Germany). In all, 64.7% had arthritis, 29.4% arthralgia and 5.9% bursitis. The knee was the most frequently affected joint. Patients with LD with no joint manifestations had skin, neurological or cardiac involvement.
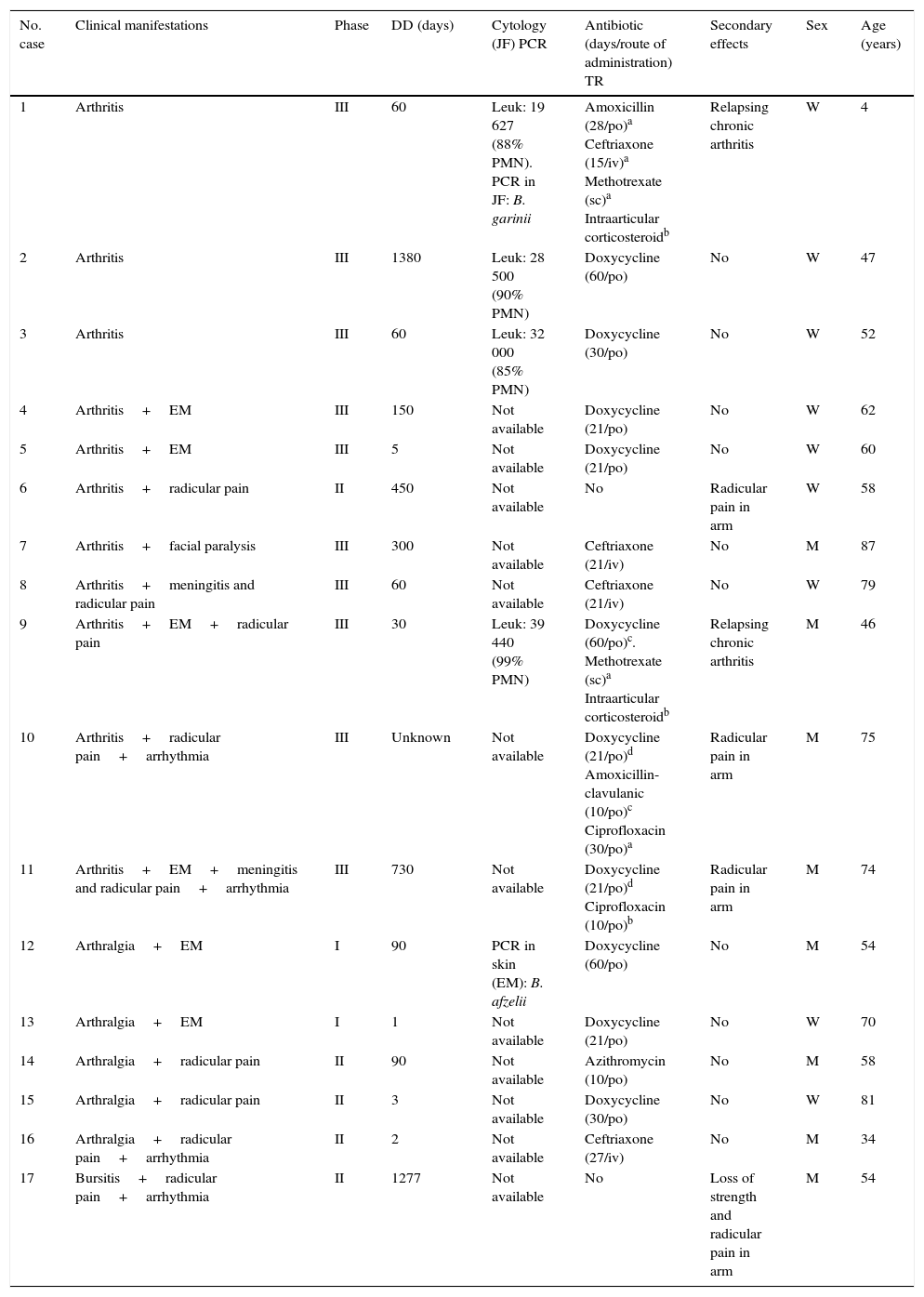
Of the 11 patients with arthritis, 72.7% associated neurological, dermatological and cardiac signs (Table 1), and the majority (90.9%) were in phase III. In 1 patient (case no. 5) in whom EM was observed, a diagnosis was performed early; in the rest, the mean delay to diagnosis was 402 days. Antibiotic therapy was administrated in 90.9% of the patients with arthritis; however, 3 had neurological secondary effects (radicular pain in arm) and 2 developed relapsing chronic arthritis, for which they received corticosteroids and methotrexate. An increase in the number of leukocytes, mostly referring to polymorphonuclear cells, was found in the joint fluid. Moreover, the synovial fluid of the pediatric child who developed relapsing chronic monoarthritis in her left knee was sent to the Spanish Microbiological Center (Instituto de Salud Carlos III, Madrid, Spain), where it was identified, by polymerase chain reaction (PCR), as Borrelia garinii (B. garinii).
Characteristics of Patients With Lyme Disease on Joint Manifestations.
| No. case | Clinical manifestations | Phase | DD (days) | Cytology (JF) PCR | Antibiotic (days/route of administration) TR | Secondary effects | Sex | Age (years) |
|---|---|---|---|---|---|---|---|---|
| 1 | Arthritis | III | 60 | Leuk: 19 627 (88% PMN). PCR in JF: B. garinii | Amoxicillin (28/po)a Ceftriaxone (15/iv)a Methotrexate (sc)a Intraarticular corticosteroidb | Relapsing chronic arthritis | W | 4 |
| 2 | Arthritis | III | 1380 | Leuk: 28 500 (90% PMN) | Doxycycline (60/po) | No | W | 47 |
| 3 | Arthritis | III | 60 | Leuk: 32 000 (85% PMN) | Doxycycline (30/po) | No | W | 52 |
| 4 | Arthritis+EM | III | 150 | Not available | Doxycycline (21/po) | No | W | 62 |
| 5 | Arthritis+EM | III | 5 | Not available | Doxycycline (21/po) | No | W | 60 |
| 6 | Arthritis+radicular pain | II | 450 | Not available | No | Radicular pain in arm | W | 58 |
| 7 | Arthritis+facial paralysis | III | 300 | Not available | Ceftriaxone (21/iv) | No | M | 87 |
| 8 | Arthritis+meningitis and radicular pain | III | 60 | Not available | Ceftriaxone (21/iv) | No | W | 79 |
| 9 | Arthritis+EM+radicular pain | III | 30 | Leuk: 39 440 (99% PMN) | Doxycycline (60/po)c. Methotrexate (sc)a Intraarticular corticosteroidb | Relapsing chronic arthritis | M | 46 |
| 10 | Arthritis+radicular pain+arrhythmia | III | Unknown | Not available | Doxycycline (21/po)d Amoxicillin-clavulanic (10/po)c Ciprofloxacin (30/po)a | Radicular pain in arm | M | 75 |
| 11 | Arthritis+EM+meningitis and radicular pain+arrhythmia | III | 730 | Not available | Doxycycline (21/po)d Ciprofloxacin (10/po)b | Radicular pain in arm | M | 74 |
| 12 | Arthralgia+EM | I | 90 | PCR in skin (EM): B. afzelii | Doxycycline (60/po) | No | M | 54 |
| 13 | Arthralgia+EM | I | 1 | Not available | Doxycycline (21/po) | No | W | 70 |
| 14 | Arthralgia+radicular pain | II | 90 | Not available | Azithromycin (10/po) | No | M | 58 |
| 15 | Arthralgia+radicular pain | II | 3 | Not available | Doxycycline (30/po) | No | W | 81 |
| 16 | Arthralgia+radicular pain+arrhythmia | II | 2 | Not available | Ceftriaxone (27/iv) | No | M | 34 |
| 17 | Bursitis+radicular pain+arrhythmia | II | 1277 | Not available | No | Loss of strength and radicular pain in arm | M | 54 |
DD, diagnosis delay; EM, erythema migrans; im, intramuscular; iv, intravenous; JF, joint fluid; Leuk, leukocytes; M, man; PCR, polymerase chain reaction; PMN, polymorphonuclear; po, per os; sc, subcutaneous; TR, treatment reiterated; W, woman.
The 6 patients who had arthralgia or bursitis were associated with dermatological, neurological and cardiac signs. In all, 33.3% were in phase I. In one of these patients (case no. 12), a biopsy was performed in the skin affected by EM, and Borrelia afzelii (B. afzelii) by PCR. In all, 83.3% received antibiotic therapy and they had no secondary effects. In contrast, one patient who had bursitis, in which there was a diagnosis delay of 1277 days, had not had treatment and presented with neurological secondary effects.
The joint signs were somewhat more superior in the women (52.9%). The age of the patients ranged between 4 and 87 years (mean and median was 58 years), and there was a minor prevalence in joint manifestations in patients under 15 years (5.9%).
All of the patients resided in the rural environment, and 52.9% remembered the tick bite. The majority of the patients (70%) were diagnosed between the months of June and August.
DiscussionIn all the patients with rheumatology clinic, the most affected joint was the knee, followed to a smaller extend by hand and ankle. The results coincide, in general, with those proposed by other authors.3,4,8,11–14
Arthralgia and bursitis, without associated arthritis, were relatively frequent, and were mostly observed in phase II of the disease.3,4
Arthritis was the predominant sign, and most of the patients were in phase III, or late diffuse phase. There was a considerable delay in the diagnosis, which agrees with other studies.2 Cytology of the joint fluid, as in septic arthritis, usually showed an increase in the leukocyte count, with a predominance of neutrophils.12 This means that, on occasions, the differential diagnosis with respect to these 2 entities was difficult, and can even signify that these patients be subjected to aggressive and unnecessary treatments, like surgical drainage.13 For this reason, in patients, in endemic LD regions, with monoarthritis or oligoarthritis, especially if the knee is the affected joint, a differential diagnosis should be done with other arthritis.12 In 2 of the patients, using PCR, B. garinii was isolated in the joint fluid and B. afzelii in skin with EM. This agrees with the fact that, in Europe, these 2 genera, together with B. burgdorferi sensu stricto, are the most frequently related to joint and dermatological signs.11 To most of the patients with arthritis with no associated neurological signs, oral doxycycline or amoxicillin was administered as the treatment of choice.4 The case of not having an adequate response to the initial therapy would mean the indication of a new antibiotic cycle of the administration, which would be 2 or 3 successive weeks. However, depending on the outcome to date, it would be of novel disease-modifying antirheumatic drugs, such as methotrexate, or intraarticular corticosteroid therapy, as was done here in cases no. 1 and 9.5,6,12 In cases no. 7 and 8 of this study, in accordance with international guidelines, intravenous ceftriaxone was administered during 21 days, given that it was associated with the neurological signs. There were no residual joint manifestations following treatment in 88.2% of the patients, a finding that agrees with the good outcome expected after therapy, according to the reports by other authors.4,5,12,14
We found no significantly different sex-related factors, coinciding with the data from other studies.2 In contrast, with respect to the age of presentation, we observed no bimodal distribution pointed out by other authors.15
All of the patients resided in rural regions, which placed them in greater risk of tick bites and, thus, in the mode of acquiring the disease.7,15 Approximately one half of the patients did not remember the history of their bites. This may be because they are not painful or because the ticks, especially if they are not fed or are young, are difficult to see.2,7
Of the results observed in this study, we deduce that, in regions with a high risk of tick bites, it is necessary to add LD to the differential diagnosis in the case of joint involvement with these manifestations, which will help to establish adequate early therapy to prevent secondary effects. A detailed case report is essential to point to the way to joint disease in the epidemiologic setting of the region.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
To the University General Secretariat for the award of a grant for structuring of competitive research groups, Redes modality (R2014/005, Xunta de Galicia, Spain), and the microbiology department of Hospital Universitario Lucus Augusti, Lugo, Spain, for their collaboration with the performance in this study.
Please cite this article as: Vázquez-López ME, Díez-Morrondo C, Sánchez-Andrade A, Pego-Reigosa R, Díaz P, Castro-Gago M. Manifestaciones articulares en enfermos de Lyme. Reumatol Clin. 2016;12:327–330.