To analyze the effect of single nucleotide polymorphisms (SNPs) with well-known functional impact of methylenetetrahydrofolate reductase (MTHFR; rs1801131 and rs1801133), the membrane transporter ABCB1 (rs1045642), the AICAR transformylase/IMP cyclohydrolase (ATIC; rs2372536) and folyl-polyglutamate synthetase (FPGS; rs1544105), on liver and bone marrow toxicity of methotrexate (MTX).
Patients and methodsWe analyzed 1415 visits from 350 patients of the PEARL (Princesa Early Arthritis Register Longitudinal) study: (732 with MTX, 683 without MTX). The different SNPs were genotyped using specific TaqMan probes (Applied Biosystems). Multivariate analyzes were performed using generalized linear models in which the dependent variables were the levels of serum alanine aminotransferase (liver toxicity), leukocytes, platelets or hemoglobin (hematologic toxicity) and adjusted for clinical variables (disease activity, etc.), analytical (renal function, etc.), sociodemographic (age, sex, etc.) and genetic variants of MTHFR, ABCB1, ATIC and FPGS. The effect of these variables on the MTX doses prescribed throughout follow-up was also analyzed through multivariate analysis nested by visit and patient.
ResultsWhen taking MTX, those patients carrying the CC genotype of rs1045642 in ABCB1 showed significantly higher GPT levels (7.1±2.0U/L; P<.001). Carrying at least one G allele of rs1544105 in FPGS was associated with lower leukocyte (−0.67±0.32; 0.038), hemoglobin (−0.34±0.11g/dL; P=.002), and platelet (−11.8±4.7; P=.012) levels. The presence of the G allele of rs1544105 in FPGS, and the T allele of rs1801133 in MTHFR, was significantly associated with the use of lower doses of MTX.
DiscussionOur data suggest that genotyping functional variants in FGPS and MTHFR enzymes and the transporter ABCB1 could help to identify patients with increased risk of MTX toxicity.
Analizar el efecto de polimorfismos de nucleótido único (SNPs) de la metilen-tetrahidrofolatorreductasa (MTHFR; rs1801131 y rs1801133), el transportador de membrana que une ATP B1 (ABCB1; rs1045642), la aicartransformilasa/IMP ciclohidrolasa (ATIC; rs2372536) y la folilpoliglutamatosintetasa (FPGS; rs1544105) en la toxicidad hepática y medular de metotrexato (MTX).
Pacientes y métodosSe analizaron 1.415 visitas (732 con MTX, 683 sin MTX) de 350 pacientes del Princesa Early Arthritis Register Longytudinal study. El genotipo de los diferentes SNP se determinó mediante sondas TaqMan (Applied Biosystems). Se realizaron análisis multivariables mediante modelos lineales generalizados en los que las variables dependientes fueron los niveles séricos de transaminasa glutámico-pirúvica (toxicidad hepática), leucocitos, plaquetas o hemoglobina (toxicidad hematológica) y se ajustaron por variables clínicas (actividad de la enfermedad, etc.), analíticas (función renal, etc.), sociodemográficas (edad, sexo, etc.) y las variantes genéticas de MTHFR, ABCB1, ATIC y FPGS. También se analizaron las variables que influyeron en las dosis de MTX administradas a lo largo del seguimiento.
ResultadosCuando recibían MTX los portadores del genotipo CC del SNP rs1045642 de ABCB1 presentaron niveles significativamente mayores de GPT (7,1±2,0U/l; p<0,001). Los portadores de al menos un alelo G de rs1544105 en FPGS presentaron niveles significativamente menores de leucocitos (−0,67±0,32; 0,038), hemoglobina (−0,34±0,11g/dl; p=0,002) y de plaquetas (−11,8±4,7; p=0,012). La presencia del alelo G de rs1544105 (FPGS) y T de rs1801133 (MTHFR) se asoció, de forma aditiva y significativa, al uso de menores dosis de MTX.
DiscusiónNuestros datos sugieren que variantes genéticas de las enzimas FGPS y MTHFR, y del transportador ABCB1, podrían ayudar a detectar pacientes con mayor riesgo de toxicidad por MTX.
Methotrexate (MTX) continues to be the cornerstone of the treatment of rheumatoid arthritis (RA), and the major guidelines for the management of that disease recommend it as the initial therapy and as a key support for treatment with biological therapies.1–3 It is not strange that in a recent international observation study, COMORA (Comorbidities in RA), 89% of the patients received treatment with MTX or had taken it over the course of their disease.4 In Spain, the EMECAR study (Study of the Morbidity and Clinical Expression of Rheumatoid Arthritis) or in the emAR II study, conducted within the last 10 years, revealed that MTX was the initial drug of choice in 55% of the cases, and between 60% and 64% received this disease-modifying drug, either alone or in combination.5,6 Despite its wide use and the extensive research on this agent, the mechanism of action of MTX in RA is not fully established. Although the drug inhibits several metabolic pathways related to folic acid (Figure 1), the available data suggest that supplements with folic acid have a greater impact on the toxicity of MTX than on its efficacy.7 It has been shown that there is a marked individual susceptibility to its immunomodulating effect as well as to its toxicity, which makes it necessary to interrupt the treatment in up to a fourth of the patients receiving it.8 This means that, although it is the synthetic disease-modifying drug with the longest survival, less than 55% of those taking it maintain it for more than 8 years.9
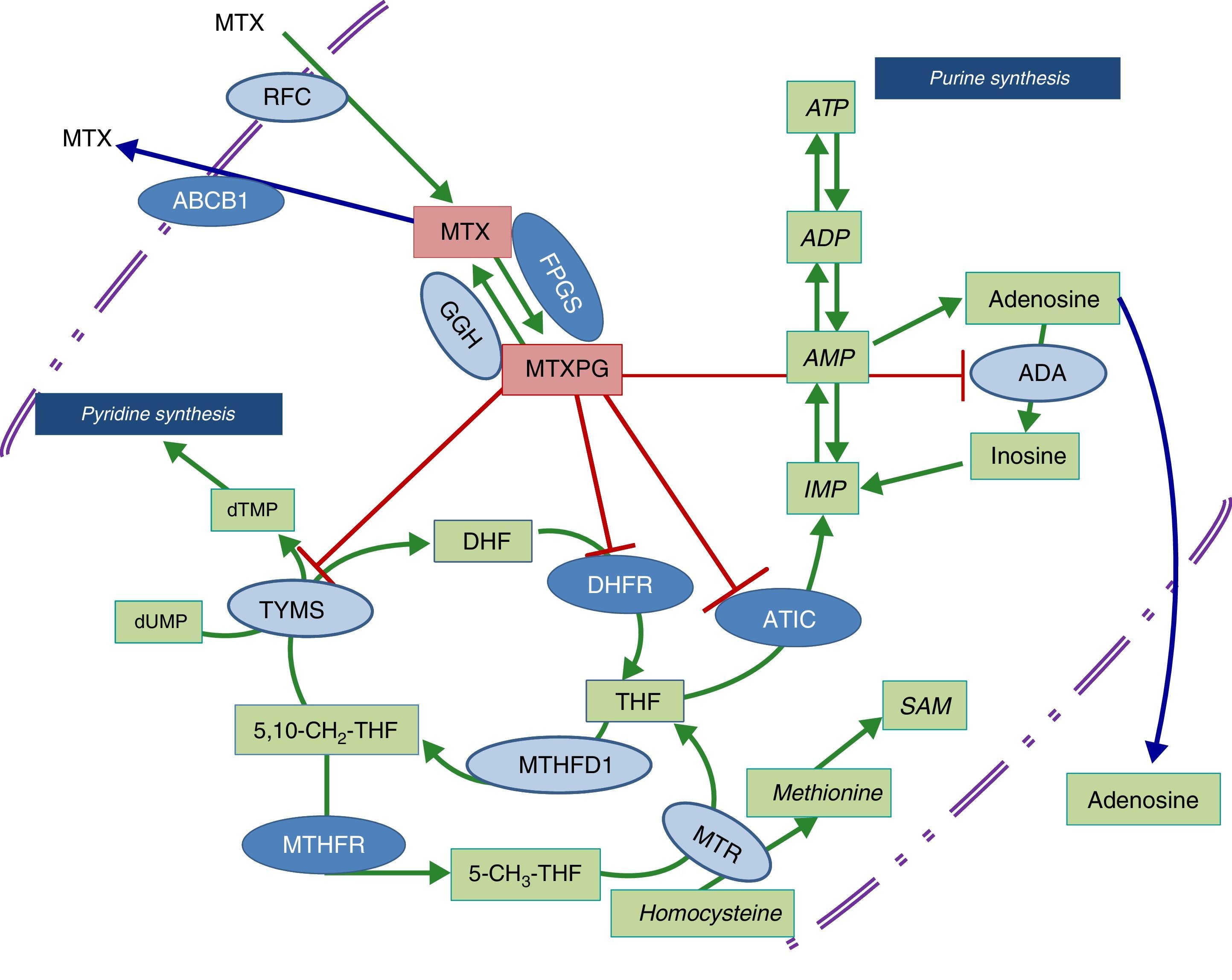
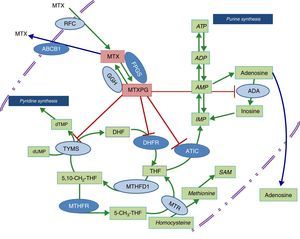
Diagram of the metabolic pathways that involve folic acid and the enzymes on which methotrexate acts. The dark blue ovals indicate the enzymes studied in the present report. The red lines point to the enzymes that are inhibited by methotrexate polyglutamates. ABCB1, transporter that bonds ATP B1; ADA, adenosine deaminase; ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATIC, AICAR transformylase/IMP cyclohydrolase; ATP, adenosine triphosphate; DHFR, dihydrofolate reductase; FPGS, folyl-polyglutamate synthetase; GGH, gamma-glutamyl hydrolase; IMP, inositol monophosphate; MTHFD1, 5,10-methylenetetrahydrofolate dehydrogenase 1; MTHFR, methylenetetrahydrofolate reductase; MTR, methionine reductase; MTX, methotrexate; MTXPG, methotrexate polyglutamates; RFC, reduced folate carrier; SAM, S-adenosyl-1-methionine; TYMS, thymidylate synthetase.
Given that early treatment in RA has been shown to be essential to achieving an optimal control of the disease, it would be of great utility to have access to biomarkers that would enable us to predict which individuals will develop toxicity that could impede their taking full therapeutic doses of MTX. The study of genetic variants in the metabolic pathway enzymes in which folic acid participates has been a question of interest that has led to the performance of a number of studies that have attempted to predict the response to or toxicity of MTX, although the information obtained until now, in patients with an inflammatory rheumatic disease, is not conclusive.10 The objective of this article is to analyze the effect of genetic variants of methylenetetrahydrofolate reductase (MTHFR; rs1801131 and rs1801133), the membrane transporter that binds adenosine triphosphate (ATP) B1 (ABCB1; rs1045642), 5′-phosphoribosyl-5-aminoimidazole-4-carboxamide (AICAR) transformylase/inositol monophosphate (IMP) cyclohydrolase (ATIC; rs2372536) and folyl-polyglutamate synthetase (FPGS; rs1544105), whose function is shown in the diagram in Fig. 1, in the toxicity related to the drug in patients with early RA (ERA) exposed to MTX.
ABCB1 belongs to the ATP-binding cassette superfamily that is responsible for transport toward the exterior of the cytoplasmic membrane of a number of molecules, among them those that are cytotoxic. Specifically, the single nuclide polymorphism (SNP) rs1045642 consists in the transition of a C for a T in exon 26 of the ABCB1 transporter gene.11 This substitution provokes mRNA instability and has been associated with a decrease in the expression and, thus, in the function of ABCB1, accompanied by higher tissue levels of numerous drugs.11 In the case of MTHFR, the SNP rs1801133 involves the transition of a C for a T in exon 4, which entails the change of an alanine for a valine in position 222 of the protein, whereas the SNP rs1801131 involves the transition of an A for a C in exon 7, provoking the change of a glutamic for an alanine in position 429 of the protein. In both cases, this results in greater thermolability of the enzyme and less functional activity. With respect to rs1544105, there is a change of a G for an A in the promotor region of the FPGS gene that has been related to lower mRNA levels of this enzyme.12 The SNP rs2372536 in ATIC means a transition of a C for a G in exon 5, which brings about the change of a threonine for a serine in position 116 of the protein.13 This information on the functional impact of these genetic variants led to their being chosen for this study, which was planned in 2008.
Patients and MethodsPatientsWe utilized data from the Princesa Early Arthritis Register Longitudinal Study (PEARL), in which the patients recruited had had inflammation in 1 or more joints for at least 4 weeks and a disease duration of less than 1 year. The registry began in September 2001 and continues at the present time. The study protocol was reviewed and approved by the clinical research ethics committee of La Princesa teaching hospital in Madrid, Spain, and all of the participants provided written informed consent when they were included in the registry.
In the database, we included those patients who, after 2 years of follow-up, met the 1987 American Rheumatism Association criteria for RA14 and those considered to have undifferentiated arthritis (UA)15 after ruling out other causes of inflammatory arthropathy, such as crystal arthritis, septic arthritis, spondyloarthritis and other connective tissue diseases. The cutoff date for the analysis of data was September 2014.
MethodsIn PEARL, in accordance with the protocol, we collected demographic and clinical data that included tender and swollen joint counts, the global assessment of the disease by the physician and by the patient using the visual analog scale and the Spanish version of the health assessment questionnaire (HAQ) to evaluate health status.16 We also recorded analytical data such as the leukocyte count, hemoglobin (Hb) concentration, platelet count and the glutamate pyruvate transaminase (GPT) level, determined using standard techniques in the hematology and biochemical departments of our center, the erythrocyte sedimentation rate (determined with the Westergren method), C-reactive protein (CRP) and rheumatoid factor (both by means of nephelometry; the latter was positive at a level of >50IU/mL), anti-cyclic citrullinated peptide (anti-CCP) antibodies (determined by ELISA, Euro-Diagnostica Immunoscan RA; positivity >50IU/mL), as well as the treatment received at each visit (Table 1 of Appendix 1 [available online]). Disease activity was evaluated by the Disease Activity Score of 28 joints (DAS28).17
The extraction of DNA was done in all of the patients of the study by means of standard procedures (QUIamp DNA Blood Mini Kit, Qiagen, Valencia, California, United States). We studied SNP rs1801131 and rs1801133 in MTHFR, rs1045642 in the ABCB1 membrane transporter, rs2372536 in ATIC and rs1544105 in FPGS, using Taqman probe kits C___1202883_20, C____850486_20, C___7586657_20, C__16218146_10 and C___8342611_10, respectively (Applied Biosystems, Foster City, California, United States). The samples were genotyped by real-time polymerase chain reaction (PCR) in a StepOnePlus Real-Time PCR thermal cycler (Applied Biosystems), following the manufacturer's instructions. Genotype assignment was done using Sequence Detection System (SDS) software (version 2.3, Applied Biosystems). Duplicate samples and negative controls were included to confirm the genotyping accuracy. The accuracy was estimated to be greater than 99%.
Statistical AnalysisTo rule out subjective considerations, both on the part of the physician and the patient, we limited the study of MTX toxicity to its effect on analytical variables routinely employed to evaluate liver and bone marrow toxicity of MTX: GPT, leukocytes, platelets and Hb. For each, we studied the association with different sociodemographic, clinical, disease-related and genetic variables. Subsequently, for each of the 4 dependent variables, we performed multivariate analysis by means of generalized linear models using the “glm” command of Stata 12 (StataCorp, College Station, Texas, United States) in the visits in which the patients were taking MTX. That is, we analyzed the details of each of the visits in which the patient had been receiving MTX. An average of 2.8 visits were analyzed per patient. The multivariate models were adjusted by initially including all of those variables with a significance level of P<.15 in the bivariate analysis and, subsequently, we excluded all those variables with a significance level of P>.15 or, in the case of categorical variables, those in which all of their categories had a P level >.15. Having established the best multivariate model for each variable related to the development of toxicity, on the basis of the visits in which the patients had received MTX, the same model was repeated in visits without MTX. Given that multiple comparisons had been carried out (GPT, Hb, leukocytes and platelets), the statistical significance was adjusted using the Bonferroni method to establish a P value<.0125. Sensitivity analysis was performed using nested generalized linear models per patient and visit with the Stata command xtgee, which gave very similar results (data not shown).
On the other hand, we performed a multivariate analysis to determine the variables associated with the MTX dose being received in each follow-up visit, using the Stata command xtgee. The model was adjusted with the option of a first-order autoregressive correlation, so that only the visits in which the patient had been receiving MTX for at least 1 year (2 consecutive visits) were considered, and thus could have reached the maximum tolerable dose. In this case, we studied an average of 3.2 visits per patient. The best fit model was obtained as was described above. Moreover, once the generic variants that were relevant in the analysis were determined, the decision was made to employ them in an additive model, as that would be the manner by which their contribution to the doses of MTX utilized would best be explained. In this analysis, the level of significance was established at P<.05.
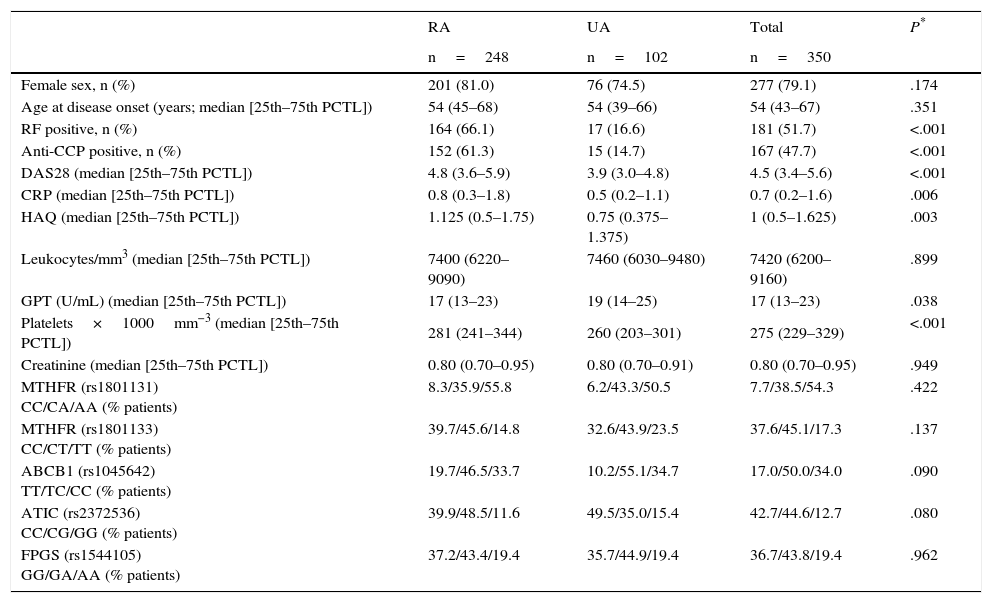
ResultsPatientsWe studied a total of 350 patients: 248 with a diagnosis of RA and 102 with UA. The baseline descriptive data are shown in Table 1. The patients who met the criteria for RA presented a significantly higher frequency of RF and anti-CCP antibodies, higher scores on DAS28 and HAQ, higher platelet counts and higher levels of CRP, in comparison with those diagnosed with UA (Table 1). With respect to treatment, 223 patients received MTX in the group of RA (89%) and 43 in the group of UA (43%), with a total of 732 visits in which the patients had been taking this drug (Table 1 of Appendix 1 [available online]).
Baseline Characteristics of the Patients.
| RA | UA | Total | P* | |
|---|---|---|---|---|
| n=248 | n=102 | n=350 | ||
| Female sex, n (%) | 201 (81.0) | 76 (74.5) | 277 (79.1) | .174 |
| Age at disease onset (years; median [25th–75th PCTL]) | 54 (45–68) | 54 (39–66) | 54 (43–67) | .351 |
| RF positive, n (%) | 164 (66.1) | 17 (16.6) | 181 (51.7) | <.001 |
| Anti-CCP positive, n (%) | 152 (61.3) | 15 (14.7) | 167 (47.7) | <.001 |
| DAS28 (median [25th–75th PCTL]) | 4.8 (3.6–5.9) | 3.9 (3.0–4.8) | 4.5 (3.4–5.6) | <.001 |
| CRP (median [25th–75th PCTL]) | 0.8 (0.3–1.8) | 0.5 (0.2–1.1) | 0.7 (0.2–1.6) | .006 |
| HAQ (median [25th–75th PCTL]) | 1.125 (0.5–1.75) | 0.75 (0.375–1.375) | 1 (0.5–1.625) | .003 |
| Leukocytes/mm3 (median [25th–75th PCTL]) | 7400 (6220–9090) | 7460 (6030–9480) | 7420 (6200–9160) | .899 |
| GPT (U/mL) (median [25th–75th PCTL]) | 17 (13–23) | 19 (14–25) | 17 (13–23) | .038 |
| Platelets×1000mm−3 (median [25th–75th PCTL]) | 281 (241–344) | 260 (203–301) | 275 (229–329) | <.001 |
| Creatinine (median [25th–75th PCTL]) | 0.80 (0.70–0.95) | 0.80 (0.70–0.91) | 0.80 (0.70–0.95) | .949 |
| MTHFR (rs1801131) CC/CA/AA (% patients) | 8.3/35.9/55.8 | 6.2/43.3/50.5 | 7.7/38.5/54.3 | .422 |
| MTHFR (rs1801133) CC/CT/TT (% patients) | 39.7/45.6/14.8 | 32.6/43.9/23.5 | 37.6/45.1/17.3 | .137 |
| ABCB1 (rs1045642) TT/TC/CC (% patients) | 19.7/46.5/33.7 | 10.2/55.1/34.7 | 17.0/50.0/34.0 | .090 |
| ATIC (rs2372536) CC/CG/GG (% patients) | 39.9/48.5/11.6 | 49.5/35.0/15.4 | 42.7/44.6/12.7 | .080 |
| FPGS (rs1544105) GG/GA/AA (% patients) | 37.2/43.4/19.4 | 35.7/44.9/19.4 | 36.7/43.8/19.4 | .962 |
ABCB1, ATP-binding cassette B1; anti-CCP, anti-cyclic citrullinated peptide; ATIC, AICAR transformylase/IMP cyclohydrolase; CRP, C-reactive protein; FPGS, folyl-polyglutamate synthetase; GPT, glutamate pyruvate transaminase; HAQ: Health Assessment Questionnaire; MTHFR, methylenetetrahydrofolate reductase; PCTL, percentile; RA, rheumatoid arthritis; RF, rheumatoid factor; UA, undifferentiated arthritis.
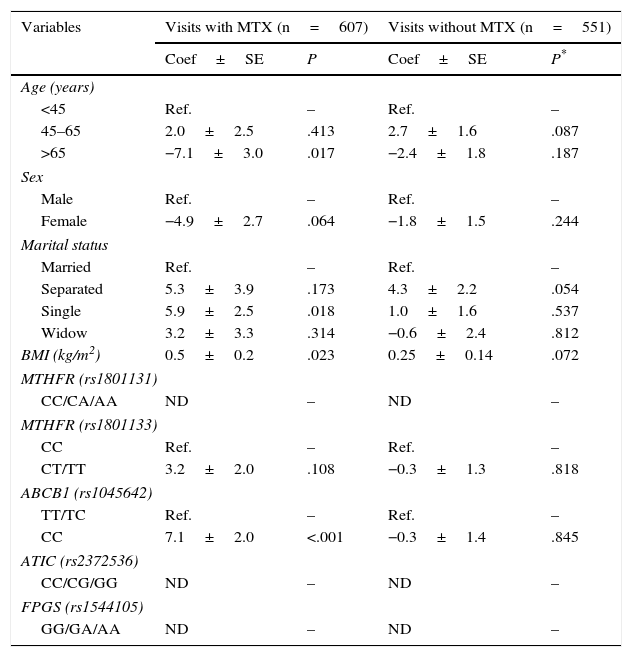
The multivariate analysis showed that in the visits in which the patients had received MTX, age, sex, being unmarried and body mass index were associated with a trend toward lower levels of GPT in the first 2 variables and higher levels in the remaining 2 (Table 2). After adjusting for these confounders, we observed that in homozygotes for the C allele of the variant rs1045642 of the ABCB1 transporter had significantly higher GPT levels than those who were carriers of, at least, a T allele (TT or CT genotypes [Table 2]). Moreover, we observed a trend, that was not statistically significant, in which carriers of at least 1 T allele of the SNP rs1801133 in MTHFR had higher levels of GPT (Table 2). The remainder of the genetic variants did not have a significant influence.
Multivariate Analysis of the Effect of Different Variables on Glutamate Pyruvate Transaminase in Patients With Early Rheumatoid Arthritis.
| Variables | Visits with MTX (n=607) | Visits without MTX (n=551) | ||
|---|---|---|---|---|
| Coef±SE | P | Coef±SE | P* | |
| Age (years) | ||||
| <45 | Ref. | – | Ref. | – |
| 45–65 | 2.0±2.5 | .413 | 2.7±1.6 | .087 |
| >65 | −7.1±3.0 | .017 | −2.4±1.8 | .187 |
| Sex | ||||
| Male | Ref. | – | Ref. | – |
| Female | −4.9±2.7 | .064 | −1.8±1.5 | .244 |
| Marital status | ||||
| Married | Ref. | – | Ref. | – |
| Separated | 5.3±3.9 | .173 | 4.3±2.2 | .054 |
| Single | 5.9±2.5 | .018 | 1.0±1.6 | .537 |
| Widow | 3.2±3.3 | .314 | −0.6±2.4 | .812 |
| BMI (kg/m2) | 0.5±0.2 | .023 | 0.25±0.14 | .072 |
| MTHFR (rs1801131) | ||||
| CC/CA/AA | ND | – | ND | – |
| MTHFR (rs1801133) | ||||
| CC | Ref. | – | Ref. | – |
| CT/TT | 3.2±2.0 | .108 | −0.3±1.3 | .818 |
| ABCB1 (rs1045642) | ||||
| TT/TC | Ref. | – | Ref. | – |
| CC | 7.1±2.0 | <.001 | −0.3±1.4 | .845 |
| ATIC (rs2372536) | ||||
| CC/CG/GG | ND | – | ND | – |
| FPGS (rs1544105) | ||||
| GG/GA/AA | ND | – | ND | – |
ABCB1, ATP-binding cassette B1, ATIC, AICAR transformylase/IMP cyclohydrolase; BMI, body mass index; Coef, coefficient; DAS28, Disease Activity Score in 28 joints; FPGS, folyl-polyglutamate synthetase; GPT, glutamate pyruvate transaminase; MTHFR, methylenetetrahydrofolate reductase; MTX, methotrexate; ND, not determined; n.s., not significant; Ref., reference; SE, standard error.
In the visits in which the patients had not received MTX, only being unmarried and body mass index were associated with a trend toward higher GPT levels (Table 2).
Hematologic toxicityAs dependent variables for the study of hematologic toxicity, we utilized Hb, leukocytes and platelet count.
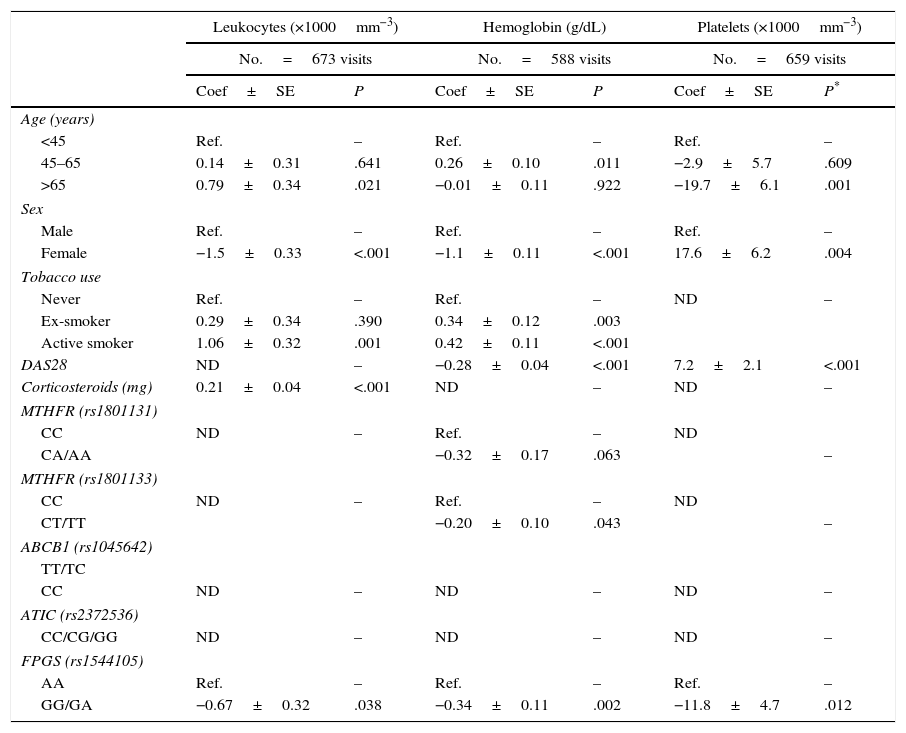
LeukocytesIn the analysis of the visits in which the patients had received MTX (Table 3) and in that dealing with the visits in which the patients had not taken MTX (Table 2 of Appendix 1 [available online]), we observed that active smokers, men, elderly patients and those taking glucocorticoids had significantly higher leukocyte counts. After adjusting for these variables, we found that in the visits in which the patients had received treatment with MTX, those who were carriers of at least 1G allele of the SNP rs1544105 in FPGS showed a nonsignificant trend toward lower leukocyte counts (Table 3), whereas that trend disappeared in the analysis of visits without MTX (Table 2 of Appendix 1 [available online]).
Multivariate Analysis of the Effect of the Different Factors on the Levels of Hematologic Variables in Visits of Patients With Early Rheumatoid Arthritis Who Were Being Treated With Methotrexate.
| Leukocytes (×1000mm−3) | Hemoglobin (g/dL) | Platelets (×1000mm−3) | ||||
|---|---|---|---|---|---|---|
| No.=673 visits | No.=588 visits | No.=659 visits | ||||
| Coef±SE | P | Coef±SE | P | Coef±SE | P* | |
| Age (years) | ||||||
| <45 | Ref. | – | Ref. | – | Ref. | – |
| 45–65 | 0.14±0.31 | .641 | 0.26±0.10 | .011 | −2.9±5.7 | .609 |
| >65 | 0.79±0.34 | .021 | −0.01±0.11 | .922 | −19.7±6.1 | .001 |
| Sex | ||||||
| Male | Ref. | – | Ref. | – | Ref. | – |
| Female | −1.5±0.33 | <.001 | −1.1±0.11 | <.001 | 17.6±6.2 | .004 |
| Tobacco use | ||||||
| Never | Ref. | – | Ref. | – | ND | – |
| Ex-smoker | 0.29±0.34 | .390 | 0.34±0.12 | .003 | ||
| Active smoker | 1.06±0.32 | .001 | 0.42±0.11 | <.001 | ||
| DAS28 | ND | – | −0.28±0.04 | <.001 | 7.2±2.1 | <.001 |
| Corticosteroids (mg) | 0.21±0.04 | <.001 | ND | – | ND | – |
| MTHFR (rs1801131) | ||||||
| CC | ND | – | Ref. | – | ND | |
| CA/AA | −0.32±0.17 | .063 | – | |||
| MTHFR (rs1801133) | ||||||
| CC | ND | – | Ref. | – | ND | |
| CT/TT | −0.20±0.10 | .043 | – | |||
| ABCB1 (rs1045642) | ||||||
| TT/TC | ||||||
| CC | ND | – | ND | – | ND | – |
| ATIC (rs2372536) | ||||||
| CC/CG/GG | ND | – | ND | – | ND | – |
| FPGS (rs1544105) | ||||||
| AA | Ref. | – | Ref. | – | Ref. | – |
| GG/GA | −0.67±0.32 | .038 | −0.34±0.11 | .002 | −11.8±4.7 | .012 |
ABCB1, ATP-binding cassette B1, ATIC, AICAR transformylase/IMP cyclohydrolase; Coef, coefficient; DAS28, Disease Activity Score in 28 joints; FPGS, folyl-polyglutamate synthetase; GPT, glutamate pyruvate transaminase; MTHFR, methylenetetrahydrofolate reductase; MTX, methotrexate; ND, not determined; n.s., not significant; Ref., reference; SE, standard error.
Among individuals with RA, men, active smokers and patients with a lower disease activity had significantly higher Hb levels, both in the analysis of visits in which the patients had received MTX (Table 3) and in those in which this drug was not being used (Table 2 of Appendix 1 [available online]). After adjusting for these variables, we again observed that carriers of at least 1 G allele of the SNP rs1544105 in FPGS presented significantly lower levels of Hb (Table 3). Moreover, carriers of an A allele in MTHFR rs1801131 or a T allele in rs1801133 showed a trend that did not reach statistical significance toward lower Hb levels (Table 3). In the 3 SNP, the association was only detected in the visits in which the patients had been taking MTX, whereas no significant association was found among the different genetic variables and the levels of Hb in the visits of patients who were not receiving MTX (Table 2 of Appendix 1 [available online]).
PlateletsPatients with a higher disease activity and women had higher platelet counts, although these variables reached statistical significance only in the visits of patients in which the individual had not taken MTX (Table 3). After adjusting for these variables, the presence of the G allele in FPGS was significantly associated with lower platelet counts in both visits with MTX and without MTX (Table 3).
Factors that influenced methotrexate dosesGiven that the effect observed in the genetic variants in MTHFR, ABCB1 and FPGS was not very marked, we wondered whether this situation could be related to a modulation in the MTX dose on the part of the prescribing physicians due to the presence of known risk factors for MTX toxicity, or to the detection of toxicity data during in-between visits not recorded in the registry. Thus, we performed a multivariate analysis to study the influence of the genetic variables of these enzymes on the MTX being employed at the time of each visit.
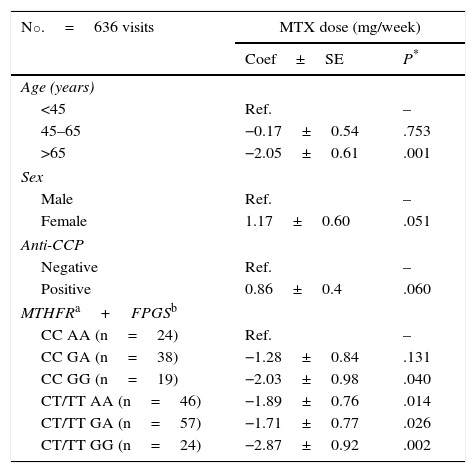
As expected, patients who were over 65 years of age were receiving significantly lower doses of MTX, whereas the presence of anti-CCP antibodies and female sex were associated with a trend toward the use of higher doses (Table 4). After adjusting for these confounders, we observed that there was an interaction between the effect of variant rs1801133 in MTHFR and of rs1544105 in FPGS on the prescribed dose of the drug. Thus, comparing with homozygotes for the common alleles of these variants, we observed that the MTX dose decreased progressively as the number of minor alleles of said proteins carried by the patient increased (Table 4).
Multivariate Analysis of the Factors That Influence the Methotrexate Dose Administered to Patients With Early Rheumatoid Arthritis (n=208).
| No.=636 visits | MTX dose (mg/week) | |
|---|---|---|
| Coef±SE | P* | |
| Age (years) | ||
| <45 | Ref. | – |
| 45–65 | −0.17±0.54 | .753 |
| >65 | −2.05±0.61 | .001 |
| Sex | ||
| Male | Ref. | – |
| Female | 1.17±0.60 | .051 |
| Anti-CCP | ||
| Negative | Ref. | – |
| Positive | 0.86±0.4 | .060 |
| MTHFRa+FPGSb | ||
| CC AA (n=24) | Ref. | – |
| CC GA (n=38) | −1.28±0.84 | .131 |
| CC GG (n=19) | −2.03±0.98 | .040 |
| CT/TT AA (n=46) | −1.89±0.76 | .014 |
| CT/TT GA (n=57) | −1.71±0.77 | .026 |
| CT/TT GG (n=24) | −2.87±0.92 | .002 |
Anti-CCP, anti-cyclic citrullinated peptide; Coef, coefficient; FPGS, folyl-polyglutamate synthetase; MTHFR, methylenetetrahydrofolate reductase; MTX, methotrexate; n, number of patients; n.s., not significant; Ref., reference; SE, standard error.
Our data suggest that functional genetic variants of proteins ABCB1, FPGS and MTHFR are associated with the presence of greater toxicity and the use of smaller doses of MTX in patients with ERA. These data are congruent with previous reports, especially in the case of MTHFR, concerning which we have greater evidence in the field of rheumatology,18,19 including the Spanish population.20–22 In patients with inflammatory rheumatic diseases, the roles of ABCB1 and FPGS have been less extensively studied, as the focus has been mostly on the efficacy of MTX and less on its toxicity.10
A noticeable aspect of our results is the differential effect of the genetic variants studied here, with SNP rs1045642 in ABCB1 transporter and rs1801133 in MTHFR mainly affecting liver toxicity, whereas SNP rs1544105 in FPGS has been associated with hematologic toxicity. There are no previous studies that investigate the differential toxicity of these genetic variants in the field of rheumatology, since the majority of the studies we cite here were based on dichotomous variables involving the discontinuation of the drug due to toxicity, and the cause was not specified.18–22
In the case of ABCB1, the cells of the patients who were carriers of the minor allele rs1045642 would not easily eliminate MTX and, consequently, there would be a higher bioavailability, which could explain the greater toxicity observed. In fact, our data confirm the findings of Gregers et al., who reported a greater liver toxicity in children with acute lymphoblastic leukemia treated with MTX when they were homozygous carriers of CC genotype of ABCB1 rs1045642, rather than carriers of CT or TT genotype.23
In the case of SNP rs1801133 in MTHFR, the infrequent presence of the T allele is associated with decreased functional activity, which is estimated to be around 30% for TT homozygotes and 70% for CT heterozygotes.24 Moreover, this genetic variant has been associated with an elevation of homocysteine levels and the corresponding increase in cardiovascular risk.25 The elevation of homocysteine in patients with low levels of MTHFR activity occurs because the catalysis of the conversion of homocysteine to methionine requires 5-CH3-THF, which is generated from 5,10-CH2-THF by MTHFR (Fig. 1). The relationship with liver toxicity would occur because these patients have a lower production of methionine and of S-adenosyl-methionine (Fig. 1), which is of great importance in hepatic metabolism.26 Although our data only show a trend toward higher levels of GPT in carriers of CT and TT genotypes of rs1801133, these findings are consistent with previous studies that associate these genotypes with an elevation of transaminases.27,28
With respect to FPGS, as we mentioned above, the infrequent presence of the rs1544105 A allele has been associated with lower levels of the enzyme12 and differences in the efficacy of MTX in both leukemia and RA.29 In relation to our findings, the presence of the ancestral G allele is associated with greater hematologic toxicity and the need for lower doses of MTX. It is probably related to a greater efficacy in MTX polyglutamylation and a higher activity of the drug on purine and pyrimidine synthesis pathways (Fig. 1), and thus greater toxicity in the 3 hematologic series.
The major limitation to this study lies in the fact that it was performed in a single population of ERA, and that local practices in the use of MTX may have biased the results. For example, the attending physician could have decided not to initiate the drug in patients with a profile that predisposed them a priori to toxicity, such as elderly patients or alcoholic individuals or those with liver disease, which could lead to the underestimation of the effect observed. However, the reproduction of factors associated with greater MTX toxicity, such as obesity in hepatotoxicity,30 or the association of genetic factors related to changes in levels of GPT, Hb, leukocytes and platelets recorded in visits in which the patients had been receiving MTX, but not in those in which this drug was not being administered, confers credibility to the approach proposed in our article.
As another limitation, it is intriguing that we cannot identify the reason why genetic variants of proteins like ABCB1 and FPGS, which act on the same levels in the management of MTX and folic acid, have different impacts in terms of MTX toxicity, involving hepatotoxicity in the former and hematologic toxicity in the latter. Finally, as a result of the organization in the collection of samples in PEARL, it was not possible to determine intracellular MTX polyglutamates, which could have provided to a better understanding of the effect of the SNP studied in FPGS.
Finally, the effect observed may have less clinical relevance than expected, since the variants of ABCB1 and FPGS that we studied, although associated with MTX toxicity, have also been related to a greater efficacy of the drug.10 However, the combination of genotypes that could best limit the MTX dose used, according to our study, would be being homozygote for the G allele of rs1544105 in FPGS and CT or TT genotype of rs1801133 in MTHFR, which was found in 22% of our patient population. In short, the percentage of carriers of the SNP that we studied is considerable, and the determination of genetic variants of ABCB1, MTHFR and FPGS could help to identify the ERA patients who should take a lower dose of MTX or replace it with another disease-modifying drug.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis study was performed with funds from projects RD12/0009/0017, PI11/00551, PI14/00442 and PIE13/00041 of the Spanish Ministry of Economy and Competiveness (Instituto de Salud Carlos III, Madrid, Spain), co-financed by the European Regional Development Fund (ERDF).
Conflicts of InterestIG-A has received funding for the last 5 years for research projects from BMS, Roche and UCB, as well as fees as a consultant and for scientific presentations from Abbvie, BMS, Lilly, Pfizer, Roche and UCB. IG-A and AMO are co-inventors of the patent entitled “Prognostic method for autoimmune disorders through genotyping vasoactive intestinal peptide variants”, code PCT/ES2015/070182.
LS, AL, EG-L and PM-F declare they have no conflicts of interest regarding this report.
We wish to thank all of the patients involved in PEARL for their enthusiastic and generous participation in the study. We would also like to acknowledge the collaboration of our nurse, Teresa Velasco, for her deep implication in the early rheumatoid arthritis clinic and the excellent technical work of Vanessa Centeno.
Please cite this article as: Sala-Icardo L, Lamana A, Ortiz AM, García Lorenzo E, Moreno Fresneda P, García-Vicuña R, et al. Impacto de variantes genéticas del transportador de membrana que une ATP B1, la aicar transformilasa/IMP ciclohidrolasa, la folilpoliglutamatosintetasa y la metilen-tetrahidrofolatorreductasa en la toxicidad de metotrexato. Reumatol Clin. 2017;13:318–325.