Eosinophilic fasciitis (EF) is a rare connective tissue disease characterized by symmetrical and painful swelling with a progressive induration and thickening of the skin and soft tissues.1 Sometimes, it presents associated with various hematological and solid malignancies.1,2
The diagnosis of EF is confirmed by histopathological examination demonstrating a thickened fascia with an inflammatory infiltration, mostly composed of lymphocytes and eosinophils. A peripheral eosinophilia is frequently present (60–90%) but is not mandatory for the EF diagnosis. At the onset, apart from histology, magnetic resonance imaging (MRI) is currently considered the ideal imaging modality for diagnosis EF.3 This technique is also useful for determining the optimal location of muscle biopsy and for follow-up purposes. The ussefulness for the diagnosis of other imaging techniques, such as [18F] FDG-PET/CT or ultrasonography, is beginning to be explored.3–5
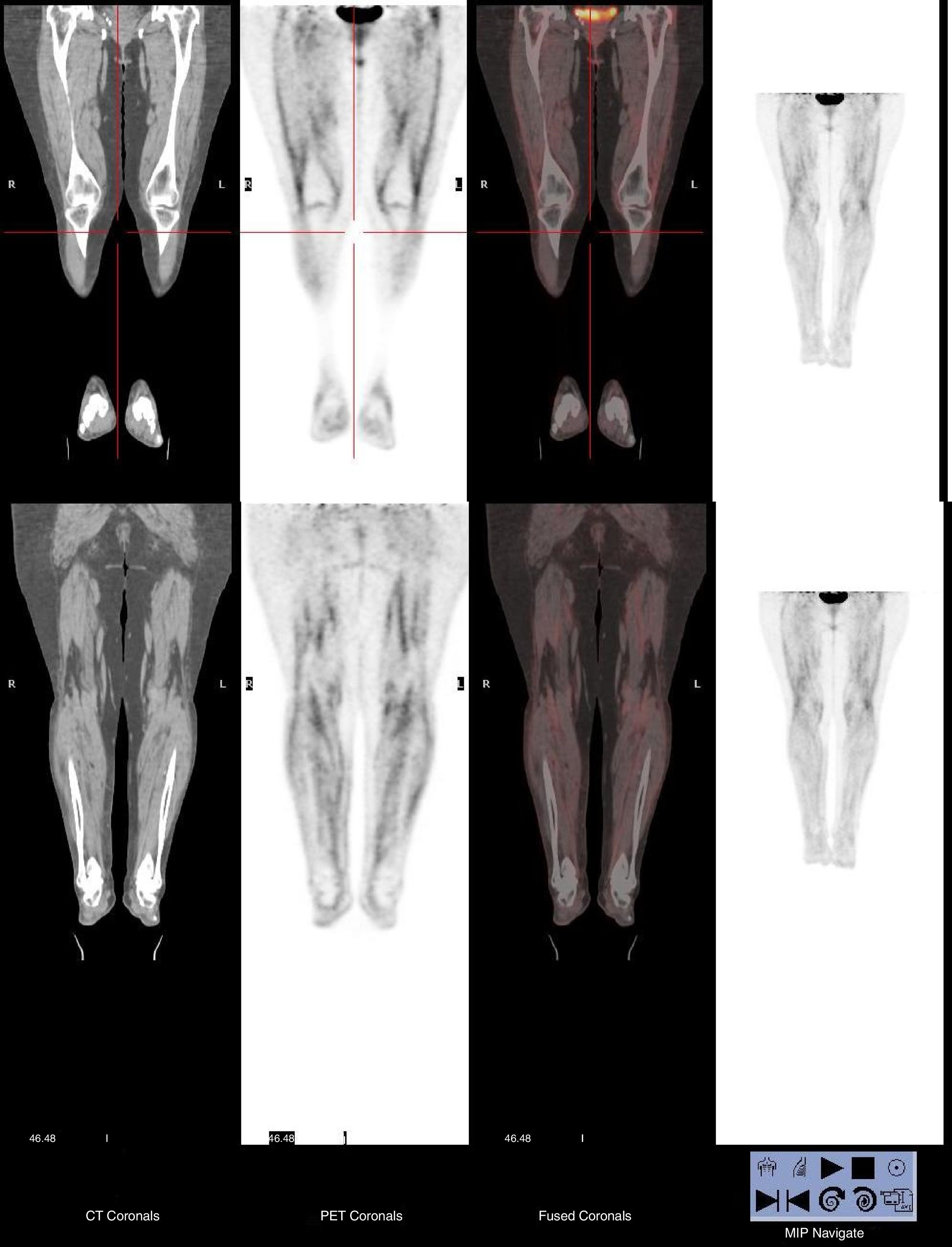
We report a case of EF in a 72-year-old woman who presented with a 1-month history of fatigue, weight loss, and rapidly progressive induration of her lower limbs without any skin manifestations. Laboratory tests showed an elevated erythrocyte sedimentation rate and hypereosinophilia. [18F] FDG-PET/CT discarded an occult neoplasia and revealed a diffuse and symmetrical FDG uptake in the fasciae of the legs and thighs (see Fig. 1). A biopsy specimen from the right leg, in which the FDG uptake was the strongest, showed marked eosinophilic infiltration in the fascia, but not in the muscle. The clinical suspicion of EF was confirmed and the patient was treated successfully with corticosteroids and methotrexate.
The association of malignancy with certain rheumatic syndromes, including FE was convincingly established.1,2 In those cases in which this possibility arises (explosive onset in advanced age or EF poorly responsive to corticosteroid therapy),6 the realization of a PET–CT could be useful to rule out occult neoplasia and to reinforce the suspected diagnosis.
Ethics approvalIn accordance with the guidelines of our institutional ethics committee, formal approval for this study was not required. The local ethics committee agreed that the findings in this report were based on normal clinical practice and were therefore suitable for dissemination. Informed consent of the patient was obtained, and her clinical record and information were anonymized. This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference for Harmonization.
Data availability statementAll relevant data are within the paper.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe authors have no support or funding to report.
Conflict of interestThe authors have declared that no competing interests exists.