In the context of the SARS-CoV-2 pandemic, the development of new vaccines and their efficacy in patients with immune-mediated rheumatic diseases has been a target to investigate. The objective of this study is to evaluate the vaccine response rate in patients with immune-mediated rheumatic diseases under treatment with immunomodulators, including rituximab (RTX), as well as the influence of possible factors involved in the vaccination response in these patients.
Material and methodsA single-centre, prospective cohort study was conducted in 130 patients with immune-mediated rheumatic disease on treatment with immunomodulators, including RTX, who received the full course of vaccination against SARS-CoV-2 with BioNTech/Pfizer, Moderna/Lonza, AstraZeneca, or Janssen between April and October 2021. Demographic factors such as age, sex, type of immune-mediated disease, immunomodulatory treatment and type of vaccine were analysed, as well as serological markers including anti-SARS-CoV-2 IgG antibody levels measured one and six months after vaccination, CD19+ lymphocyte levels and the presence or absence of hypogammaglobulinemia. A statistical analysis was performed to assess the influence of the different variables collected in the study on the antibody titres.
ResultsA sample of 130 patients was studied, 41 under treatment with RTX and 89 with other immunomodulators. A lower vaccination response rate was observed in patients with RTX (12/34, 36.7%) one month after the primary vaccination compared to 96.5% (82/85) of patients who did not receive this drug and did respond. In the analysis of secondary variables, hypogammaglobulinemia was significantly associated with lack of development of a vaccine response. The administration of the last RTX cycle in the 6 months prior to vaccination and low CD19+ levels (<20 mg/dL) also had a negative influence on the development of a vaccine response. In the group of patients who were not receiving RTX treatment, the vaccination response was like that observed in the general population. We did not observe statistically significant differences in the vaccine response based on immunomodulatory treatment other than RTX, concomitant corticosteroid treatment, type of immune-mediated pathology, age, or sex.
Discussion and conclusionsIn patients with rheumatic diseases receiving immunomodulatory treatment, the response to vaccination against SARS-CoV-2 is comparable to the general population, except in the case of patients receiving RTX, who have a lower response rate (around 36.7%) which is associated with factors such as hypogammaglobulinemia, pre-vaccination CD19+ lymphocyte levels, and a period between vaccination and the last dose of RTX of less than 6 months. It is important to take these factors into consideration to optimize vaccination in these patients.
En el contexto de la pandemia por SARS-CoV-2 el desarrollo de nuevas vacunas y su eficacia en pacientes con enfermedades reumáticas inmunomediadas ha sido motivo de estudio. El objetivo de este trabajo es evaluar la tasa de respuesta vacunal en pacientes con enfermedades reumáticas inmunomediadas en tratamiento con inmunomoduladores, incluyendo rituximab (RTX) así como la influencia de posibles factores implicados en la respuesta vacunal en estos pacientes.
Material y métodosSe realizó un estudio de cohortes prospectivo, unicéntrico, en 130 pacientes con enfermedad reumática inmunomediada en tratamiento con inmunomoduladores, incluyendo RTX que recibieron la pauta completa de vacunación frente a SARS-CoV-2 con BioNTech/Pfizer, Moderna/Lonza, AstraZeneca o Janssen entre abril y octubre de 2021. Se analizaron factores demográficos como la edad, sexo, tipo de enfermedad inmunomediada, tratamiento inmunomodulador y tipo de vacuna así como marcadores serológicos incluyendo los niveles de anticuerpos anti-SARS-CoV-2 IgG al mes y a los 6 meses desde la vacunación, niveles de linfocitos CD19+ y la presencia o no de hipogammaglobulinemia. Se realizó un análisis estadístico para evaluar la influencia en los títulos de anticuerpos de las diferentes variables recogidas en el estudio.
ResultadosSe obtuvo una muestra de 130 pacientes, 41 en tratamiento con RTX y 89 con otros inmunomoduladores. Se observó una menor tasa de respuesta vacunal en los pacientes con RTX (12/34, 36,7%) al mes de la primovacunación con respecto al 96,5% (82/85) de pacientes que no recibieron este fármaco y sí alcanzaron respuesta. En el análisis de variables secundarias, la hipogammaglobulinemia se asoció de forma significativa a la ausencia de desarrollo de respuesta vacunal. La administración del último RTX en los 6 meses previos a la vacunación y niveles bajos de CD19+ (<20 mg/dL) también influyeron de forma negativa en el desarrollo de respuesta vacunal. En el grupo de pacientes que no estaban en tratamiento con RTX, la respuesta vacunal fue similar a la observada en la población general. No observamos diferencias estadísticamente significativas en la respuesta vacunal en función del tratamiento inmunomodulador diferente al RTX, el tratamiento corticoideo concomitante, el tipo de patología inmunomediada, la edad o el sexo.
Discusión y conclusionesEn los pacientes con enfermedades reumáticas en tratamiento inmunomodulador la respuesta a la vacunación frente al SARS-CoV-2 es superponible a la de la población general salvo en el caso de pacientes en tratamiento con RTX, que es alrededor de un tercio (36,7%), los cuales tienen una tasa de respuesta menor asociada a factores como la hipogammaglobulinemia, los niveles de linfocitos CD19+ previos a la vacunación y el tiempo entre la vacunación y la última dosis de RTX inferior a 6 meses. Es importante tener en consideración dichos factores para optimizar la vacunación en estos pacientes.
SARS-CoV-2 infection has been a major public health problem with a major impact on the management of healthcare resources and a high impact on morbidity and mortality in the general population and, in particular, in immunocompromised individuals.1,2
There are numerous publications on the efficacy and safety of different vaccines in patients with immune-mediated rheumatic diseases (IMRD).3,4 Most of them describe a good level of safety and similar efficacy to that observed in healthy controls in the same studies. Del Porto et al.3 collected data on influenza vaccination in patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and healthy controls without observing significant differences in the antibody titres achieved after vaccination. However, other studies have reported lower efficacy, especially in patients treated with rituximab (RTX). Oren et al.5 studied the response to influenza vaccine in 43 RA patients, 14 on RTX treatment and 21 healthy controls, observing that at 4 weeks the proportion of responders to one of the vaccine antigens was significantly lower in the RTX group than in the non-RTX group (21% vs. 67%, p = .006).
With regard to the SARS-CoV-2 vaccine, several studies have been published to date analysing the vaccine response in healthy individuals, but in the first months of the pandemic there was little information; thus, Lusting et al.6 in Israel showed that with the BNT162b2 vaccine (Pfizer/BioNTech) adequate antibody titres were achieved in 96.5% of the 2,607 healthcare workers, most of them healthy, who were included in the study. However, clinical trials of these vaccines excluded patients with LRTI, so data were not available until post-marketing. In a multicentre study, the efficacy and safety of the same vaccine was evaluated in a population with MRSA (n = 686), compared with a healthy population (n = 121), and an adequate serological response was observed in these patients, except in those on treatment with RTX, glucocorticoids, abatacept and mycophenolate mofetil (MMF), which were associated with a lower vaccine response rate.7
The primary objective of this study is to determine the rate of humoral response to the SARS-CoV-2 vaccine in patients with immune-mediated rheumatic disease on immunomodulatory therapy, including RTX patients at our centre. The secondary objectives of the study were to determine the persistence of the serological response to the vaccine at 6 months and to identify factors that may influence this response.
Material and methodsA prospective observational study was conducted with 2 cohorts of patients under follow-up in the Rheumatology Department of the Hospital Universitario Doctor Peset, diagnosed with ERIM and treated with immunomodulators, without natural immunity to SARS-CoV-2. The 2 cohorts differed in the type of immunomodulatory treatment: the first included patients treated with RTX and the second with synthetic immunomodulators (classical or targeted) and/or biologics, excluding RTX.
Patients included were those undergoing primary vaccination against SARS-CoV-2 who had received the full regimen (one or two doses of vaccine) according to the Spanish Ministry of Health protocols at the time of the study with BioNTech/Pfizer (2 doses 4–8 weeks apart), Moderna/Lonza (2 doses 8 weeks apart), AstraZeneca (2 doses 4–12 weeks apart) or Janssen (single dose) vaccines. A booster dose was also recorded for patients who had not developed a humoral response with the previous regimen.
The main variable studied was the humoral response rate determined by anti-SARS-CoV-2 IgG antibody levels (considered a response if levels were greater than 50 AU/Ml)a one month and 6 months after completing the vaccination regimen. Secondary variables analysed were as follows: the type of vaccine (BioNTech/Pfizer, Moderna/Lonza, AstraZeneca, Janssen), the rate of patients maintaining protective levels of anti-SARS-CoV-2 IgG II antibodies 6 months after receiving the vaccine, the type of treatment received, as well as the number of RTX, considering the presence of hypogammaglobulinaemia as gamma globulin levels below 0.71 g/dl, according to the reference laboratory.
Proportions were calculated for qualitative variables; mean with standard deviation; and median with interquartile range (IQR) for continuous quantitative variables. Qualitative variables were compared using Pearson's Chi-square test, applying the continuity correction if necessary. The Student's t-statistic (or Kruskal–Wallis if more appropriate) was used to compare means. In all analyses, statistical significance was assumed at p < 0.05.
ResultsPatients were recruited between April and October 2021. In total, data from 130 patients were analysed, 41 in the RTX group and 89 in the non-RTX group.
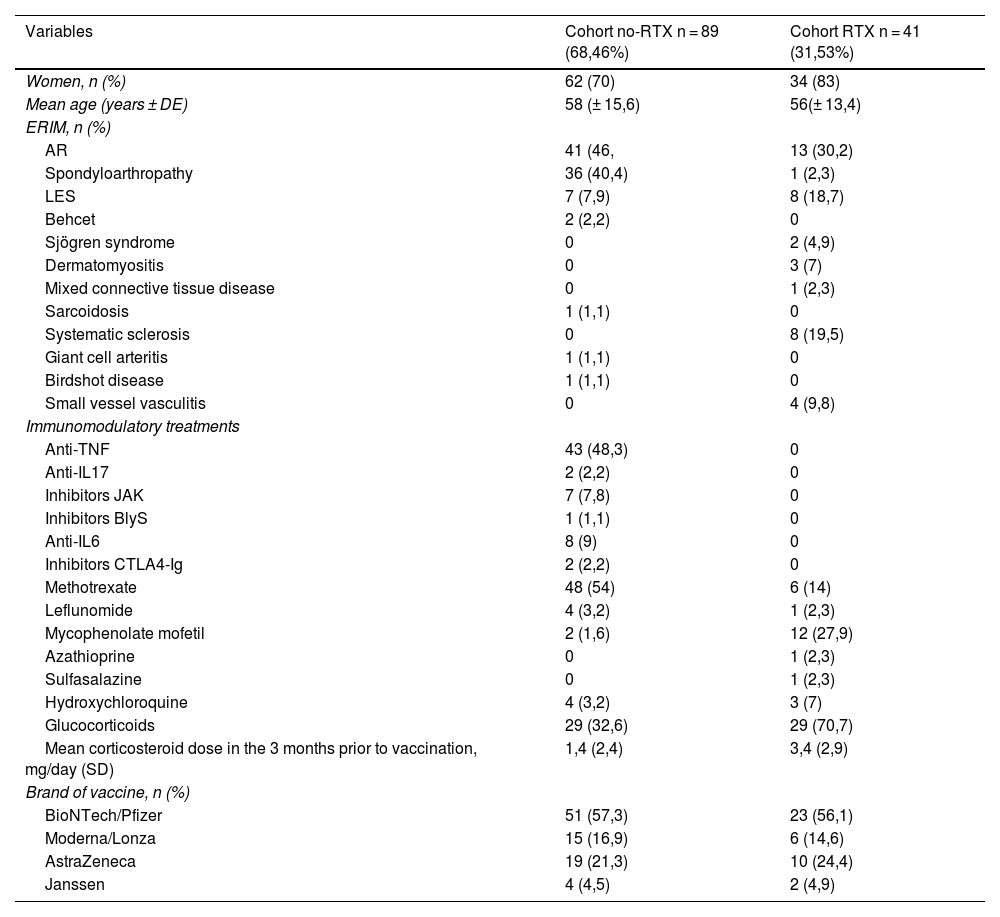
Demographic data and the distribution of diseases and treatments in both cohorts analysed are shown in Table 1.
Demographic data and distribution of diseases and treatments in the cohorts analysed.
| Variables | Cohort no-RTX n = 89 (68,46%) | Cohort RTX n = 41 (31,53%) |
|---|---|---|
| Women, n (%) | 62 (70) | 34 (83) |
| Mean age (years ± DE) | 58 (± 15,6) | 56(± 13,4) |
| ERIM, n (%) | ||
| AR | 41 (46, | 13 (30,2) |
| Spondyloarthropathy | 36 (40,4) | 1 (2,3) |
| LES | 7 (7,9) | 8 (18,7) |
| Behcet | 2 (2,2) | 0 |
| Sjögren syndrome | 0 | 2 (4,9) |
| Dermatomyositis | 0 | 3 (7) |
| Mixed connective tissue disease | 0 | 1 (2,3) |
| Sarcoidosis | 1 (1,1) | 0 |
| Systematic sclerosis | 0 | 8 (19,5) |
| Giant cell arteritis | 1 (1,1) | 0 |
| Birdshot disease | 1 (1,1) | 0 |
| Small vessel vasculitis | 0 | 4 (9,8) |
| Immunomodulatory treatments | ||
| Anti-TNF | 43 (48,3) | 0 |
| Anti-IL17 | 2 (2,2) | 0 |
| Inhibitors JAK | 7 (7,8) | 0 |
| Inhibitors BlyS | 1 (1,1) | 0 |
| Anti-IL6 | 8 (9) | 0 |
| Inhibitors CTLA4-Ig | 2 (2,2) | 0 |
| Methotrexate | 48 (54) | 6 (14) |
| Leflunomide | 4 (3,2) | 1 (2,3) |
| Mycophenolate mofetil | 2 (1,6) | 12 (27,9) |
| Azathioprine | 0 | 1 (2,3) |
| Sulfasalazine | 0 | 1 (2,3) |
| Hydroxychloroquine | 4 (3,2) | 3 (7) |
| Glucocorticoids | 29 (32,6) | 29 (70,7) |
| Mean corticosteroid dose in the 3 months prior to vaccination, mg/day (SD) | 1,4 (2,4) | 3,4 (2,9) |
| Brand of vaccine, n (%) | ||
| BioNTech/Pfizer | 51 (57,3) | 23 (56,1) |
| Moderna/Lonza | 15 (16,9) | 6 (14,6) |
| AstraZeneca | 19 (21,3) | 10 (24,4) |
| Janssen | 4 (4,5) | 2 (4,9) |
IMRD, Immune-mediated rheumatic disease; n, Sample size; SD, Standard Deviation.
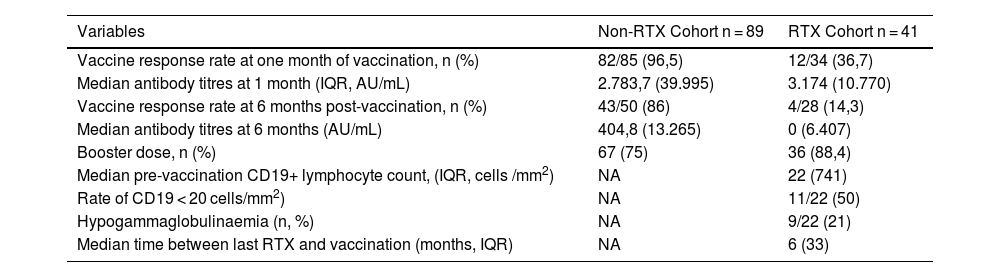
In the RTX cohort, of the patients who had serology at one-month post-vaccination, 36.7% (12/34) managed to develop titres above the cut-off point considered a vaccine response with a median of 3,174 AU/mL (IQR 10770) (Table 2). Of the 19 patients (46.3%) for whom data were available at 1 month and 6 months, 10.6% (2/19) maintained a vaccine response at 6 months, one patient (5.2%) lost response and the remaining 84.2% (16/19) did not develop a response in either of the 2 data collections. There were 9 patients with data at 6 months only, with a response rate of 22% (2/9). The overall median antibody at 6 months was 0 AU/mL (IQR 6407). 88.4% (36/41) received a booster dose one month after the last dose of the usual vaccination schedule, but failed to produce a response in any patient who had not developed a response with previous doses (Table 2). 88.8% of patients with hypogammaglobulinaemia (8/9) had a statistically significant lack of vaccine response (p = 0.04). The mean time between the administration of the last RTX and the complete vaccination regimen was 8.1 months (SD 6.34), and it was observed that the vaccination response rate in patients who had received the last course of RTX in the 6 months prior to vaccination tended to be lower (4/19, 21%) relative to those who received RTX beyond 6 months pre-vaccine (9/22, 41%), but statistical significance was not reached (p = 0.058). Antibody titres which had developed one-month post-vaccination correlated positively and statistically significantly with the time between the last RTX cycle and vaccination (r = 0.423; p = 0.014) and with absolute pre-vaccination CD19+ lymphocyte levels (median 22 cells/mm3; IQR 741) (r = .514; p = .029), but these differences were not maintained at 6 months. No significant differences were found in relation to the number of previous RTX cycles, the type of immune-mediated disease, concomitant treatment with classical synthetic immunomodulators or corticosteroids, or the type of vaccine received.
Summary of vaccine response rates and antibody levels at 1- and 6-months post-vaccination.
| Variables | Non-RTX Cohort n = 89 | RTX Cohort n = 41 |
|---|---|---|
| Vaccine response rate at one month of vaccination, n (%) | 82/85 (96,5) | 12/34 (36,7) |
| Median antibody titres at 1 month (IQR, AU/mL) | 2.783,7 (39.995) | 3.174 (10.770) |
| Vaccine response rate at 6 months post-vaccination, n (%) | 43/50 (86) | 4/28 (14,3) |
| Median antibody titres at 6 months (AU/mL) | 404,8 (13.265) | 0 (6.407) |
| Booster dose, n (%) | 67 (75) | 36 (88,4) |
| Median pre-vaccination CD19+ lymphocyte count, (IQR, cells /mm2) | NA | 22 (741) |
| Rate of CD19 < 20 cells/mm2) | NA | 11/22 (50) |
| Hypogammaglobulinaemia (n, %) | NA | 9/22 (21) |
| Median time between last RTX and vaccination (months, IQR) | NA | 6 (33) |
NA, not affected.
In the non-RTX cohort 96.5% (82/85) of patients with serology at one month achieved a vaccine response with median antibody levels of 2783.7 AU/mL (IQR 39995). The percentage of responders decreased at 6 months (43/50, 86%) and median levels decreased by 85% (404.8 AU/mL; IQR 13265) (Table 2).
All patients treated with anti-TNF, anti-IL17, JAK inhibitors and BLyS inhibitors (belimumab) showed a response at one-month post-vaccination. The percentage dropped for anti-IL6 (7/8, 87.5%) and CTLA4-Ig inhibitors (abatacept) (1/2, 50%). 96.5% (56/58) of patients on classic DMARDs achieved response at one month. The percentage of responders decreased at 6 months (43/50, 86%) and median Ac levels decreased by 85% (404.8 AU/mL). No association was found between the development of vaccine response and baseline disease, corticosteroid therapy or age in this group of patients. However, in the RTX group it was observed that of the 21 patients over 50 years only one (4.7%), maintained antibodies at protective titre levels (p = .008).
DiscussionThe rate of humoral response to the SARS-CoV-2 vaccine in patients receiving immunomodulatory treatment other than RTX in our cohort (82/85 [96.5%]) can be superimposed upon the response expected in the general population, which is over 95% in the different studies in which this response was measured in the healthy population, as well as patients with ERIM and most immunmodulatory treatments.1,6,7 Therefore, we can assume that the standard vaccination regimen against SARS-CoV-2 is adequate in this patient group. However, studies in patients with IMRD and immunosuppressive treatment have found that certain drugs are associated with a lower vaccination response rate, such as RTX, but also abatacept, MMF and glucocorticoids.6–8 In our study, we observed that patients treated with RTX had a lower vaccination response rate (12/34 [36.7%]) than patients without RTX (82/85 [96.5%]) at one month after vaccination, which is consistent with what has been observed in other studies, such as that of Furer et al.,7 where the response rate of patients with RTX was 41% (36/87) at one month after vaccination. In the analysis of variables that may have influenced the lower response of patients in the RTX group, we found that three factors were significantly related to this fact.
Firstly, hypogammaglobulinaemia prior to receiving the vaccines (p = 0.044), which had already been described in studies on the efficacy of these vaccines in patients with haematological malignancies, where hypogammaglobulinaemia was one of the factors negatively associated with the development of vaccine response.9 With regard to this, it could be suggested that the presence of hypogammaglobulinaemia in patients being treated with RTX may be a predictor of a low response to the vaccine.
Secondly, the time between the last dose of RTX and vaccination has a decisive influence on the development of vaccine response. Thus, Furer et al. in their cohort of RTX patients describe a seropositivity rate of 20% in those patients who received the last cycle of RTX in the 6 months prior to vaccination and 50% in those who were vaccinated at least one year after the drug was administered, being similar in our study (21% and 41% respectively). In this regard, the most recent EULAR/ACR recommendations10,11 advocate vaccination before 4 weeks prior to the next cycle of RTX and waiting 2–4 weeks after vaccination to administer the drug if disease activity permits. However, they do not specify whether in case of non-response to vaccine doses it is appropriate to postpone RTX and revaccinate with a third or fourth dose. It is possible that in patients with severe disease and with few treatment options, taking into account the risk-benefit ratio, it would be advisable to prioritise the administration of RTX despite a poor humoral response to the vaccine and the risk of infection that it entails.12 Based on the data from our study, no patients with RTX who received booster doses developed a vaccine response, but longer-term studies with a larger sample size are needed to establish solid recommendations on this issue.
Finally, patients with CD19+ lymphocyte depletion prior to vaccination had lower vaccine response than patients with levels >20 cells/mm3 at the time of vaccination. In the study by Stefansky et al.8 they set the minimum CD19 level at a minimum of 10 cells/mm3 in order to develop an immune response and propose it as a possible biomarker for predicting response. Some authors point out that in patients receiving RTX, cell-mediated immunity to SARS-CoV-2 can develop after a second and third dose of the vaccine, although humoral immunity is not detected, which confers a certain degree of protection against infection.13 Although there is no specific recommendation in this regard in the guidelines, measuring the cellular response in certain patients could be of help in making more individualised decisions.
Regarding the influence of other immunomodulators on the development of vaccine response, we did not observe significant differences in either of the two cohorts analysed. The use of MMF, abatacept and glucocorticoids have been reported to reduce seroconversion rates,7 although not as significantly as RTX. In our study, with respect to MMF, 50% (6/6) did not achieve a non-significant vaccination response in the RTX group (concomitant treatment), while in the non-RTX group, the 2 patients on MMF treatment (combined with adalimumab and belimumab respectively) developed a vaccination response at one month (100%). With respect to abatacept only one of the 2 patients developed a vaccination response (50%), but due to the small sample size statistical significance cannot be established. With regard to corticosteroid treatment, we found no significant differences in vaccine response rates, as reported in some studies7,8; however, in these studies the mean doses were 6.7 and 6.5 mg/day, whereas in our study they were 1.5 and 3.4 mg/day. Studies in patients with RA or spondyloarthropathies with low doses of corticosteroids have shown no influence on seroconversion to influenza vaccine.4 However, it is not clear by what mechanism glucocorticoids may reduce the vaccine response to SARS-CoV-2, but the concomitant use of other immunosuppressants and the activity of the underlying disease that requires the use of higher doses of corticosteroids may have a negative influence,8,14,15 although further studies are needed. With respect to other variables such as sex and age, negative data on vaccine response have been reported in male patients and those over 66 years of age,6 but in our cohorts we did not observe any statistically significant differences according to sex or age.
The limitations of this study include the low sample size, due to the single-centre nature of the study, and logistical limitations due to health restrictions that hindered access to medical consultations, analyses and vaccination in a timely manner. It should also be mentioned that it was not possible to analyse the influence of disease activity or comorbidities on the vaccination response of these patients.
This study has been approved by the CEIC at the Hospital Doctor Peset.
ConclusionsIn our study, SARS-CoV-2 vaccines have demonstrated an effectiveness rate in patients with MRIA similar to that in the general population (82/85 [96.5%]).1,6,7 Treatment with RTX decreases the response rate to vaccination, even after administration of booster doses (12/34 [36.7%]) and is similar to the response rate observed in other studies.6–8 The presence of hypogammaglobulinaemia and CD19 levels below 20 cells/mm3 prior to vaccination, as well as the time between the last RTX cycle and vaccination of less than 6 months had a negative influence on antibody development. No differences were found for treatment with other immunomodulators (including abatacept and MMF) or corticosteroids. We also found no association with age or gender, probably due to the sample size of the study.
In patients with ERIM treated with RTX who have not developed a response to the SARS-CoV-2 vaccine and require revaccination due to a high risk of infection, it should be taken into account that, in order to optimise the possibility of generating antibodies, it is advisable to wait 6 months after the last dose of RTX and to administer the vaccine before 4 weeks prior to infusion, if disease activity permits, following the EULAR/ACR recommendations.10,11 CD19+ lymphocyte levels over 20 cells/mm3 and the absence of hypogammaglobulinaemia are proposed as additional factors that could be taken into account to predict vaccine response.
FundingThis study was funded by a 2022 grant from the Fundación Valenciana de Reumatología.
Conflict of interestsNone.
To José Miguel Nogueira Coito (Microbiology Dept., Hospital Universitari Doctor Peset).