Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are autoimmune diseases. Premature atherosclerosis and cardiovascular diseases are two of the most important complications of these diseases. Anti-carbamylated protein antibody (Anti-carP Ab) is one of the antibodies which was studied in RA and SLE. In our study, we studied the relation between anti-carP Ab, disease activity and insulin resistance in RA and SLE patients.
Methods90Patients with SLE and RA were enrolled and subjected to history taking, clinical examination and assessment of disease activity using SLE disease activity index 2000 (SLEDAI-2K) scoring for SLE patients and disease activity score 28 (DAS28-ESR) for RA patients. Samples were examined for complete blood count (CBC), creatinine, inflammatory markers, Tumour necrosis factor alpha (TNF alpha), fasting insulin, fasting blood sugar (FBS), lipid profile and anti-carPAb. HOMA-IR (homeostasis model assessment for insulin resistance) was calculated.
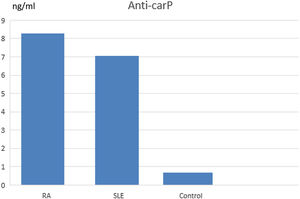
ResultsPatients with RA and SLE showed higher levels of anti-carPAb in comparison with healthy subjects (8.25ng/ml for RA, 7ng/ml for SLE and 0.6ng/ml for healthy subjects with p value <0.001). There was a positive correlation between anti-carPAb and disease activity of RA (p value <0.001) and a positive correlation between anti-carPAb and TNF alpha in RA. In SLE, there was no correlation of anti-carP Ab with disease activity while, HOMA-IR showed a positive correlation with nephritis (p value 0.04).
ConclusionAnti-carP antibody is a marker of disease activity in RA patients and has high specificity for both RA and SLE detection.
La artritis reumatoide (AR) y el lupus eritematoso sistémico (LES) son enfermedades autoinmunes. La aterosclerosis prematura y las enfermedades cardiovasculares son 2 de las complicaciones más importantes de estas enfermedades. El anticuerpo anti-proteína carbamilada (Anti-carPAb) es uno de los anticuerpos que se estudió en AR y LES. En nuestro estudio analizamos la relación entre los Ac anti-carP, la actividad de la enfermedad y la resistencia a la insulina en pacientes con AR y LES.
MétodosSe inscribieron 90 pacientes con LES y AR y se sometieron a la historia clínica, el examen clínico y la evaluación de la actividad de la enfermedad utilizando el índice de actividad de la enfermedad de LES 2000 (SLEDAI-2K) para los pacientes con LES y la calificación de actividad de la enfermedad 28 (DAS28-ESR) para los pacientes con AR. Las muestras se examinaron para hemograma completo, creatinina, marcadores inflamatorios, factor de necrosis tumoral alfa, insulina en ayunas, azúcar en sangre en ayunas, perfil de lípidos y anti-carPAb. Se calculó HOMA-IR (evaluación del modelo de homeostasis para la resistencia a la insulina).
ResultadosLos pacientes con AR y LES mostraron niveles más altos de anti-carP Ab encomparación con sujetos sanos (8,25ng/ml para AR, 7ng/ml para LES y 0,6ng/ml para sujetos sanos con un valor de p<0,001). Hubo una correlación positiva entre anti-carP Ab y la actividad de la enfermedad de la AR (valor de p<0,001) y una correlación positiva entre anti-carPAb y factor de necrosis tumoral alfa en la AR. En el LES no hubo correlación de anti-carPAb con la actividad de la enfermedad, mientras que HOMA-IR mostró una correlación positiva con la nefritis (valor de p=0,04).
ConclusiónEl anticuerpo anti-carP es un marcador de la actividad de la enfermedad en pacientes con AR y tiene una alta especificidad para la detección de AR y LES.
RA is a chronic inflammatory disease that leads to severe joint destruction. In addition, RA patients have higher risk of developing cardiovascular disease (CVD) and this is related to chronic inflammation,1 Systemic chronic inflammation and proinflammatory cytokines have been proposed as major protagonists in the pathogenesis of insulin resistance (IR), an important factor for CVD.2 Abnormalities in glucose metabolism have been well documented in RA patients and may also correlate with Disease Activity Score evaluating 28 joints (DAS 28) and corticosteroids treatment.3
Anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF) are important serological markers in the diagnostic procedure of RA.4 Just recently, anti-carP Ab has been described in RApatients.5,6 Anti-carP Ab can be detected in serum many years before the clinical diagnosis of RA and is independently associated with increased joint damage at the baseline of RA diagnosis.7 In SLE, it is more frequently found than anti-cyclic citrullinated protein antibody (anti-CCP) and mainly identify a different subgroup of cases compared with anti-CCP positives. They are associated significantly with erosive disease.8
Aim of work: To study the relation between anti-carP Ab, disease activity and insulin resistance in RA and SLE patients.
Patients and methodsThis cross-sectional study included 90 patients divided in to 3 groups: Group I: 30 patients with RA fulfilling ACR/EULAR criteria 2010,9Group II: 30 patients with SLEfulfilling the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for SLE,10Group III: 30 normal healthy volunteers. All patients were chosen from Kasr El Aini University Hospital, Internal Medicine Department and Clinic after the approval of the institutional ethical committee.
None of the patients were smokers, none of the RA patients were on steroids while among the SLE group 22 patients were on steroids, average dose of 20mg with average duration of 2 months
Exclusion criteria: Patients with diabetes mellitus, angina, myocardial infarction, stroke, transient ischaemic attack, heart failure, peripheral vascular disease and obesity.
Methods: Patients were subjected to the following:
Full history taking, clinical examination and assessment of disease activity using DAS 28 score for RA and SLEDAI-2k score for SLE.
Laboratory investigations include: CBC, kidney function tests, Erythrocyte sedimentation rate (ESR) and C reactive protein (CRP), FBS and insulin levels, HOMA-IR calculation, total cholesterol (TC), triglycerides (TG),Low density lipoprotein (LDL) and High-density lipoprotein (HDL) levels, TNF alpha ELISA according to manufacture steps and Anti-carP Ab using ELISA Kits (Cloud Clone CORP,Wuhan,China).
Reference ranges: For TNF alpha: 110–189ng/l,insulin level: 0.5–3ng/ml and anti-carP Ab: less than 5ng/ml.
DAS28-ESRscore: is composed of number of swollen joints (out of 28), number of tender joints (out of 28), patient's global assessment of health and ESR level. Score ≤2.6 means disease in remission, >2.6–≤3.2 means in low activity, >3.2–≤5.1 means moderately active while score >5.1 means highly active disease.3
SLEDAI-2K score: is based on the presence of 24 descriptors in nine organ systems over the preceding 30 days. Descriptors of SLEDAI-2K are documented as present or absent. Each of the descriptors has a weighted score and the total score of SLEDAI-2K is the sum of all 24 descriptor scores. The total SLEDAI-2K score falls between 0 and 105, with higher scores representing higher disease activity (usually above 4).11
The research was approved by the local ethical committee and patients provided an informed consent to participate.
Statistical analysisData were coded and entered using SPSS (Statistical Package for the Social Sciences) version 24. Data was summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Kruskal–Wallis and Mann–Whitney tests. For comparing categorical data, Chi square (v2) test was performed. Exact test was used instead when the expected frequency is less than 5. Correlations between quantitative variables were done using Spearman correlation coefficient and multivariate linear regression analysis done. p-Values <0.05 were considered significant.
ResultsThis study included 30 patients with RA (groupI), 30 SLE patients (groupII) and 30 matched age and sex control (groupIII).
Demographic and laboratory data for group I (RA group) and II (SLE group) are shown in Tables 1 and 2.
Demographic and clinical features of RA & SLE groups.
| RA | SLE | |||
|---|---|---|---|---|
| Count | % | Count | % | |
| Sex | ||||
| M | 9 | 30.0% | 6 | 20.0% |
| F | 21 | 70.0% | 24 | 80.0% |
| Arthritis | 23 | 76.7% | 16 | 53.3% |
| Interstitiallung disease | 3 | 10.0% | 0 | 0% |
| Skin | ||||
| Malar rash | 3 | 10% | 18 | 60% |
| Photosensitivity | 15 | 50% | ||
| Oral ulcers | 8 | 26.7% | ||
| Hair fall | 11 | 36.7% | ||
| Peripheral neuropathy | 2 | 6.7% | 0 | 0 |
| Nephritis | 0 | 0% | 8 | 26.7% |
| Cerebritis | 0 | 0% | 1 | 3.3% |
| Haemolytic anaemia | 0 | 0% | 3 | 10.0% |
| Other haematological abnormalities | 0 | 0% | 5 | 16.7% |
Laboratory features of RA and SLE groups:.
| RA SLE | ||||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 47.37 | 13.79 | 26.93 | 10.91 |
| TLC | 7.62 | 3.66 | 7.10 | 5.82 |
| Hb | 11.45 | 2.02 | 9.82 | 2.10 |
| PLT | 284.80 | 82.88 | 200.03 | 114.11 |
| Creatinine | 0.92 | 0.42 | 1.63 | 2.02 |
| ESR | 60.03 | 33.67 | 63.27 | 36.00 |
| Cholesterol | 185.00 | 49.05 | 178.60 | 65.53 |
| LDL | 116.67 | 46.45 | 102.03 | 49.28 |
| HDL | 47.60 | 7.89 | 43.50 | 9.96 |
| TG | 112.07 | 66.46 | 174.73 | 122.32 |
| FBS | 94.90 | 7.34 | 89.63 | 6.68 |
| Insulin | 0.20 | 0.16 | 0.25 | 0.21 |
| TNF alpha | 195.49 | 459.39 | 167.34 | 406.18 |
| Anti-carP | 8.25 | 15.98 | 7.04 | 15.52 |
| HOMA-IR | 1.20 | 0.97 | 1.42 | 1.23 |
| TG/HDL | 2.42 | 1.60 | 3.94 | 2.52 |
| Albumin/creatinine ratio | 911.43 | 1676.64 | ||
| C3 | 73.48 | 35.57 | ||
| C4 | 15.10 | 10.72 | ||
A comparative study was done between RA group and control showing higher levels of ESR, FBS andTG among the RA group with high statistically significant difference, p value (<0.001). HDL level was lower among RA group with high statistically significant difference, p value (<0.001). TG/HDL ratio was higher also among the RA group with high statistically significant difference, p value (<0.001).
Anti-carPAb was more prevalent among the RA group with high statistically significant difference, p value (<0.001) (Table 3) and Fig. 1.
comparative study between RA group and control.
| RA | Control | p Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Minimum | Maximum | Mean | SD | Median | Minimum | Maximum | ||
| TLC | 7.62 | 3.66 | 6.75 | 3.40 | 18.00 | 6.74 | 1.21 | 6.60 | 4.50 | 9.00 | 0.836 |
| Hb | 11.45 | 2.02 | 11.85 | 5.80 | 14.00 | 12.03 | 1.96 | 12.60 | 8.30 | 14.50 | 0.283 |
| PLT | 284.80 | 82.88 | 268.00 | 130.00 | 516.00 | 305.27 | 48.71 | 296.50 | 225.00 | 395.00 | 0.124 |
| Creatinine | 0.92 | 0.42 | 0.80 | 0.50 | 3.00 | 0.79 | 0.14 | 0.80 | 0.50 | 1.01 | 0.129 |
| ESR | 60.03 | 33.67 | 52.50 | 15.00 | 140.00 | 5.87 | 0.97 | 6.00 | 5.00 | 8.00 | <0.001 |
| Cholesterol | 185.00 | 49.05 | 179.00 | 110.00 | 320.00 | 198.07 | 9.52 | 195.00 | 185.00 | 222.00 | 0.010 |
| LDL | 116.67 | 46.45 | 107.50 | 46.00 | 222.00 | 110.27 | 13.63 | 106.00 | 88.00 | 151.00 | 0.807 |
| HDL | 47.60 | 7.89 | 47.00 | 35.00 | 69.00 | 74.20 | 7.53 | 75.00 | 52.00 | 87.00 | <0.001 |
| TG | 112.07 | 66.46 | 87.00 | 50.00 | 313.00 | 65.40 | 10.98 | 62.50 | 50.00 | 95.00 | <0.001 |
| FBS | 94.90 | 7.34 | 92.50 | 82.00 | 110.00 | 81.73 | 9.49 | 80.50 | 70.00 | 99.00 | <0.001 |
| Insulin | 0.20 | 0.16 | 0.13 | 0.10 | 0.84 | 0.22 | 0.12 | 0.18 | 0.10 | 0.48 | 0.710 |
| TNF alpha | 195.49 | 459.39 | 62.65 | 10.50 | 2150.00 | 110.57 | 107.20 | 81.70 | 10.20 | 406.00 | 0.212 |
| Anti-carP | 8.25 | 15.98 | 1.20 | 0.48 | 58.00 | 0.67 | 0.28 | 0.60 | 0.15 | 1.35 | <0.001 |
| HOMA-IR | 1.20 | 0.97 | 0.80 | 0.50 | 5.40 | 1.07 | 0.61 | 0.85 | 0.40 | 2.40 | 0.485 |
| TG/HDL | 2.42 | 1.60 | 2.00 | 1.00 | 8.90 | 0.90 | 0.25 | 0.90 | 0.70 | 1.80 | <0.001 |
A comparative study was done between SLE group and control showing lower levels of haemoglobin (Hb), platelet (PLT) and HDL among SLE group with high statistically significant difference with p value (<0.001). Levels of creatinine, ESR, TG and FBS were higher among SLE group with high statistically significant difference, p value (<0.001). Anti-carPAb was more prevalent among SLE group with high statistically significant difference with p value (<0.001). TG/HDL ratio was higher among SLE group with high statistically significant difference (Table 4 and Fig. 1).
Comparative study between SLE group and control.
| SLE | Control | p Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Minimum | Maximum | Mean | SD | Median | Minimum | Maximum | ||
| TLC | 7.10 | 5.82 | 5.65 | 1.20 | 27.00 | 6.74 | 1.21 | 6.60 | 4.50 | 9.00 | 0.092 |
| Hb | 9.82 | 2.10 | 9.55 | 6.40 | 13.50 | 12.03 | 1.96 | 12.60 | 8.30 | 14.50 | <0.001 |
| PLT | 200.03 | 114.11 | 195.50 | 5.00 | 506.00 | 305.27 | 48.71 | 296.50 | 225.00 | 395.00 | <0.001 |
| Creatinine | 1.63 | 2.02 | 0.85 | 0.40 | 7.80 | 0.79 | 0.14 | 0.80 | 0.50 | 1.01 | 0.047 |
| ESR | 63.27 | 36.00 | 60.00 | 3.00 | 140.00 | 5.87 | 0.97 | 6.00 | 5.00 | 8.00 | <0.001 |
| Cholesterol | 178.60 | 65.53 | 169.00 | 89.00 | 352.00 | 198.07 | 9.52 | 195.00 | 185.00 | 222.00 | 0.379 |
| LDL | 102.03 | 49.28 | 93.00 | 41.00 | 244.00 | 110.27 | 13.63 | 106.00 | 88.00 | 151.00 | 0.211 |
| HDL | 43.50 | 9.96 | 44.00 | 30.00 | 69.00 | 74.20 | 7.53 | 75.00 | 52.00 | 87.00 | <0.001 |
| TG | 174.73 | 122.32 | 125.50 | 56.00 | 501.00 | 65.40 | 10.98 | 62.50 | 50.00 | 95.00 | <0.001 |
| FBS | 89.63 | 6.68 | 89.00 | 77.00 | 108.00 | 81.73 | 9.49 | 80.50 | 70.00 | 99.00 | 0.002 |
| Insulin | 0.25 | 0.21 | 0.13 | 0.05 | 0.78 | 0.22 | 0.12 | 0.18 | 0.10 | 0.48 | 0.864 |
| TNF alpha | 167.34 | 406.18 | 39.35 | 10.00 | 1950.0 | 110.57 | 107.2 | 81.70 | 10.20 | 406.00 | 0.069 |
| Anti-carP | 7.04 | 15.52 | 1.00 | 0.50 | 60.00 | 0.67 | 0.28 | 0.60 | 0.15 | 1.35 | <0.001 |
| HOMA-IR | 1.42 | 1.23 | 0.70 | 0.40 | 4.50 | 1.07 | 0.61 | 0.85 | 0.40 | 2.40 | 0.608 |
| TG/HDL | 3.94 | 2.52 | 3.20 | 1.30 | 9.60 | 0.90 | 0.25 | 0.90 | 0.70 | 1.80 | <0.001 |
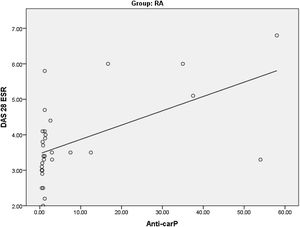
Studying the correlations between anti-carP Ab and HOMA-IR among the RA groupas regards clinical and laboratory variables, we found a highly significant positive correlationbetween anti-carP Ab and DAS 28-ESR (Table 5) (Fig. 2). There was also a significant positive correlation between TNF alpha and both anti-carP Ab and HOMA-IR. A highly significant positive correlation was found between HOMA-IR and TG.
Correlations between anti-carP Ab and HOMA-IR with clinical and laboratory variables in RA group.
| RA | Anti-carP | HOMA-IR | |
|---|---|---|---|
| Anti-carP | r | 1.000 | 0.271 |
| p Value | . | 0.147 | |
| N | 30 | 30 | |
| HOMA-IR | r | 0.271 | 1.000 |
| p Value | 0.147 | . | |
| N | 30 | 30 | |
| Age | r | 0.097 | 0.192 |
| p Value | 0.610 | 0.308 | |
| N | 30 | 30 | |
| TLC | r | -0.026- | -0.336- |
| p Value | 0.890 | 0.070 | |
| N | 30 | 30 | |
| Hb | r | -0.120- | -0.145- |
| p Value | 0.528 | 0.443 | |
| N | 30 | 30 | |
| PLT | r | -0.253- | -0.272- |
| p Value | 0.177 | 0.145 | |
| N | 30 | 30 | |
| Creatinine | r | 0.173 | 0.285 |
| p Value | 0.360 | 0.127 | |
| N | 30 | 30 | |
| ESR | r | 0.000 | 0.310 |
| p Value | 0.999 | 0.096 | |
| N | 30 | 30 | |
| Cholesterol | r | -0.296- | 0.119 |
| p Value | 0.112 | 0.531 | |
| N | 30 | 30 | |
| LDL | r | -0.211- | 0.023 |
| p Value | 0.264 | 0.906 | |
| N | 30 | 30 | |
| HDL | r | -0.185- | 0.221 |
| p Value | 0.329 | 0.239 | |
| N | 30 | 30 | |
| TG | r | 0.037 | 0.601 |
| p Value | 0.847 | <0.001 | |
| N | 30 | 30 | |
| FBS | r | 0.085 | 0.179 |
| p Value | 0.656 | 0.345 | |
| N | 30 | 30 | |
| Insulin | r | 0.245 | 0.947 |
| p Value | 0.192 | <0.001 | |
| N | 30 | 30 | |
| TNF alpha | r | 0.432 | 0.405 |
| p Value | 0.017 | 0.026 | |
| N | 30 | 30 | |
| DAS 28 ESR | r | 0.609 | 0.355 |
| p Value | <0.001 | 0.054 | |
| N | 30 | 30 | |
| TG/HDL | r | 0.093 | 0.116 |
| p Value | 0.627 | 0.541 | |
| N | 30 | 30 | |
Studying the correlations between anti-carPAbs and HOMA-IR among the SLE group as regards clinical and laboratory variables, we found a significant negative correlation between Hb level and HOMA-IR (Table 6). There was a highly significant positive correlation between HOMA-IR and FBS. A significant positive correlation was present between HOMA-IR and albumin creatinine ratio.
Correlations between anti-carp ab and HOMA-IR with clinical and laboratory variables in SLE group.
| SLE | Anti-carP | HOMA-IR | |
|---|---|---|---|
| Anti-carP | r | 1.000 | 0.243 |
| p Value | . | 0.196 | |
| N | 30 | 30 | |
| HOMA-IR | r | 0.243 | 1.000 |
| p Value | 0.196 | . | |
| N | 30 | 30 | |
| Age | r | 0.253 | -0.197- |
| p Value | 0.177 | 0.298 | |
| N | 30 | 30 | |
| TLC | r | -0.190- | -0.116- |
| p Value | 0.315 | 0.542 | |
| N | 30 | 30 | |
| Hb | r | -0.238- | -0.409- |
| p Value | 0.205 | 0.025 | |
| N | 30 | 30 | |
| PLT | r | -0.089- | 0.145 |
| p Value | 0.639 | 0.444 | |
| N | 30 | 30 | |
| Creatinine | r | 0.251 | 0.268 |
| p Value | 0.181 | 0.152 | |
| N | 30 | 30 | |
| ESR | r | 0.246 | 0.017 |
| p Value | 0.190 | 0.927 | |
| N | 30 | 30 | |
| Cholesterol | r | -0.094- | -0.287- |
| p Value | 0.620 | 0.124 | |
| N | 30 | 30 | |
| LDL | r | -0.089- | -0.212- |
| p Value | 0.641 | 0.262 | |
| N | 30 | 30 | |
| HDL | r | 0.100 | -0.022- |
| p Value | 0.598 | 0.908 | |
| N | 30 | 30 | |
| TG | r | 0.012 | -0.267- |
| p Value | 0.951 | 0.154 | |
| N | 30 | 30 | |
| FBS | r | -0.013- | 0.602 |
| p Value | 0.947 | <0.001 | |
| N | 30 | 30 | |
| Insulin | r | 0.234 | 0.984 |
| p Value | 0.213 | <0.001 | |
| N | 30 | 30 | |
| TNF alpha | r | -0.260- | 0.203 |
| p Value | 0.165 | 0.283 | |
| N | 30 | 30 | |
| SLEDAI-2k | r | 0.047 | -0.014- |
| p Value | 0.805 | 0.942 | |
| N | 30 | 30 | |
| TG/HDL | r | 0.036 | 0.000 |
| p Value | 0.851 | 0.999 | |
| N | 30 | 30 | |
| Albumin creatinine ratio | r | 0.124 | 0.410 |
| p Value | 0.514 | 0.024 | |
| N | 30 | 30 | |
| C3 | r | -0.336- | -0.274- |
| p Value | 0.070 | 0.142 | |
| N | 30 | 30 | |
| C4 | r | -0.278- | -0.299- |
| p Value | 0.137 | 0.109 | |
| N | 30 | 30 | |
Studying the correlation between anti-carP Abs and SLEDAI-2K, there was no significant correlation between them (Table 6).
The sensitivity and specificity of anti-carP antibodies for detection of RA is 73.3% and 76.7 respectively.
The sensitivity and specificity of anti-carP Abs for detection of SLE is 70% and 86.7% respectively. The test showed higher specificity for SLE reaching 86.7%.
DiscussionRA is a chronic autoimmune disease characterized by inflammation within the joints that eventually leads to destruction of cartilage and bone.12 Presentations ranged from mild to severe, although the typical patient had a progressive course leading to functional limitations.13 Inflammation plays a central role in the pathogenesis of atherosclerosis, and the increase in CV disease and mortality in RA may be partly explained by inflammatory factors associated with RA, even after adjustment for traditional CV risk factors.14
SLE is associated with an increased prevalence of atherosclerosis. Traditional risk factors for atherosclerosis are common among patients with SLE. However, atherosclerosis occurs prematurely in patients with SLE, independently of traditional risk factors for CV.15
Anti-carP Abs were detected in various studies in several patients with RA.16
In our study we found that FBS was higher in patients with rheumatoid arthritis with high statistically significant difference in comparison with the control group, while fasting insulin level and calculated HOMA-IR were higher in the RA group with no statistically significant difference.
In contrast to our study, Montagna and his colleagues found statistically significant difference in HOMA-IR between RA patients and control,17 this can be explained that our patients were younger, of lower body mass index (BMI) and most of them were not on steroids in contrast to the study done by Montagna and his colleagues, where nearly 50% of his group were on steroid therapy.
Previous results from studies done by Hussein, Gazareen and their colleagues supported our results and found that the FBS was higher in the RA group with a high statistically significant difference.18,19
In our study,patients with RA had higher levels of TG and TG/HDL ratio and lower levels of HDL and TC with high statistically significant difference in comparison with the control group while the LDL level showed no difference between the two groups.
These results were supported by the study done by Gazareen and his colleagues who found higher levels of TG among the RA group.19 This was also in agreement with the study done by Kim and his colleagues who found markedly increased levels of TG with increased TG/HDL ratio in RA patients and lower levels of HDL with high statistically significant difference. The LDL levels showed no difference between the two groups.20
Patients with RA had high levels of anti-carP Abs with high statistically significant difference in comparison with the control group.
Previous results obtained from studies done by, Mohamed, El-Shorbagy, Elsawy and their colleagues supported our study and found higher levels of anti-carP Abs among the RA group with very high statistically significant difference.21,22 This was also in agreement with the study done by Ozdemir et al.23,24
Our study showed a highly significant positive correlation between anti-carP antibody and DAS 28-ESR in RA patients. Previous results from studies done by Kumar, Elsayed,ELsawy and their colleagues supported these results.23,25,26
In our study there was a highly significant positive correlation between TNF alpha and both anti-carP Ab and HOMA-IR.
These results were supported by the results of the study done by Hussein, Chung and their colleagues who found a strong positive correlation between TNF alpha level and HOMA-IR.18
Patients with SLE had higher levels of FBS with high statistically significant difference in comparison with the control group, the fasting insulin levels and HOMA-IR were higher but without significant difference.
These results were in agreement with the study done by Koca and his colleagues who found no significant difference in HOMA-IR between the SLE group and the control.27
In contrast to our study, Gazareen and his colleagues found a high significant level of fasting insulin and HOMA-IR among the SLE group and no significant difference in the FBS levels.19 This can be explained by that our study included SLE patients younger with lower BMI and different disease activity.
Patients with SLE had higher levels of anti-carP Abs with high statistically significant difference in comparison with the control group.
Previous results from studies done by Ozdemir, Pecani and their colleagues supported the results of our study and found a highly significant level of anti-carP antibody among the SLE group.23,28
There was no significant correlation between anti-carP Ab and SLEDAI-2K, this was in agreement with the study done by Spinelli and his colleagues who found the same result.29
ConclusionAnti-carP Ab could provide valuable information about disease activity of RA and had high specificity for both RA and SLE detection. HOMA-IR was high among RA & SLE patients; hence their blood sugar should be frequently checked.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.