Immune-mediated inflammatory diseases (IMID) predispose to a higher infection risk by modifying the host's immune response, which acts as a key factor in SARS-CoV-2 infection resolution. Recent publications show that IMID patients and its treatments do not worsen the outcome of SARS-CoV-2 infection.
ObjectivesTo describe the clinical characteristics and outcomes of patients with IMID who required hospital admission due to SARS-CoV-2 infection. Secondly, to compare clinical characteristics and outcomes between patients who required hospital admission due to SARS-CoV-2 infection with IMID and those who were not affected.
MethodsWe performed an observational retrospective cohort study, including admitted patients with suspected SARS-CoV-2 infection, treated according to medical criteria and local protocols based on the best available scientific evidence. Clinical data were collected from their electronical clinical history. Statistical analysis determined the differences in the characteristics and clinical outcome of the infection in IMID patients.
ResultsOf a total number of 612 revised patients, 23 had an IMID and 9 of them were positive for the SARS-CoV-2 infection. We did not observe a correlation between these two disorders. There was a higher frequency of obesity and cardiovascular disease among IMID patients, but without statistical significance. The clinical outcomes were no different between hospitalized IMID and non IMID patients.
ConclusionIMID and its treatments do not determine the outcome of patients admitted with SARS-CoV-2 infection.
Las enfermedades inflamatorias inmunomediadas (IMID) predisponen a un aumento del riesgo infeccioso al modificar la respuesta inmune del huésped, que resulta crucial para la resolución de la infección por SARS-CoV-2. Las últimas publicaciones indican que los pacientes con IMID y sus tratamientos de base no empeoran el pronóstico de la infección por SARS-CoV-2.
ObjetivosDescribir las características clínicas y la evolución de pacientes con IMID que requirieron ingreso hospitalario por infección por SARS-CoV-2. En segundo lugar, comparar las características clínicas y la evolución entre pacientes que requirieron ingreso hospitalario por infección por SARS-CoV-2 con IMID y aquellos que no la presentaban.
MétodosEstudio observacional de cohortes retrospectivo, que incluyó pacientes ingresados por sospecha de SARS-CoV-2, tratados según el criterio médico y los protocolos basados en la evidencia científica. La recogida de datos clínicos se realizó por descarga directa o mediante revisión manual de la historia clínica electrónica. El análisis estadístico determinó las diferencias de características y evolución clínica de la infección en pacientes con IMID.
ResultadosDe los 612 pacientes revisados, 23 padecían IMID y 9 de ellos fueron diagnosticados de infección por SARS-CoV-2. No se observó correlación entre infección por SARS-CoV-2 e IMID. Los pacientes con IMID presentaban mayor prevalencia de enfermedad cardiovascular y obesidad, aunque no significativamente. Asimismo, los pacientes con IMID no presentaron una evolución clínica durante el ingreso hospitalario diferente respecto al resto de pacientes.
ConclusiónLas IMID y los tratamientos de las mismas no determinan el pronóstico del ingreso hospitalario de la infección por SARS-CoV-2.
On 31 December 2019, the Chinese health authorities informed the World Health Organization (WHO) of a series of pneumonia cases linked to a live animal market in the city of Wuhan. The WHO declared global spread of COVID-19, caused by the SARS-CoV-2 coronavirus, a pandemic on 11 March 2020.1–3 Since then, a wide range of clinical manifestations and severity has been observed in patients, from asymptomatic or paucisymptomatic positive to life-threatening cases with severe respiratory involvement.2,4,5 Severe lung damage may be caused by cytokine storms that produce hyperinflammation and are also responsible for the inflammatory activity of immune-mediated inflammatory diseases (IMID).4,6,7
We know that the pathophysiology of IMID predisposes to an increased risk of infection by modifying the host immune response, which is crucial for the outcome of SARS-CoV-2 infection.2,3 Different therapies have been used to treat IMID over the years, although biological disease-modifying antirheumatic drugs and small molecule Janus kinase (JAK) inhibitors, which act by blocking the expression of proinflammatory cytokines such as interleukin IL-1, IL-6, tumour necrosis factor-alpha (TNFα) that use JAK pathways to transmit intracellular signals, have revolutionised the management and control of flares in recent decades.4,5,8 Therefore, these biological agents and small molecules,2 such as tocilizumab, anakinra and baricitinib, have been introduced into SARS-CoV-2 treatment protocols to improve the prognosis of the most severe cases, which, from a more theoretical point of view, can modify the course of the disease and improve the prognosis of patients affected by SARS-CoV-2.
There is limited evidence on the risk of contracting SARS-CoV-2 infection and its prognosis in patients with IMID. To date, both the prevalence and behaviour of these inflammatory diseases have been reported not to differ in this population group,9–13 and the baseline treatments used for their treatment do not worsen the prognosis of the infection.9–13 However, a recent publication in a Spanish population observed that having an IMID, and the different therapies used, have some impact on SARS-CoV-2 infection.14 In addition, the rapid spread of COVID-19 infection worldwide raises questions about the impact of the virus on pregnant patients. Recently, the Center for Disease Control and Prevention (CDC), based on current evidence, concluded that pregnant women are at increased risk of COVID-19 infection compared to the general population and at increased risk of preterm delivery.15 To compound this, pregnant patients diagnosed with rheumatic diseases have an immunocompromised status, which added to the use of disease-modifying treatments, may mean this group of patients are more vulnerable to coronavirus infection.16 However, the number of reported cases of pregnant patients with inflammatory diseases is limited and the impact of coronavirus on these patients is unknown.
Our study analyses the demographic and clinical characteristics and prognosis of patients with IMID within a cohort of patients hospitalised in our centre for SARS-CoV-2 infection.
MethodsWe conducted a retrospective observational cohort study in a Spanish university hospital with 258 inpatient beds, including 12 in the Intensive Care Unit (ICU), serving a population of about 190,000 inhabitants. Patients were included aged 18 years or older admitted to a university hospital consecutively with SARS-CoV-2 infection from 26 February to 20 May 2020, both dates inclusive. SARS-CoV-2 infection was confirmed by polymerase chain reaction (PCR) diagnostic test on a nasopharyngeal exudate or sputum sample. If the result was negative, a second test was performed if the patient's clinical manifestations were highly suggestive of the disease and/or they had laboratory or radiological criteria suggestive of infection.17 Patients who refused to give their informed consent to treatments for SARS-CoV-2 infection approved in the hospital protocol were excluded, as were readmissions of the same patient, only the first hospital admission was considered. Pregnant or breastfeeding women were included. Patients were treated according to medical criteria and local treatment protocols, based on the recommendations of the health authorities, in line with the scientific evidence available at the time.18
The demographic data, comorbidities, clinical symptoms, laboratory results, radiological tests and treatment of each patient were obtained from the hospital's electronic medical records. The IMID included were rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), polymyalgia rheumatica (PRM), giant cell arteritis (GCA), Sjögren's syndrome (SS), vasculitis, systemic scleroderma (SSc), psoriasis (P), Crohn's disease (CD), ulcerative colitis (UC) and other autoimmune diseases (including Behçet's disease and inflammatory myopathies). PsA and Pso were included under psoriatic disease (PD). Data were also collected from patients receiving inflammatory disease treatment as a dichotomous yes/no variable, including biological treatments and JAK inhibitors. Clinical data were collected by direct download or by individualised manual review of the electronic health records. A secure data collection form was designed in the corporate health record software for the remaining demographic data, comorbidities, clinical symptoms, and radiological tests. To ensure correct collection, these forms were validated by the principal investigators, MRV and DEQ, before they were entered into the database, to improve the quality of the data collected and reduce missing data to a minimum. Personal data were dissociated and pseudo-anonymised in the database for further statistical analysis by an independent expert.
Statistical analysisFor the statistical analysis, quantitative variables are expressed as medians and interquartile range (IQR). The Student’s t-test for normally distributed variables and the Mann-Whitney U-test for non-normally distributed variables were used to analyse mean differences. Categorical variables are represented as absolute frequencies and percentages. Pearson’s χ2 test or Fisher's exact test was used to compare categorical variables, and if necessary, the Mantel-Haenszel test for trend. The level of statistical significance established for all the comparison tests was P < .05. The SPSS statistical package (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp.) was used for the statistical analysis and processing of the data.
The study protocol was approved on 22 June by the Ethics Committee for Research with Medicines (CEIm) prior to its implementation and classified by the Spanish Agency for Medicines and Health Products (AEMPS). Exemption was obtained from requesting written informed consent from patients.
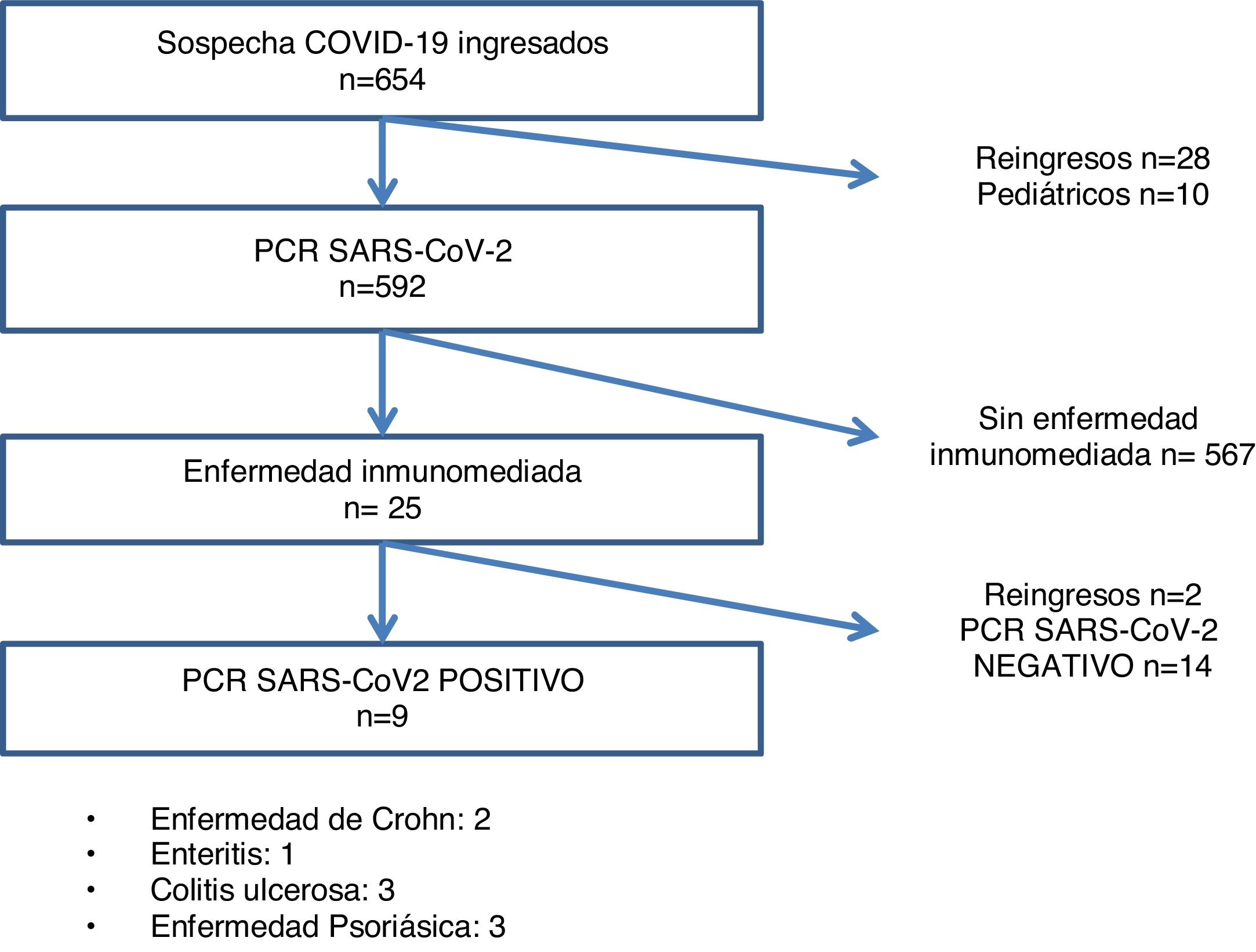
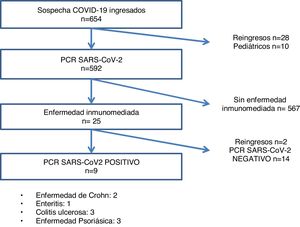
ResultsOf the 612 patients with suspected SARS-CoV-2 infection at the start of admission, 246 were PCR positive, and 23 had IMID, 9 of whom were confirmed as infectious disease cases by PCR test (Fig. 1). The most frequent IMID were the following in this order: psoriatic disease (43.5%), ulcerative colitis (26.1%), Crohn's disease (17.4%), and enteritis (13%) (Table 1).
Demographic characteristics and comorbidities of patients with immune-mediated disease stratified according to COVID-19.
| N (%) | Total (n = 23) | Not infected (n = 14) | Infected (n = 9) | P |
|---|---|---|---|---|
| Immune-mediated disease | ||||
| Crohn’s disease | 4 (17.4) | 2 (14.3) | 2 (22.2) | |
| Enteritis | 3 (13.0) | 2 (14.3) | 1 (11.1) | |
| Ulcerative colitis | 6 (26.1) | 3 (21.4) | 3 (33.3) | .55a |
| Psoriatic disease | 10 (43.5) | 7 (50.0) | 3 (33.3) | |
| Sex | ||||
| Female | 12 (52.2) | 7 (50.0) | 5 (55.6) | |
| Male | 11 (47.8) | 7 (50.0) | 4 (44.4) | 1.00 |
| Smoker | 2 (8.7) | 2 (14.3) | 0 (.0) | .50 |
| Obesity (BMI≥30 kg/m2) | 7 (30.4) | 2 (14.3) | 5 (55.6) | .07 |
| Hypertension | 11 (47.8) | 6 (42.9) | 5 (55.6) | .68 |
| Dyslipidaemia | 10 (43.5) | 7 (50.0) | 3 (33.3) | .67 |
| Diabetes | 4 (17.4) | 2 (14.3) | 2 (22.2) | 1.00 |
| Chronic kidney disease | 2 (8.7) | 2 (14.3) | 0 (0.0) | .50 |
| Cardiovascular disease | 5 (21.7) | 1 (7.1) | 4 (44.4) | .06 |
| COPD | 3 (13.0) | 3 (21.4) | 0 (.0) | .25 |
| Asthma | 3 (13.0) | 1 (7.1) | 2 (22.2) | .54 |
| Other chronic lung diseases | 5 (21.7) | 4 (28.6) | 1 (11.1) | .61 |
| Pulmonary embolism or deep vein thrombosis | 0 (.0) | 0 (.0) | 0 (.0) | NA |
| Cancer | 3 (13.0) | 3 (21.4) | 0 (.0) | .25 |
| HIV | 0 (.0) | 0 (.0) | 0 (.0) | NA |
| Home treatment | ||||
| Anticoagulant | 1 (4.3) | 1 (7.1) | 0 (.0) | 1.00 |
| ACEis or ARBs | 9 (39.1) | 5 (35.7) | 4 (44.4) | 1.00 |
| Biological drugs | 2 (8.7) | 1 (7.1) | 1 (11.1) | 1.00 |
| DMARDs | 13 (56.5) | 6 (42.9) | 7 (77.8) | .10 |
| Methotrexate | 4 (17.4) | 3 (21.4) | 1(11.1) | .63 |
| Leflunomide | 1 (4.3) | 0 (.0) | 1 (11.1) | .39 |
| Azathioprine | 3 (13.0) | 2 (14.3) | 1 (11.1) | 1.00 |
| Mesalazine | 5 (21.7) | 1 (7.1) | 4 (44.4) | .06 |
| Systemic glucocorticoids | 2 (8.7) | 0 (.0) | 2 (22.2) | .14 |
| No treatment | 3 (13.0) | 2 (14.3) | 1 (11.1) | 1.00 |
| Mean glucocorticoid dose/day (mg) (Prednisone or equivalent) | 0 | 5 | ||
ACEis: Angiotensin-converting enzyme inhibitors; ARBs: Angiotensin II receptor blockers; BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; DMARDs: Disease modifying anti-rheumatic drugs; HIV: Human Immunodeficiency Virus; NA: Not assessable.
Of the patients admitted with suspected SARS-CoV-2 infection (n = 612), 23 had IMID, 12 women (44.7%) and 11 men (55.3%), and there were no pregnant women. Of the 23 patients with IMID, 9 were diagnosed with SARS-CoV-2 infection, compared to 14 patients in whom SARS-CoV-2 infection was ruled out. There was no correlation between SARS-CoV-2 infection and IMID (P = .55). Similarly, no statistical differences were observed by age or sex between the infected and non-infected patients admitted with IMID: 5 infected women (55.6%) vs. 7 non-infected women (50%), 4 infected men (36.4%) vs. 7 non-infected men (50%).
Among the infected patients, PD was the most prevalent of the IMID, but no statistically significant differences were observed with respect to the other IMID detected.
The symptoms at the onset of the infection presented by the patients with IMID were similar in frequency to those of the rest of the patients studied. Fever was the most frequent symptom observed on admission (78.3%), followed by dyspnoea (43.5%) and dry cough (43.5%) (data pending publication).
On analysis of the comorbidities presented by the patients with IMID, no differences were observed with respect to the rest of the patients admitted in terms of demographic characteristics, cardiovascular risk factors or chronic lung diseases. However, there was a trend towards a higher percentage of obesity (38.5% vs. 22.7%) and treatment with angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin II receptor blockers (ARBs) (65.4% vs. 42.8%), compared to other patients admitted for suspected SARS-CoV-2 infection. Therefore, although the differences were not statistically significant, patients with IMID were more likely to have cardiovascular risk factors.
When analysing the 23 patients with IMID, differences that did not reach statistical significance between infected and non-infected individuals were observed in relation to cardiovascular disease (44.4% in infected vs. 7.1% in non-infected; P = .06), and obesity (55.6% in infected vs. 14.3% in non-infected P = .07). Regarding the presence of thromboembolic disease, arterial hypertension (HTN), diabetes mellitus (DM) or ischaemic heart disease, no differences were observed between infected and non-infected patients. Nor was there a difference in the use of home treatment with ACE inhibitors, anticoagulants, glucocorticoids (GC), anti-rheumatic disease-modifying drugs, or biological therapies.
None of the IMID patients had a flare of their underlying disease during their hospital stay.
We collected data in our cohort on the number of symptomatic days prior to hospital admission and the number of days of hospital stay. On analysis of this information, we observed no significant differences between patients with and without IMID. There were also no differences in ICU admissions between patients with and without IMID (3.8% vs. 3.9%). In terms of outcomes of death, which occurred in 43 infected patients (16.9%), we observed no differences between patients with or without IMID (19.2% vs. 16%, P = .73) either (Table 2).
Demographic characteristics and comorbidities of patients admitted for confirmed COVID-19 to the la Plana hospital.
| N (%) | Without immune-mediated disease | With immune-mediated disease | P |
|---|---|---|---|
| (n = 246) | (n = 9) | ||
| Age in years, median (IQR) | 70.6 (55.6−82.2) | 59.8 (58.6−63.6) | .23 |
| Sex | |||
| Female | 110 (44.7) | 5 (55.6) | .74 |
| Male | 136 (55.3) | 4 (44.4) | |
| Hypertension | 143 (58.1) | 5 (55.6) | 1.00 |
| Diabetes | 63 (25.6) | 2 (22.2) | 1.00 |
| Ischaemic heart disease | 22 (8.9) | 1 (11.1) | .58 |
| Chronic kidney disease | 49 (19.9) | 0 (.0) | .21 |
| COPD | 21 (8.5) | 0 (.0) | 1.00 |
| Asthma | 17 (6.9) | 2 (22.2) | .14 |
| Other chronic lung diseases | 23 (9.3) | 1 (11.1) | .60 |
| Heart failure | 21 (8.5) | 0 (.0) | 1.00 |
| Cirrhosis | 3 (1.2) | 1 (11.1) | .14 |
| Cancer | 31 (12.6) | 0 (.0) | .61 |
| Cardiovascular disease | 67 (27.2) | 4 (44.4) | .27 |
| Cerebrovascular disease | 17 (6.9) | 1 (11.1) | .49 |
| Dyslipidaemia | 105 (42.7) | 3 (33.3) | .74 |
| Smoker | 23 (9.3) | 0 (.0) | 1.00 |
| Obesity (BMI≥30 kg/m2) | 57 (23.2) | 5 (55.6) | .04 |
| HIV | 4 (1.6) | 0 (.0) | 1.00 |
| Dementia | 27 (11.0) | 0 (.0) | .60 |
| Pulmonary embolism or deep vein thrombosis | 4 (1.6) | 0 (.0) | 1.00 |
| Treatment | |||
| Anticoagulant | 30 (12.2) | 0 (.0) | .60 |
| ACEis or ARBs | 111 (45.1) | 4 (44.4) | 1.00 |
| Biological drugs | 1 (.4) | 1 (11.1) | .07 |
| Symptoms | |||
| Fever | 181 (73.6) | 9 (100.0) | .12 |
| Dyspnoea | 126 (51.2) | 4 (44.4) | .75 |
| Dry cough | 150 (61.0) | 6 (66.7) | 1.00 |
| Expectoration | 35 (14.2) | 1 (11.1) | 1.00 |
| Sore throat | 18 (7.3) | 1 (11.1) | .51 |
| Myalgia | 52 (21.1) | 1 (11.1) | .69 |
| Headache | 27 (11.0) | 1 (11.1) | 1.00 |
| Dizziness | 18 (7.3) | 1 (11.1) | .51 |
| Diarrhoea | 56 (22.8) | 2 (22.2) | 1.00 |
| General malaise | 128 (52.0) | 4 (44.4) | .74 |
| Anosmia | 17 (6.9) | 1 (11.1) | .49 |
| Ageusia | 18 (7.3) | 1 (11.1) | .51 |
| Chest pain | 16 (6.5) | 2 (22.2) | .13 |
| Outcome variable | |||
| Hospital discharge | 192 (78.0) | 6 (66.7) | .42 |
| Deaths | 41 (16.7) | 2 (22.2) | .65 |
| Median days of hospital stay (IQR) | 8.0 (4.0−15.0) | 9.0 (6.0−13.0) | .67 |
ACEis: Angiotensin-converting enzyme inhibitors; ARBs: Angiotensin II receptor blockers; BMI: Body Mass Index; COPD: Chronic Obstructive Pulmonary Disease; DMARDs: Disease modifying anti-rheumatic drugs; HIV: Human Immunodeficiency Virus; IQR: Interquartile Range; NA: Not assessable.
Unlike studies published to date, in our cohort we assessed the epidemiological and clinical characteristics of patients with IMID, who, due to severity on arrival at the hospital emergency department, required hospitalisation for suspected SARS-CoV-2 infection. The articles published to date on the prevalence of SARS-CoV-2 infection in patients with IMID in different geographical areas are mainly European.9,13,19 Furthermore, they describe the characteristics of patients with a history of IMID and the impact of the disease itself or related treatments on the morbidity and mortality of SARS-CoV-2 disease, which does not demonstrate an association between immunosuppressive treatments and respiratory complications associated with COVID-19 infection.20 Our study does not enable us to establish the population prevalence of SARS-CoV-2 disease among patients with IMID in our area, but it does allow us to describe the prevalence of SARS-CoV-2 infection among patients with IMID hospitalised for suspected infection, and their clinical course.
According to the distribution by disease observed in our study population, 23 patients had diseases included in the IMID spectrum. The diseases identified among patients admitted with IMID were PsA, Pso, CD and CU. No admissions were collected from patients with RA, AS, SLE, or other connective tissue diseases. These results are in line with those of other working groups,8,14,19 where the aggregate data of patients with IMID showed more cases of SARS-CoV-2 infection than those with other autoimmune diseases. No patients with PMR were recorded, even though this is a typical disease of advanced age, a factor associated with greater severity of viral infection, and for which the baseline treatment is GC. It could be that following health recommendations or due to mobility difficulties, the patients attended hospital less frequently. It is noteworthy that the prevalence of infection in SLE was lower than in other diseases, perhaps because using GC in SLE patients acts as a protective factor against severe COVID-19 disease, as treatment with GC in severe COVID-19 cases has been shown to be beneficial for the outcome of the infection.21,22 However, hydroxychloroquine, which is the standard treatment for SLE, has not been shown to improve the clinical course of SARS-CoV-2,23 despite the fact that it was administered to infected patients at the beginning of the pandemic under the hypothesis of improving the prognosis of the disease. The small number of IMID patients in our cohort means we cannot draw firm conclusions about the effect of inflammatory disease or underlying immunosuppressive therapies on the course of COVID-19 infection. However, it is striking that the immunocompromised patients do not have higher morbidity or mortality from SARS-CoV-2 compared to the other patients, as has been reported in other series.8,12
No pregnant patients with rheumatic diseases requiring admission due to SARS-CoV-2 infection were recorded in our cohort, as in other European groups,16 despite the greater predisposition to infection due to the association between pregnancy, IMID and immunosuppressive treatments.
The clinical presentation of the patients with IMID, as well as severity on admission, are like those of the other patients in our study cohort. Likewise, patients with rheumatological diseases in other series presented more frequently with dry cough, dyspnoea, and fever.8,12,19 However, as has been mentioned in other series of patients with chronic inflammatory diseases,24 in our population, patients with IMID have more comorbidities, and specifically there is a higher percentage of obesity. Obesity is a determining factor in cardiovascular disease in IMID, and has been recognised as a major cause of morbidity and mortality in these diseases. Obesity is also a risk factor for more severe SARS-CoV-225 infection and therefore a worse clinical outcome.
The baseline immunosuppressive treatment was discontinued in the patients admitted with IMID, in accordance with the recommendations of scientific societies.26,27 Nevertheless, no flare of the underlying disease was reported. The treatment received by the patients was identical to that of the other patients hospitalised for the infection, following the recommendations of the national guidelines and our hospital’s protocol. The mean number of days of hospital and ICU stay, and case fatality did not differ from that of the other infected patients (data pending publication). These results suggest the determinant role of IMID, and immunomodulatory or immunosuppressive treatments previously received by these patients in the management of SARS-CoV-2 infection and in the outcomes of these patients. Most immunosuppressive drugs used in IMID have a long half-life, and therefore withdrawal after diagnosis of the infection does not guarantee immediate elimination of the drug from the blood. Nevertheless, most of the patients affected by SARS-CoV-2 had a good clinical outcome, as has been demonstrated in other series,28 with no differences in the outcome of SARS-CoV-2 between patients with and without IMID.
As a limitation of our study, we should mention that we were only able to identify the most clinically severe cases of IMID patients since, on the recommendations of the health authorities, patients with milder symptoms were instructed to remain at home and only patients who consulted the hospital emergency department with admission criteria underwent PCR testing. Therefore, we were unable to establish the true prevalence of the infection in our study population or to make more realistic associations.
As this is a retrospective study, no causal relationships can be extracted from it. Moreover, as it was conducted in a single hospital, our results cannot be directly extrapolated to other healthcare settings.
From this study, we can conclude that immune-mediated diseases and their treatments do not seem to determine clinical outcomes after the hospital admission of patients infected with SARS-CoV-2.
FundingThis study was funded by a “Beca de Ayuda a la Investigación en Reumatología 2020” (Grant for Research Assistance in Rheumatology), awarded by the Fundación Valenciana de Reumatología.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank our colleagues at the hospital who were on the front line in treating patients during the COVID-19 pandemic. They also contributed to the data collection that was required to undertake this study.
Please cite this article as: Robustillo-Villarino M, Álvarez-Arroyo L, Carrera-Hueso FJ, Barreda-Altaba I, Nieto-Cid M, Girona-Sanz AM, et al. Características de pacientes con enfermedades inflamatorias inmunomediadas hospitalizados por infección por SARS-CoV-2. Reumatol Clin. 2022;18:331–337.