Inflammatory biomarkers have been used for the diagnosis and management of multisystemic inflammatory syndrome in children (MIS-C). We aimed to compare the clinical and laboratory findings of MIS-C cases versus other febrile cases cataloged as potentially suspected bacterial infection (non-MIS-C).
MethodsUnicentric ambispective observational cohort study (June 2020–February 2022). We analyzed demographics, clinical symptoms and laboratory findings in MIS-C cases and in non-MIS-C cases with febrile processes of patients under 15 years of age admitted to hospital.
ResultsWe enrolled 54 patients with potential suspected bacterial infection and 20 patients with MIS-C for analysis. Fever (100%), gastrointestinal (80%) and mucocutaneous findings (35%) were common in MIS-C patients, also hypotension (36.8%) and tachycardia (55%). Laboratory findings showed significantly elevated proBNP (70%), ferritin (35%), D-dimer (80%) and lymphopenia (55%) and thrombocytopenia (27.8%) in MIS-C cases. IL-6 values were high in non-MIS-C patients (92.6%).
ConclusionsIn the management of MIS‐C patients, the dynamic monitoring of proBNP, ferritin, D-dimer, lymphocytes and platelets could be helpful to pediatricians to effectively evaluate the progress of MIS‐C in the early phases, not IL-6 values. The applicability of the IL-6 level as a prognostic biomarker in MIS-C patients may require closer discussion. In addition, the optimal laboratory markers, as stated in our study, can help establish a biomarkers model to early distinguish the MIS-C versus non-MIS-C in patients who are admitted to febrile syndrome.
Los biomarcadores inflamatorios se han utilizado para el diagnóstico y tratamiento del síndrome inflamatorio multisistémico en niños (SIM-PedS). Nuestro objetivo fue determinar cómo se comportan estos biomarcadores inflamatorios en pacientes con síndrome febril orientados inicialmente como infección bacteriana potencialmente grave (IBPG) y comparar los hallazgos clínicos y de laboratorio con los casos SIM-PedS.
MétodosEstudio de cohorte observacional ambispectivo unicéntrico (junio 2020-febrero 2022). Analizamos la demografía, los síntomas clínicos y los hallazgos de laboratorio en casos SIM-PedS y en casos de síndrome febril de otras etiologías infecciosas de pacientes menores de 15 años con ingreso hospitalario.
ResultadosIncluimos a 54 pacientes con sospecha analítica de infección bacteriana y 20 pacientes con SIM-PedS para el análisis. La fiebre (100%), los hallazgos gastrointestinales (80%) y mucocutáneos (35%) fueron más frecuentes en los pacientes con SIM-PedS, también la hipotensión (36,8%) y la taquicardia (55%). Los hallazgos de laboratorio mostraron niveles significativamente elevados de proBNP (70 %), ferritina (35 %), dímeros D (80 %) así como linfopenia (55 %) y trombocitopenia (27,8 %) en los casos de SIM-PedS. Los valores de IL-6 fueron elevados en pacientes no SIM-PedS. (92,6%).
ConclusionesEn el manejo de pacientes con SIM-PedS, la monitorización dinámica de proBNP, ferritina, dímero D, linfocitos y plaquetas podría ser útil para evaluar efectivamente el progreso de la enfermedad en las primeras fases. Los valores de IL-6 pueden elevarse significativamente en pacientes con síndrome febril de otras etiologías, así como los dímeros D. El uso de diversos biomarcadores de laboratorio, podría ayudar a determinar precozmente la evolución de los pacientes con síndrome febril.
The determination of specific biomarkers of bacterial infection makes it easier to manage paediatric patients with febrile syndrome.
In December 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first appeared in China. In most children, COVID-19 infection was asymptomatic or symptoms were mild during the acute phase of infection.1 However, a new clinical condition emerged that was initially termed Multisystem Inflammatory Syndrome in Children (MIS-C) linked to SARS-CoV-2. This included cases of paediatric patients with clinical features that overlapped with other well-known paediatric conditions, such as Kawasaki disease or toxic shock syndrome.2
Due to the progressive increase in cases described with similar manifestations, the Royal College of Paediatrics and Child Health produced an initial description and named it multisystem inflammatory syndrome temporally associated with SARS-CoV-2. At the same time, the United States CDC (Center for Disease Control and Prevention) defined this as a multisystem inflammatory syndrome in children (MIS-C).
Along with the definition of this new condition, inflammatory analytical parameters were set down within the diagnostic criteria to try to stratify the severity of this and the risk of admission to intensive care units. Certain laboratory abnormalities, such as leukocytopenia, thrombocytopaenia, elevated B-type natriuretic propeptide (pro-BNP), and high ferritin and C-reactive protein (CRP) levels, appeared to be more closely associated with the occurrence of shock due to myocardial dysfunction.3 As a result of this, children who presented with this pro-inflammatory response required an accurate diagnosis and early initiation of immunosuppressive treatment.
New studies confirmed the presence of a hyperinflammatory syndrome in patients with MIS-C and differences were identified when comparing MIS-C with severe/non-severe COVID-19 infection.3 Analytical parameters were evaluated to determine prognostic markers of severity, such as pro-BNP, interleukin-6 (IL-6), and D-dimers (DD). These have been used previously in paediatric and adult patients as indicative values of inflammation in infectious processes. Some studies have been published in which IL-6 was used as a predictor of a high risk of sepsis in newborns and cancer patients with febrile neutropenia.4,5 In the same vein, Pro-BNP has been analysed as a prognostic value of paediatric septic shock, ventricular dysfunction, and cardiac markers in paediatric patients with sepsis.6,7
These inflammatory biomarkers may be useful in determining clinical severity in paediatric patients with severe multiorgan dysfunction, however threshold values in children with bacterial or viral infections are not clearly defined.
For these reasons, we considered it important to ascertain how these inflammatory biomarkers could behave in paediatric patients affected by infectious diseases unrelated to COVID-19 and who require hospitalisation. In addition, this could enable a more accurate estimate of the values used to stratify the risk of MIS-C and determine its severity and management.
MethodsThis was a single-centre, ambispective, observational study of patients in a second-level hospital during the period from June 2020 to February 2022. Included were patients under 15 years of age diagnosed with MIS-C during hospitalisation and patients under 15 years of age who met clinical or laboratory criteria for possible serious bacterial infection (PSBI) and who required admission to our centre for observation or treatment.
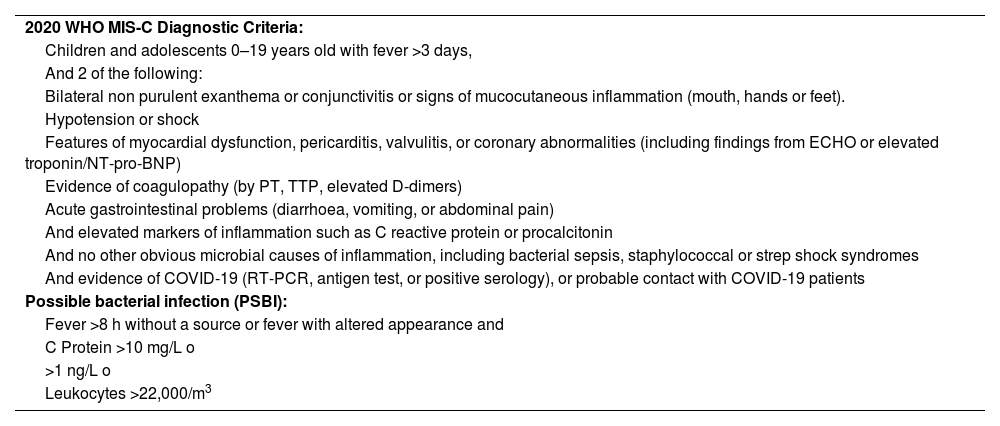
The inclusion parameters of MIS-C patients were based on the WHO MIS-C diagnostic criteria8 shown in Table 1. Patients in the PSBI group were treated using the clinical-analytical criteria for our centre (Table 1).
2020 WHO MIS-C Diagnostic Criteria and Criteria for Potentially Serious Bacterial Infection (PSBI).
| 2020 WHO MIS-C Diagnostic Criteria: |
| Children and adolescents 0–19 years old with fever >3 days, |
| And 2 of the following: |
| Bilateral non purulent exanthema or conjunctivitis or signs of mucocutaneous inflammation (mouth, hands or feet). |
| Hypotension or shock |
| Features of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including findings from ECHO or elevated troponin/NT-pro-BNP) |
| Evidence of coagulopathy (by PT, TTP, elevated D-dimers) |
| Acute gastrointestinal problems (diarrhoea, vomiting, or abdominal pain) |
| And elevated markers of inflammation such as C reactive protein or procalcitonin |
| And no other obvious microbial causes of inflammation, including bacterial sepsis, staphylococcal or strep shock syndromes |
| And evidence of COVID-19 (RT-PCR, antigen test, or positive serology), or probable contact with COVID-19 patients |
| Possible bacterial infection (PSBI): |
| Fever >8 h without a source or fever with altered appearance and |
| C Protein >10 mg/L o |
| >1 ng/L o |
| Leukocytes >22,000/m3 |
The Ethics Committee of our hospital approved the collection of data, analysis and dissemination of results in line with the Code of Best Practice.
The information on patients with MIS-C admitted from June 2020 to February 2021 was collected retrospectively through their electronic medical record. Patients diagnosed with MIS-C after February 2021 were prospectively incorporated into the study. Patients with suspected PSBI were included from February 2021 until the end of the study.
Epidemiological data recorded were as follows > sex, age, ethnicity, clinical manifestations (fever, gastrointestinal, neurological, mucocutaneous, respiratory symptoms), clinical signs (hypotension, tachycardia), laboratory parameters (C-reactive protein, procalcitonin, blood count values), therapeutic requirements and patients’ evolution.
One medical indication was to determine inflammatory biomarkers in all patients with clinical suspicion of MIS-C. In the case of patients with suspected PSBIs at the time of the initial blood test to determine the aetiology of the possible infection, we added the biochemical parameters of the study (IL-6, pro-BNP, DD, ferritin). Prior to this, informed consent was obtained from the legal representatives, along with the assent of the minors who were included.
These analytical parameters (IL-6, proBNP, DD, ferritin), in patients who did not meet the criteria for MIS-C, were determined for assessment within the study only and were not used for any therapeutic action or changes in the usual management, nor did they involve extraordinary, additional tests. Clinical and laboratory values were adjusted using normal age-specific ranges. In order to be considered abnormally elevated, the cut-off points for inflammatory biomarkers were taken from the review by Miller et al., where threshold values were set using the longest series of MIS-C published to date. Clinical parameters were recorded on admission. The laboratory results corresponded to admission and, if control tests were required during the clinical course, these were determined once again.
The final diagnoses of the patients studied were divided into 2 categories: MIS-C or non-MIS-C patients. Non-MIS-C patients were subclassified into bacterial infection, viral infection, suspected bacteraemia without microbiological confirmation, and others (parasitic infections or autoimmune diseases). Bacterial infections were diagnosed through cultures of biological samples. In viral infections, the germ was identified on nasopharyngeal swabs by reverse transcriptase polymerase chain reaction (RT-PCR) for respiratory viruses. We classified patients with laboratory suspicion of bacteraemia without microbiological confirmation as those in whom blood, urine and stool cultures were negative; those in whom no virus was isolated in the nasopharyngeal swab; and thirdly, cases where no positive serological results or any other aetiology causing fever had been identified. Parasitic infection was diagnosed with the detection of Plasmodium in the blood by RT-PCR.
The patients were included in the study prior to the use of the COVID-19 vaccine in children.
All related variables were analysed with SPSS software, using tests for the data distribution study (Kolmogorov–Smirnov) and comparing quantitative data (Student's t and Mann–Whitney U tests) as well as qualitative data (χ2, contingency table, Fisher's exact test). Continuous variables were expressed as mean and standard deviation, or as median and interquartile range (IQR), depending on their distribution. Categorical variables were expressed as absolute and relative frequencies (percentages). P values below 0.05 were considered significant. To measure the linear correlation between 2 data sets, we used Pearson's correlation coefficient (Spearman's rho in nonparametric variables).
ResultsEpidemiological dataA total of 54 patients with suspected PSBI and 20 patients with MIS-C were included for analysis. None of the patients died (Table 2). Of the patients included in the group with suspected PSBIs (non-MIS-C patients), the final diagnosis was bacterial infection in 40% (n = 22), viral infection in 25.9% (n = 14), laboratory suspicion of bacteraemia without microbiological confirmation in 22.2% (n = 12) and others in 11.1% (n = 6) of patients. Included in this category were infection by parasites (malaria, n = 3) and the final diagnosis of autoimmune disease.
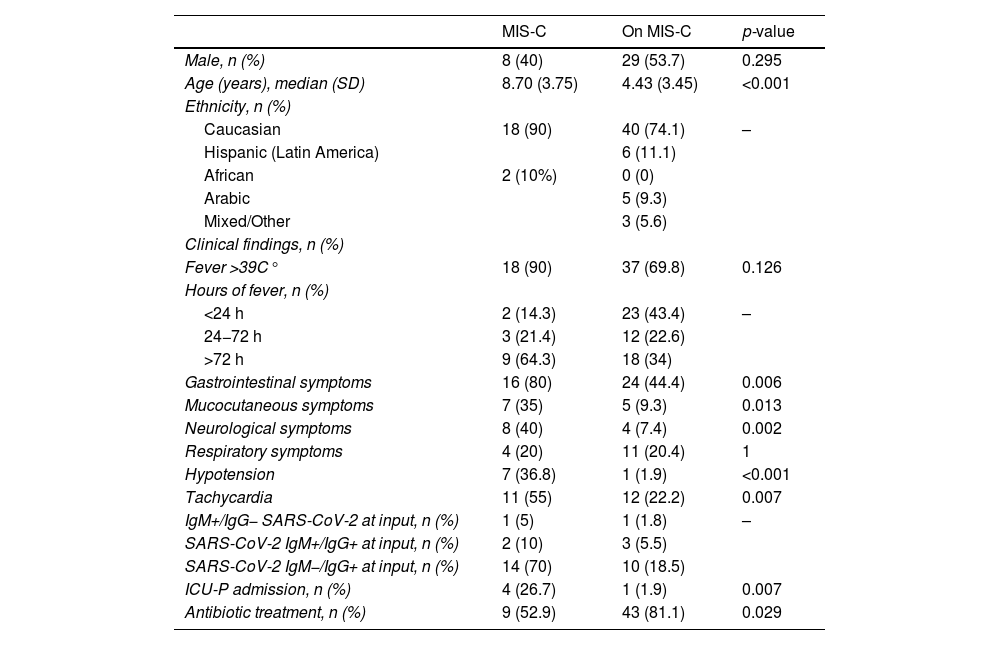
Multivariate analysis of MIS-C and non-MIS-C patients for epidemiological and clinical values.
| MIS-C | On MIS-C | p-value | |
|---|---|---|---|
| Male, n (%) | 8 (40) | 29 (53.7) | 0.295 |
| Age (years), median (SD) | 8.70 (3.75) | 4.43 (3.45) | <0.001 |
| Ethnicity, n (%) | |||
| Caucasian | 18 (90) | 40 (74.1) | – |
| Hispanic (Latin America) | 6 (11.1) | ||
| African | 2 (10%) | 0 (0) | |
| Arabic | 5 (9.3) | ||
| Mixed/Other | 3 (5.6) | ||
| Clinical findings, n (%) | |||
| Fever >39C° | 18 (90) | 37 (69.8) | 0.126 |
| Hours of fever, n (%) | |||
| <24 h | 2 (14.3) | 23 (43.4) | – |
| 24−72 h | 3 (21.4) | 12 (22.6) | |
| >72 h | 9 (64.3) | 18 (34) | |
| Gastrointestinal symptoms | 16 (80) | 24 (44.4) | 0.006 |
| Mucocutaneous symptoms | 7 (35) | 5 (9.3) | 0.013 |
| Neurological symptoms | 8 (40) | 4 (7.4) | 0.002 |
| Respiratory symptoms | 4 (20) | 11 (20.4) | 1 |
| Hypotension | 7 (36.8) | 1 (1.9) | <0.001 |
| Tachycardia | 11 (55) | 12 (22.2) | 0.007 |
| IgM+/IgG− SARS-CoV-2 at input, n (%) | 1 (5) | 1 (1.8) | – |
| SARS-CoV-2 IgM+/IgG+ at input, n (%) | 2 (10) | 3 (5.5) | |
| SARS-CoV-2 IgM−/IgG+ at input, n (%) | 14 (70) | 10 (18.5) | |
| ICU-P admission, n (%) | 4 (26.7) | 1 (1.9) | 0.007 |
| Antibiotic treatment, n (%) | 9 (52.9) | 43 (81.1) | 0.029 |
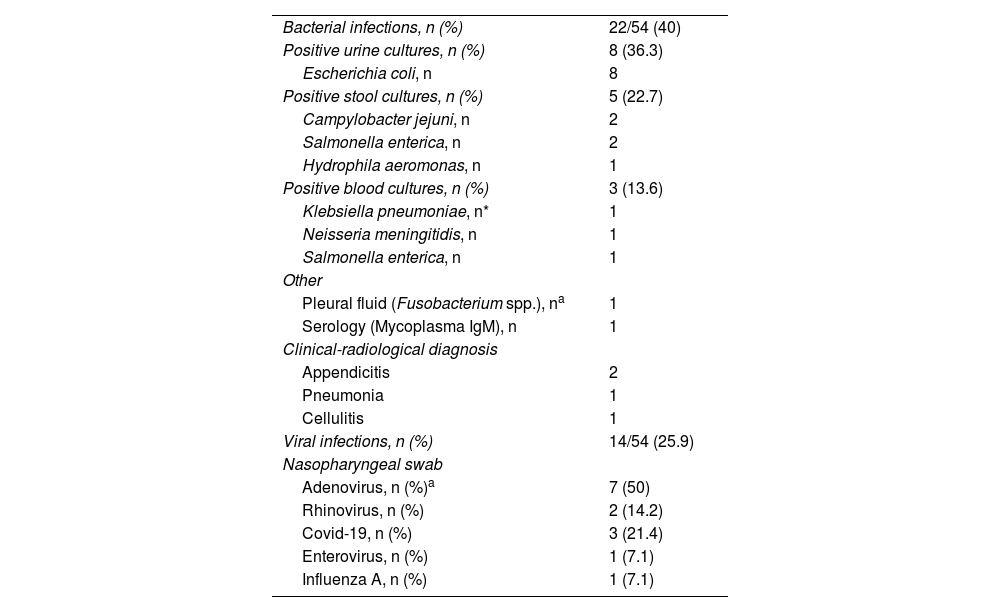
Patients with bacterial infections were distributed as follows: 36.3% (8/22) had urinary tract infection; 22.7% (5/22), enteroinvasive gastroenteritis; 18.1% (4/22) suspected pneumonia; 9% (2/22), appendicitis; 9% (2/22) had sepsis and 4.5% (1/22) had cutaneous cellulitis (Table 3).
Results of biological samples from patients with suspected PSBI.
| Bacterial infections, n (%) | 22/54 (40) |
| Positive urine cultures, n (%) | 8 (36.3) |
| Escherichia coli, n | 8 |
| Positive stool cultures, n (%) | 5 (22.7) |
| Campylobacter jejuni, n | 2 |
| Salmonella enterica, n | 2 |
| Hydrophila aeromonas, n | 1 |
| Positive blood cultures, n (%) | 3 (13.6) |
| Klebsiella pneumoniae, n* | 1 |
| Neisseria meningitidis, n | 1 |
| Salmonella enterica, n | 1 |
| Other | |
| Pleural fluid (Fusobacterium spp.), na | 1 |
| Serology (Mycoplasma IgM), n | 1 |
| Clinical-radiological diagnosis | |
| Appendicitis | 2 |
| Pneumonia | 1 |
| Cellulitis | 1 |
| Viral infections, n (%) | 14/54 (25.9) |
| Nasopharyngeal swab | |
| Adenovirus, n (%)a | 7 (50) |
| Rhinovirus, n (%) | 2 (14.2) |
| Covid-19, n (%) | 3 (21.4) |
| Enterovirus, n (%) | 1 (7.1) |
| Influenza A, n (%) | 1 (7.1) |
Fever was identified in all patients upon admission. The median duration of fever was 4 days in both groups, with an IQR of2–7 in non-MIS-C patients and an IQR of 3–7 in MIS-C. Gastrointestinal symptoms (abdominal pain, diarrhoea, vomiting) were the most common clinical findings in both groups. MIS-C patients also had mucocutaneous (rash, conjunctivitis, lymphadenopathy) (35%) and neurological (confusion, headache, seizures) (40%) symptoms, with statistically significant differences between this group and the non-MIS-C group (Table 2).
SARS-CoV-2 antibodies (IgM or IgG) were positive in 85% (n = 17) of patients with MIS-C. Only one of the patients had active infection (IgM positive and IgG negative) and the remaining 16 had positive IgG with negative IgM. Of the non-MIS-C patients, one of these had positive IgM with negative IgG and only 24% of them had had previous COVID-19 infection (IgG positive for SARS-CoV-2). The diagnosis of COVID-19 in the acute phase (diagnosed by RT-PCR or rapid antigen test) had only been reached in 18.5% of non-MIS-C patients and 25% of MIS-C patients (Table 2).
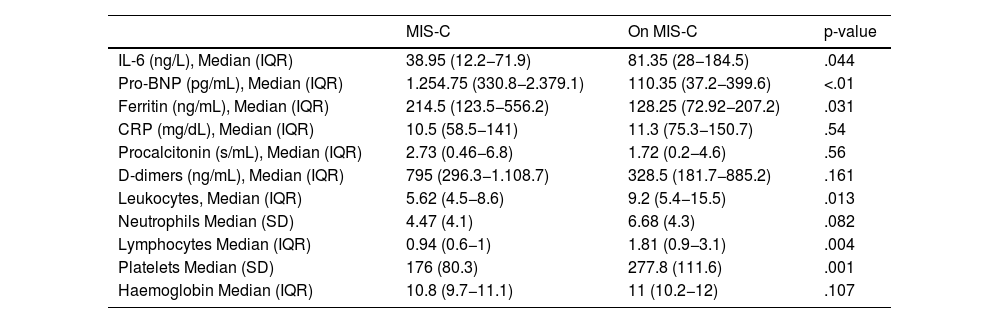
Lab resultsThe results of the laboratory tests are shown in Table 4. Elevated IL-6 levels were >30 ng/L in 90% (n = 18) of patients with MIS-C and 92.6% (n = 50) of non-MIS-C patients. Abnormally elevated inflammatory markers observed in patients with MIS-C included pro-BNP (defined by >400 pg/mL) in 70% of cases in that group, compared to non-MIS-C patients, where this was elevated in only 24.1% of cases. In patients with MIS-C, ferritin was elevated in 35% of patients (defined as >300 ng/mL), C-reactive protein in 75% (defined as >0.6 mg/dL), procalcitonin (PCT) in 75% (defined as >0.5 ng/mL), and DDs in 80% (defined as >250 ng/mL). However, in non-MIS-C patients, ferritin was elevated in 13% of cases, C-reactive protein in 83.3%, PCT in 68.5%, and DDs in 63%. We compared the median pro-BNP in patients with tachycardia, which stood at 336 pg/mL (IQR: 47.4−1,999.5), while in the non-tachycardia cases this was 132.4 pg/mL (IQR 46.6–494) with p = 0.052 in the Mann-Whitney U test. Otherwise, the median pro-BNP in patients with hypotension was 863.6 pg/mL (IQR: 329−2.684), while in non-hypotensive patients this was 141.4 pg/mL (IQR: 47–522.3) with p = 0.028.
Laboratory findings, multivariate analyses, comparing MIS-C and non-MIS-C cases.
| MIS-C | On MIS-C | p-value | |
|---|---|---|---|
| IL-6 (ng/L), Median (IQR) | 38.95 (12.2−71.9) | 81.35 (28−184.5) | .044 |
| Pro-BNP (pg/mL), Median (IQR) | 1.254.75 (330.8−2.379.1) | 110.35 (37.2−399.6) | <.01 |
| Ferritin (ng/mL), Median (IQR) | 214.5 (123.5−556.2) | 128.25 (72.92−207.2) | .031 |
| CRP (mg/dL), Median (IQR) | 10.5 (58.5−141) | 11.3 (75.3−150.7) | .54 |
| Procalcitonin (s/mL), Median (IQR) | 2.73 (0.46−6.8) | 1.72 (0.2−4.6) | .56 |
| D-dimers (ng/mL), Median (IQR) | 795 (296.3−1.108.7) | 328.5 (181.7−885.2) | .161 |
| Leukocytes, Median (IQR) | 5.62 (4.5−8.6) | 9.2 (5.4−15.5) | .013 |
| Neutrophils Median (SD) | 4.47 (4.1) | 6.68 (4.3) | .082 |
| Lymphocytes Median (IQR) | 0.94 (0.6−1) | 1.81 (0.9−3.1) | .004 |
| Platelets Median (SD) | 176 (80.3) | 277.8 (111.6) | .001 |
| Haemoglobin Median (IQR) | 10.8 (9.7−11.1) | 11 (10.2−12) | .107 |
Leukocytopenia (<1.0 × 10 9/L) was present in 55% (n = 11) of MIS-C cases, and 35% also had thrombocytopenia (<15 × 109/L) compared to 27.8% and 13%, respectively, of non-MIS-C patients.
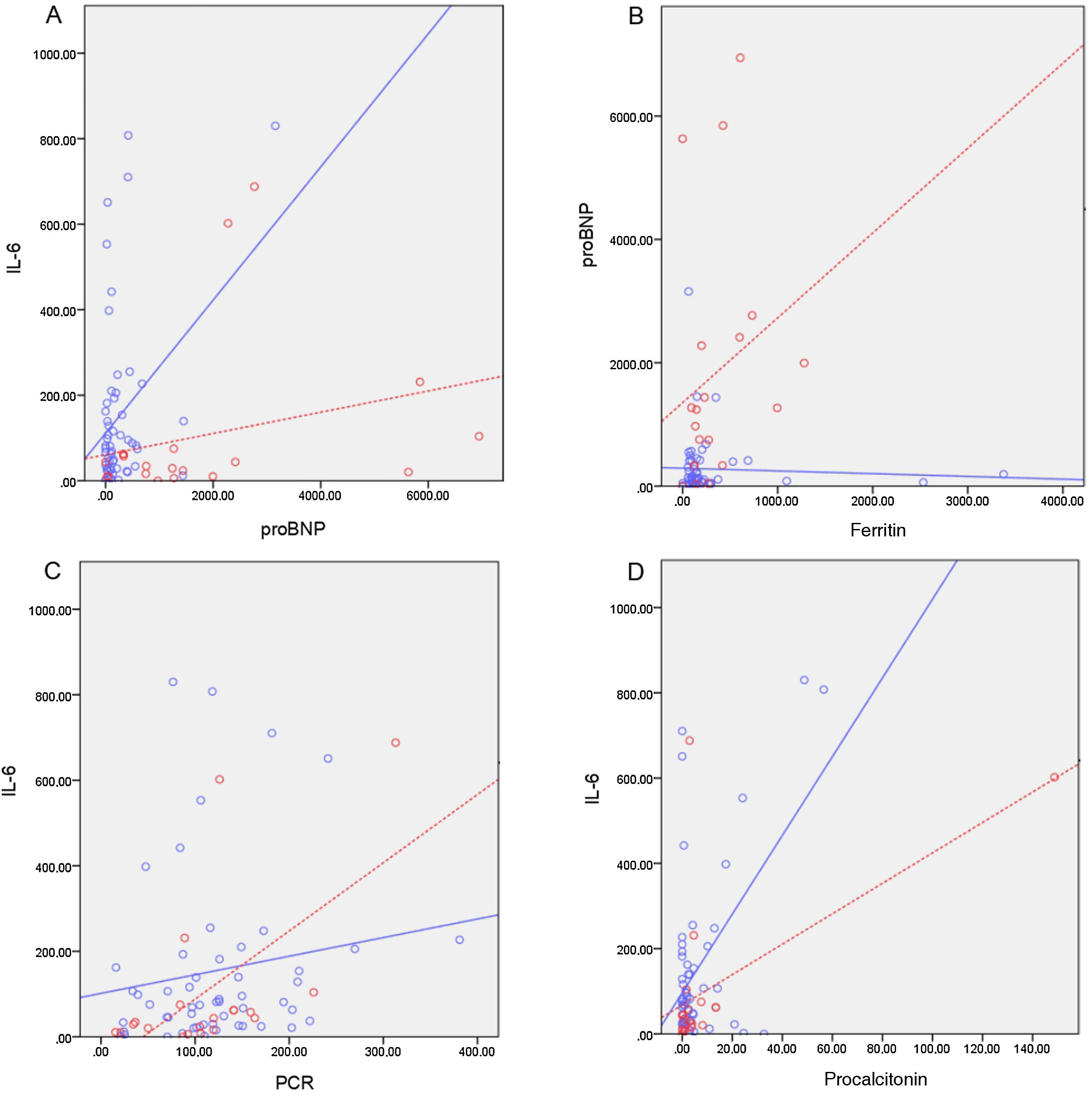
In the bivariate correlation, we found no significant correlation between the 2 predominant inflammatory parameters (IL-6 and pro-BNP) in MIS-C patients (rho = 0.364; p = 0.115) or in non-MIS-C patients (rho = 0.185; p = 0.182). In MIS-C patients, there is a significant correlation between IL-6 and CRP (rho = 0.569; p = 0.009), IL-6 and PCT (rho = 0.562; p = 0.01) and between pro-BNP and ferritin (rho = 0.452; p = 0.045) (Fig. 1).
Bivariate correlation between inflammatory markers in patients with MIS-C diagnosis (dashed line) vs. patients with suspected PSBI (solid line). (A) Correlation IL-6/proBNP MIS-C patients (rho = 0.364, p = 0.115), non-MIS-C(rho = 0.185; p = 0.182). (B) MIS-C(rho = 0.452; p = 0.045), non-MIS-C(rho = 0.127; p = 0.361) pro-BNP/ferritin patients correlation. (C) Correlation IL-6/CRP MIS-Cpatients (rho = 0.569; p = 0.009), non-MIS-C(rho = 0.217; p = 0.116). (D) Correlation MIS-C IL-6/PCT patients (rho = 0.562; p = 0.01), non-MIS-C(rho = -0.026; p = 0.854).
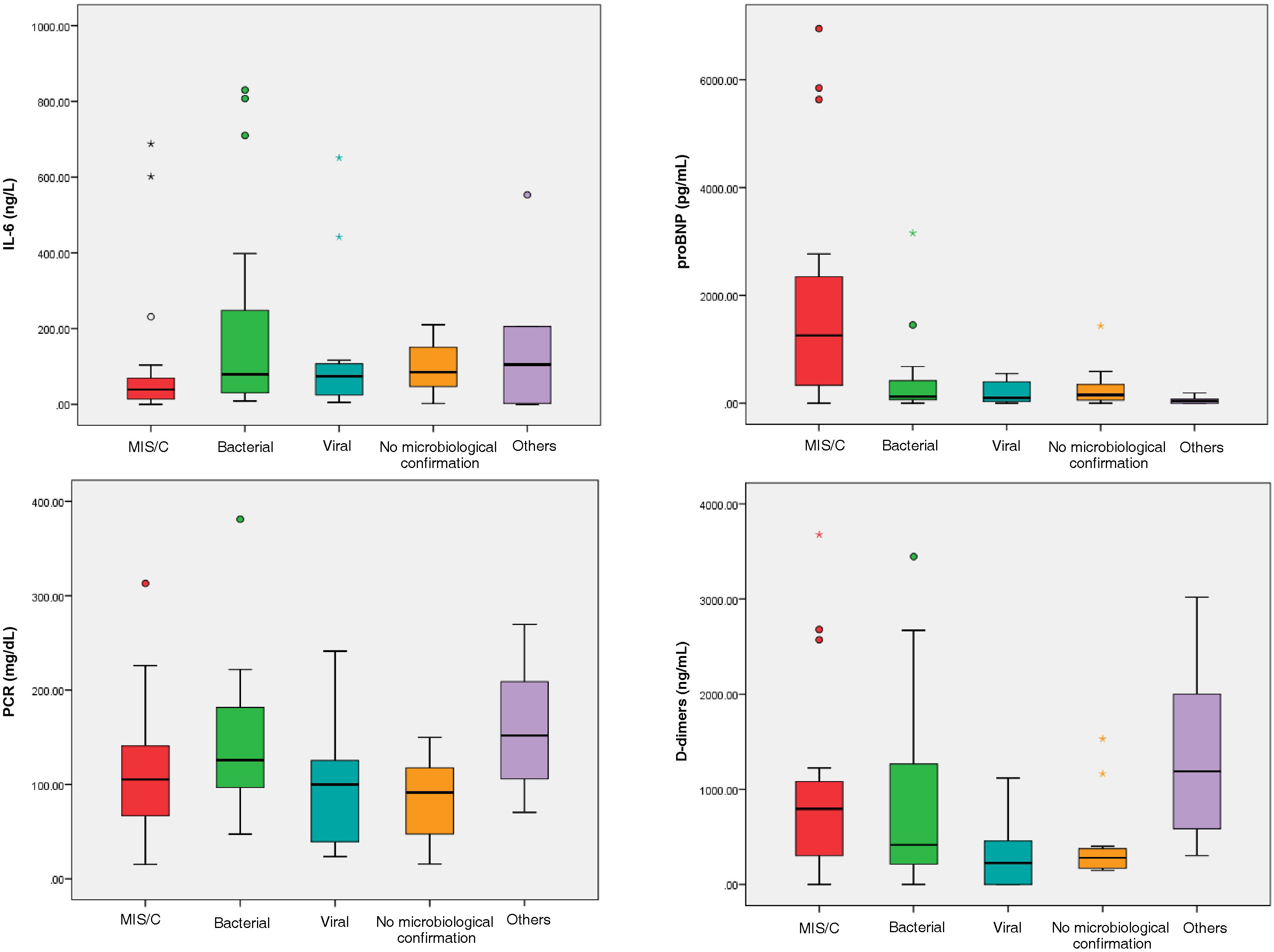
We analysed inflammatory markers by subcategories in non-MIS-C patients compared to MIS-C patients and observed that the median IL-6 was higher in bacterial infections (78.95 ng/L) and autoimmune diseases (107.87 ng/L) than in MIS-C patients (38.95 ng/L) (Fig. 2). The median pro-BNP was markedly higher in patients with MIS-C (1,254.75 pg/mL) than in other subgroups. However, the median CRP was similar in all patients in the group and median DDs were elevated in patients with MIS-C (795 ng/mL) as well as in autoimmune disease in non-MIS-C patients (1,190 ng/mL).
DiscussionWith regard to COVID-19 infection, the new condition of MIS-C was defined and clinical criteria set, with analytical values being related to this data, as possible predictors of the severity of this syndrome.3
Given the use of these biomarkers for the MIS-C, we considered the possibility of determining whether these analytical parameters could also be used in other paediatric patients with febrile syndrome to determine their usefulness as markers of severity. In this study, we compared the differences between paediatric patients with MIS-C and children with febrile syndrome with elevated inflammatory markers.
After review, higher levels of pro-BNP and ferritin and lower levels of lymphocytes and platelets were reported in patients with MIS-C than in other aetiologies of febrile syndromes.
In our sample, we found that the median age in children with MIS-C was higher (8.7 years) than in non-MIS-C children (4.4 years), a finding which was statistically significant. The median age of patients with MIS-C was similar to that reported in other reviews.9 Our hypothesis is that infectious processes tend to be more frequent in early childhood, while this new condition predominantly affects older children. The median number of days of fever at the time of consultation was the same for both groups, but the IQR was 2–7 days in non-MIS-C patients, perhaps associated with the fact that they were the youngest patients, who tend to present for examination earlier as they are a more vulnerable age group. There were also differences in relation to gender: MIS-C predominated in females (60%), unlike previously published series.10 The proportion of children of African ethnicity with MIS-C (10%) was higher than in the other infectious aetiologies but lower than in other reports in the literature.10
The most frequently presented symptoms in patients with MIS-C were fever (100%), high fever (>39) which was particularly notable and in 90°% of cases, compared to febrile syndromes of other causes (69.8%). Gastrointestinal (80%) and mucocutaneous (35%) symptoms were more frequent in the MIS-C group. In our cases, we detected 40% of neurological symptoms. This figure was higher than in other reviews, where it is usually10%, however headache was also included as a neurological symptom in our sample, a frequent manifestation in other series (40%).11 We found that the presence of hypotension and tachycardia was more frequent in patients with MIS-C (36.8% and 55%, respectively) than in patients with non-MIS-C febrile syndrome (1.9% and 22.2%), with values similar to the study carried out by Miller et al., with the largest sample of MIS-C published.11 These signs could be taken as risk factors for developing more severe disease with possible cardiovascular involvement, as has already been described in patients with MIS-C.12
Our lab results, which compared IL-6 values determined by MIS-C with non-MIS-C, appeared not to be good predictors of severe disease in patients with MIS-C. We found that in non-MIS-C patients, IL-6 values had a higher median (81.95 ng/L) than in MIS-C (38.95 ng/L).
Some published studies reveal that in severe cases of COVID-19 there is an overactivated immune response and that IL-6 values predict the severity of the disease.13,14 However, Leisman et al.15 determined in a meta-analysis conducted in adult patients that IL-6 concentrations in patients with severe COVID-19 were lower than those reported in patients with sepsis or respiratory distress without COVID-19, consistent with our results. Although IL-6 has been identified as a biomarker in patients with MIS-C in studies conducted only in patients with this clinical condition, there are no comparative studies of this analytical parameter in paediatric patients with febrile syndrome. In our case series, although IL-6 was elevated in MIS-C, we saw that this was even higher in patients with febrile syndrome due to another aetiology, as has been reported in studies conducted in the adult population.15 From this point of view, the applicability of IL-6 levels as a prognostic biomarker in patients with MIS-C might require further discussion.
Conversely, in our study, several non-cytokine biomarkers, including pro-BNP, ferritin, and DD, were more elevated in patients with MIS-C than in patients with non-MIS-C.
Median pro-BNP values were higher in MIS-C patients (1,254.75 pg/mL) than in non-MIS-C patients (110.35 pg/mL). In addition, a statistically significant association was found between elevated pro-BNP values and the presence of cardiovascular signs, such as tachycardia and hypotension. Because MIS-C is a pro-inflammatory state, cardiac markers are monitored in the management of the disease. In the meta-analysis by Zhao et al.,12 the results indicated that the pro-BNP levels of patients with severe MIS-C were higher than those of patients with non-severe MIS-C, as we have seen in our results.
Ferritin is a parameter related to pro-inflammatory states and has been used as a biomarker of sepsis in children.16 In our study, only 35% of patients with MIS-C had elevated ferritin, however, we observed significantly higher values in patients with MIS-C (214.5 ng/mL) than in patients with fever of a different aetiology (128.25 ng/mL). The increase in ferritin appears to be an inflammatory response mediated by stimulation of pro-inflammatory cytokines such as IL-6.16 Our results show that the main biomarkers present in patients with MIS-C (pro-BNP and ferritin) also had a significant correlation (Fig. 2).
We found higher DD values in patients with MIS-C (795 ng/mL), without significant differences but with much higher values than in bacterial infections (416.5 ng/mL). In patients with autoimmune diseases or malaria these were much higher (1,150.5 ng/mL). Studies have detected elevated DD in patients with sepsis and have considered it a specific marker,17 although in our sample we did not analyse patients with sepsis but rather patients with fever and suspected infection.
Our results showed no significant differences in CRP and PCT between patients with or without MIS-C. However, a correlation was observed in elevation between IL-6 and CRP, and IL-6 and PCT in patients with MIS-C. This may be useful for interpreting results in patients with MIS-C but it may not be a prognostic marker. The increase in CRP levels is mainly induced by IL-6, which would justify its correlation in our study.18
Patients with MIS-C in our sample had significant values of leukocytopaenia (55%) and thrombocytopenia (35%) compared to other febrile syndromes. These results are similar to those of a large study with 4,901 patients with MIS-C in which 60% of patients with MIS-C were found to have severe haematological involvement, including thrombocytopaenia (42.3%) and lymphopaenia (35.3%).11 These findings appear to be due to changes in the immune landscape during the course of MIS-C, the acute phase of which is characterised by activation of the innate immune system with T-cell and B-cell lymphocytopaenia, which normalise during the recovery process.19
This study has limitations. In all patients, the laboratory parameters were determined on admission, however not homogeneously in successive examinations. When comparing patients, it was not possible to be sure that all of the patients were at the same stage of clinical evolution. The number of children with MIS-C (n = 20) included is a limitation and weakness of this study. The sample, although producing significant differences in the results, had low statistical power. We did not analyse information on the time-frame in relation to hours of fever for most of the laboratory tests; therefore, laboratory findings can be used more as indicative markers, rather than predictors, of severe outcomes.
However, we believe that from this study it would be possible to increase the sample through other patient series and evaluate the use of time-based associations between laboratory markers and the evolution of febrile syndrome to determine the predictive potential of biomarkers. By increasing the sample size, this could even be useful to determine threshold values or biomarker cut-off points for severity and the possibility of developing a prognostic index or initial management of these patients.
ConclusionsDue to the severity of MIS-C cases, it was necessary to determine analytical parameters that would be useful in guiding the initial management and possible evolution of this new condition. In children, the presence of fever and its association with sepsis can be difficult. Therefore, trying to determine reliable biomarkers to distinguish the severity of febrile illness will be extremely useful.
Through our descriptive and comparative study of the different cohorts, we tried to identify which parameters could be most useful to distinguish MIS-C in patients with febrile syndrome and those that are not MIS-C. Initially, a history of COVID-19 infection or serological detection was helpful in diagnosis, however now a large part of the population has a history of infection or positive antibodies, in addition to possible vaccination. In addition, we attempted to determine if some of the analytical values that had been reported in other studies as severity factors of MIS-C could also be useful as biomarkers with greater association to bacterial infections in febrile syndromes in children.
After our analysis, dynamic monitoring of pro-BNP, ferritin, DD, lymphocytes and platelets will be useful for paediatricians as indicative markers of cases of MIS-C in the early stages, however IL-6 values will be less useful in the management of patients with MIS-C.
We believe that additional studies with larger sample sizes using time-based associations could help determine plasma biomarkers for the initial management and prognosis of paediatric patients with febrile syndrome.
FinancingThis research has not received any specific support from public sector agencies, the commercial sector or non-profit bodies.
Conflict of interestThe authors declare they have no conflict of interest.