To assess the evolution of cost per patient/year and the cost per patient/year/drug in patients with rheumatoid arthritis (RA) receiving biological treatments. To analyze and quantify the factors influencing this evolution, such as the optimization of the biological drugs, the use of biosimilars, and official discounts and discounts obtained after negotiated procedures. In addition, to assess specific clinical parameters of disease activity in these patients.
MethodsRetrospective, observational study conducted in a Spanish tertiary hospital. Adult patients diagnosed with RA under treatment from 2009 to 2017 were included.
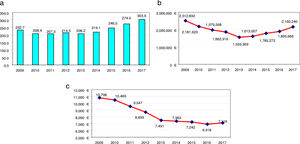
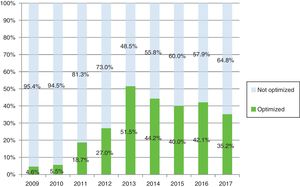
Results320, 270 and 389 patients were included in 2009, 2013 and 2017, respectively. The patient/year cost decreased from 10,789€ in 2009, 7491€ in 2013 to 7116€ in 2017. In 2017, due to the established competition, discounts of 14% and 29.5% were achieved on etanercept and its biosimilar; 11.5%, 17.8%, 17.9%, 17.3% on adalimumab, certolizumab, golimumab and tocilizumab IV respectively, and 24.6% and 43.1% on infliximab and its biosimilar. The percentage of patients optimized in 2017 was 35.2%. The annual saving in 2017 was 1,288,535€ (830,000€ due to dose optimization and/or administration regimens, 249,666€ corresponding to 7.5% of the official discount and 208,868€ after negotiated procedures).
ConclusionThe annual cost per patient in RA decreased considerably due to different factors, such as discounts on the purchase of drugs due to official discounts and negotiated procedures, together with the optimization of therapies, the latter being the factor that contributed most to this decrease.
Evaluar la evolución del coste por paciente/año y del coste por paciente/año/medicamento en pacientes en tratamientos con biológicos con artritis reumatoide (AR). Analizar y cuantificar los factores influyentes en dicha evolución tales como la optimización de medicamentos biológicos, el uso de biosimilares y los descuentos oficiales y los obtenidos tras procedimientos negociados. Además, evaluar parámetros clínicos de la actividad propios de la enfermedad en dichos pacientes.
MétodosEstudio retrospectivo, observacional, realizado en un hospital terciario español. Se incluyeron pacientes adultos diagnosticados de AR en tratamiento con biológicos desde 2009 a 2017.
ResultadosSe incluyeron 320, 270 y 389 pacientes en 2009, 2013 y 2017, respectivamente. El coste paciente/año disminuyó de 10.798€ en 2009, 7.491€ en 2013 a 7.116€ en 2017. En 2017, debido a la competencia establecida, se alcanzaron descuentos del 14 y del 29,5% en etanercept y su biosimilar; 11,5, 17,8, 17,9 y 17,3% en adalimumab, certolizumab, golimumab y tocilizumab IV, respectivamente, así como un 24,6% y 43,1% en infliximab y su biosimilar. El porcentaje de pacientes optimizados en 2017 alcanzó el 35.2%. El ahorro anual en 2017 fue de 1.288.535€ (830.000€ debido a la optimización de dosis y/o pautas de administración, 249.666€ correspondiente al 7,5% del descuento oficial y 208.868€ tras procedimientos negociados).
ConclusiónEl coste anual por paciente en AR disminuyó considerablemente debido a diferentes factores, tales como, descuentos en la adquisición de medicamentos debido a descuentos oficiales y procedimientos negociados, junto a la optimización de terapias, siendo este último el factor que más contribuyó en dicho descenso.
Rheumatoid arthritis (RA) is a chronic, progressive auto immune disease that causes severe articular damage and functional impotence in the affected joints.1 The prevalence of RA is reported to be 0.5%–1% in developed countries, with a higher prevalence among females (ratio 2:1).1,2 Its prevalence is 0.5% in Spain.3 The therapy of RA aims at early disease control and induction of sustained remission; successful treatment is reflected by sustained quality of live and ability to work.4
Treatment with nonsteroidal anti-inflammatory drugs, conventional disease-modifying antirheumatic drugs and biologic treatment have been assessed in individuals with RA.5 Biologics drugs (BD) approved for use in RA include TNF inhibitors (TNFi), Tocilizumab (Tcz), Rituximab (Rtx), Abatacept (Aba) and Janus kinase inhibitors tofacitinib and baricitinib (Jak).6–8 The TNFi registered for the indication of RA are adalimumab (Ada), certolizumab pegol (Ctz), etanercept (Etn), golimumab (Goli), and infliximab (Ifx)5,7,9; TNFi have improved outcomes for patients who are refractory or intolerant to conventional treatments, inducing long-term remission in some cases.10,11 If TNFi fails, switching to another TNFi or an agent with another mode of action should be considered.6–9 The cost of BD for treating rheumatic diseases has dramatically increased in Spanish hospitals.12 Due to the high cost of BD, it is important to evaluate real costs of use of these agents.13
Aims of the studyThe main objective was to calculate the annual cost per patient and the cost of each biological treatment of patients with RA in real practice in a tertiary hospital in Spain for eighteen years (2009–2017). Other secondary objectives were to analyze factors related to treatment costs (the prescription of biosimilars instead of original drugs, discounts and negotiated rebates or biologic regimes optimization according to drug and anti-drug antibodies serum levels.14–16
MethodsWe conducted a retrospective observational study between 2009 and 2017 approved by Ethics Committee of La Paz University hospital in April 2017.
Patients diagnosed of RA who were dispensed BD by the pharmacy department in the study period were included. These dispensations were recorded in a CPOE program (FarmaTools 2.5 Dominion). This software allows pharmacists to register regimes, drugs and unit-drugs used by patient and related them with costs.
Inclusion criteriaAdult patients with RA followed in the rheumatology unit in our hospital were included.
Clinical data were obtained from the La Paz Biological Registry of Rheumatology database, created by the hospital's rheumatology department. Disease activity was measured by the Disease Activity Score 28 (DAS28) and the Simplified Disease Activity Index (SDAI).17 Remission was defined as achieving a DAS28<2.6 and SDAI≤3; low disease activity were defined as ≥2.6 DAS28<3.2, and >3.3 SDAI≤11; moderate activity were defined as ≥3.2 DAS28<5.1, and >11 SDAI≤26; and high activity were defined as DAS28>5.1 and SDAI>26.6,17 These parameters, as well as C-reactive protein (C-RP) and erythrocyte sedimentation rate, were measured every 3–6 months for clinical disease assessment.
Costs were calculated according to direct cost of BD dispensed. Drugs prices used were those set out by the Spanish Medicines Agency.18 Costs associated with concomitant medications, laboratory tests or a switch from initial therapy that affected the overall cost were excluded.
Main variables and secondary variablesTo calculate main outcomes such as average-dispensed-patient, the annual cost per average-patient and annual cost per average-patient per drug we applied a standardized methodology used by the public health system of the Community of Madrid. In addition, other variables such the theoretical cost per drug(units) acquired, annual theoretical cost per drug, total cost savings and the cost savings as a result of biological therapy optimization were calculated.15,16
Biological therapy optimization by monitoring drug and ADA serum levelsWe also evaluated annual costs per patient and per drug savings due to biologic therapies optimization. Optimized therapies were defined as those in which the dosing interval were extended and/or the dose of biological drug was reduced. Total percentages of patients with optimized therapies and per drug were calculated.
Statistical analysisThe results were expressed as percentage, mean and standard deviation (SD). All tests were performed using IBM SPSS version 19.0. Differences in patients’ characteristics were examined using the analysis of variance (ANOVA) model or a t-test for continuous variables (age, DAS28, SDAI, C-RPC, ESR). Differences in costs were examined using analysis of trends (Joinpoint Regression Program® 4.5.01-June, 2017). Significant values were defined as P<.05.
ResultsIn 2009, 2013, and 2017 were treated with BD 320, 270, and 389 patients respectively. Patient's characteristics are shown in Table 1. No statistically significant difference was found between study groups except in DAS28.
Characteristics of patients.
| 2009 | 2013 | 2017 | P(*) (between groups) | |
|---|---|---|---|---|
| Dispensed patient | 320 | 270 | 389 | |
| Ages (years) | 56.94 (14.51) | 57.87 (13.25) | 58.20 (14.72) | .562 (NS) |
| Gender (female) | 236 (73.75%) | 218 (80.74%) | 321 (85.55%) | |
| DAS28 | 3.57 (1.35) | 3.31 (1.25) | 3.21 (1.29) | .012 (S) |
| SDAI | 11.86 (11.85) | 10.93 (11.13) | 9.97 (9.55) | .303 (NS) |
| CRP-C | 5.69 (9.09) | 6.51 (13.62) | 6.39 (12.89) | .366 (NS) |
| ESR | 22.09 (15.92) | 18.93 (13.28) | 21.18 (16.17) | .086 (NS) |
Data are expressed as mean (SD) for continuous variables and frequencies (percentage) for categorical variables
DAS28: Disease Activity Score28; SDAI: Simplified Disease Activity Index.
CRP-C: C-Reactive Protein; ESR Erythrocite Sedimentation Rate.
Statistical signification P<.05; (*) ANOVA test.
Fig. 1 shows the evolution of the results for RA per average-dispensed-patient, annual cost, and annual cost per average-dispensed-patient. “We observed an average-dispensed-patient decrease from 2009 to 2013, and an upward trend from 2014 to 2017 (Fig. 1a)”. The same tendency in terms of annual cost of RA was observed, with a minimum data in 2013 (Fig. 1b)”. However, the annual cost per patient decreased (P<.001 from 2009 to 2013) (Fig. 1c).
When evaluating costs according to each drug used a similar trend was observed (Table 2). Annual cost per patient from 2009 to 2017 decreased significantly for Ifx and Eta (P<.001), Ctz and Aba IV (P<.05), and from 2009 to 2013 for Ada (<.001) and Toci IV (P<.05).
Economic data evolution per drug from 2009 to 2017 in rheumatoid arthritis.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|---|---|---|---|
| Adalimumab | |||||||||
| Average dispensed patient | 62.12 | 59.91 | 59 | 52.7 | 41.54 | 35.52 | 35.17 | 34.24 | 30.73 |
| Annual cost (€) | 781.698 | 722.026 | 596.887 | 488.699 | 301.058 | 239.691 | 245.295 | 233.874 | 215.512 |
| Annual cost per average patient (€) | 12.584 | 12.052 | 10.117 | 9.273 | 7.247 | 6.748 | 6.975 | 6.830 | 7.013 |
| Incremental difference annual cost | −4.2% | −16.1% | −8.3% | −21.8% | −6.9% | 3.4% | −2.1% | 2.7% | |
| Etanercept | |||||||||
| Average dispensed patient | 73.58 | 68.58 | 66.53 | 70.44 | 73.48 | 69.92 | 70.46 | 78.91 | 86.16 |
| Annual cost (€) | 802.414 | 737.450 | 660.645 | 633.196 | 543.150 | 556.287 | 554.835 | 549.650 | 579.680 |
| Annual cost per average patient (€) | 10.905 | 10.753 | 9.930 | 8.989 | 7.392 | 7.956 | 7.874 | 6.966 | 6.728 |
| Incremental difference annual cost | −1.4% | −7.7% | −9.5% | −17.8% | 7.6% | −1.0% | −11.5% | −3.4% | |
| Certolizumab | |||||||||
| Average dispensed patient | – | 0.5 | 8.6 | 17.95 | 22.23 | 24.75 | 33 | 37.53 | 44.48 |
| Annual cost (€) | – | 4.260 | 87.869 | 172.010 | 178.747 | 194.765 | 246.084 | 281.660 | 335.283 |
| Annual cost per average patient (€) | – | 8.520 | 10.217 | 9.583 | 8.041 | 7.869 | 7.457 | 7.505 | 7.538 |
| Incremental difference annual cost | −6.2% | −16.1% | −2.1% | −5.2% | 0.6% | 0.4% | |||
| Golimumab | |||||||||
| Average dispensed patient | – | – | 1.83 | 1 | 1.41 | 3.9 | 4.6 | 9.16 | 11.38 |
| Annual cost (€) | – | – | 14.521 | 9.564 | 14.573 | 33.965 | 37.326 | 85.081 | 96.171 |
| Annual cost per average patient (€) | – | – | 7.935 | 9.564 | 10.335 | 8.709 | 8.114 | 9.288 | 8.451 |
| Incremental difference annual cost | 8.1% | −15.7% | −6.8% | 14.5% | −9.0% | ||||
| Infliximab | |||||||||
| Average dispensed patient | 64.54 | 47.83 | 37.72 | 31.6 | 22.78 | 25.29 | 29.98 | 24.09 | 27.68 |
| Annual cost (€) | 628.903 | 439.037 | 283.243 | 187.865 | 123.575 | 142.822 | 188.394 | 103.430 | 92.414 |
| Annual cost per average patient (€) | 9.744 | 9.179 | 7.509 | 5.945 | 5.425 | 5.647 | 6.284 | 4.293 | 3.339 |
| Incremental difference annual cost | −5.8% | −18.2% | −20.8% | −8.7% | 4.1% | 11.3% | −31.7% | −22.2% | |
| Tocilizumab IV | |||||||||
| Average dispensed patient | – | 3.87 | 10.66 | 16.2 | 23.79 | 27.17 | 25.35 | 17.52 | 20.92 |
| Annual cost (€) | – | 46.437 | 118.381 | 167.048 | 199.503 | 210.997 | 185.996 | 121.669 | 158.076 |
| Annual cost per average patient (€) | – | 11.999 | 11.105 | 10.312 | 8.386 | 7.766 | 7.337 | 6.945 | 7.556 |
| Incremental difference annual cost | −7.5% | −7.1% | −18.7% | −7.4% | −5.5% | −5.3% | 8.8% | ||
| Tocilizumab SC | |||||||||
| Average dispensed patient | – | – | – | – | – | – | 9.38 | 20.91 | 28.9 |
| Annual cost (€) | – | – | – | – | – | – | 68.410 | 154.759 | 240.921 |
| Annual cost per average patient (€) | – | – | – | – | – | – | 7.293 | 7.401 | 8.336 |
| Incremental difference annual cost | 1.5% | 12.6% | |||||||
| Abatacept IV | |||||||||
| Average dispensed patient | 7.16 | 4.6 | 7.08 | 8.93 | 7.95 | 7.81 | 8.5 | 8.46 | 10.21 |
| Annual cost (€) | 81.131 | 53.252 | 81.497 | 89.371 | 66.972 | 67.728 | 82.066 | 69.025 | 90.261 |
| Annual cost per average patient (€) | 11.331 | 11.577 | 11.511 | 10.008 | 8.424 | 8.672 | 9.655 | 8.159 | 8.840 |
| Incremental difference annual cost | 2.2% | −0.6% | −13.1% | −15.8% | 2.9% | 11.3% | −15.5% | 8.3% | |
| Abatacept SC | |||||||||
| Average dispensed patient | – | – | – | – | – | – | 8.52 | 12.26 | 12.86 |
| Annual cost (€) | – | – | – | – | – | – | 80.151 | 99.619 | 102.692 |
| Annual cost per average patient (€) | – | – | – | – | – | – | 9.407 | 8.126 | 7.985 |
| Incremental difference annual cost | −13.6% | −1.7% | |||||||
| Rituximab | |||||||||
| Average dispensed patient | 24.4 | 21.99 | 15.67 | 15.43 | 14.98 | 23.58 | 21.54 | 29.71 | 29.51 |
| Annual cost (€) | 210.095 | 169.293 | 134.357 | 115.163 | 128.359 | 135.331 | 177.886 | 202.985 | 242.484 |
| Annual cost per average patient (€) | 8.610 | 7.699 | 8.574 | 7.464 | 8.569 | 5.739 | 8.258 | 6.833 | 8.217 |
| Incremental difference annual cost | −10.6% | 11.4% | −12.9% | 14.8% | −33.0% | 43.9% | −17.3% | 20.3% | |
| Baricitinib VO | |||||||||
| Average dispensed patient | – | – | – | – | – | – | – | – | 0.33 |
| Annual cost (€) | – | – | – | – | – | – | – | – | 2.508 |
| Annual cost per average patient (€) | – | – | – | – | – | – | – | – | 7.600 |
| Tofacitinib VO | |||||||||
| Average dispensed patient | – | – | – | – | – | – | – | – | 0.42 |
| Annual cost (€) | – | – | – | – | – | – | – | – | 3.266 |
| Annual cost per average patient (€) | – | – | – | – | – | – | – | – | 7.776 |
Biologics acquired by our center (units) as well as the data for BD dispensed (units) to patients with RA are shown in Table 3. We detected that there was an increase in the number of marketed BD and total savings per drug. In order of appearance, official discounts and negotiated rebates in 2017 were 11.5% for Ada, 15.5% for Eta, 30.9% for Ifx, 17.9% for Goli, 17.8 for Ctz and 17.4% for Rtx, and 19.8% for Aba SC, 15.9% for Tcz, 10.3% for Bari and 7.5% for Tofa, the latest released biological. We checked that the release of a biosimilar infliximab increased the rebates up to 43.1% in 2017, with a gradual increase in bonus units over time while original Ifx rebate reached a 24.6%.
Official discounts and negotiated rebates from 2009 to 2017.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Adalimumab | Total acquired | |||||||||
| Rebates (€) | 3.161 | 183,172 | 374,030 | 301,885 | 306,498 | 322,854 | 305,937 | 531,078 | 360,828 | |
| Rebates (% Unit) | 0.2% | 6.7% | 12.8% | 11.1% | 11.5% | 12.1% | 11.6% | 16.7% | 11.5% | |
| Bonus (U) | 0 | 0 | 314 | 200 | 202 | 128 | 166 | 606 | 282 | |
| Dispensed units (U) in RA | 1.489 | 1.438 | 1.260 | 1.023 | 635 | 501 | 487 | 551 | 508 | |
| Rebates in RA (€) | 1.198 | 5.415 | 87,209 | 61,100 | 40,038 | 33,454 | 26,629 | 46,500 | 27,326 | |
| Etanercept | Total acquired | |||||||||
| Rebates (€) | 37,680 | 152,295 | 202,444 | 240,499 | 209,525 | 228,183 | 262,751 | 260,054 | 297,629 | |
| Rebates (% Unit) | 1.5% | 6.1% | 8.8% | 10.3% | 10.3% | 10.6% | 12.6% | 12.4% | 15.5% | |
| Bonus (U) | 0 | 0 | 0 | 0 | 0 | 0 | 2.400 | 23,500 | 26,600 | |
| Dispensed units (U) in RA | 165,425 | 160,050 | 146,925 | 143,075 | 122,960 | 128,700 | 132,775 | 141,500 | 160,750 | |
| Rebates in RA (€) | 15,061 | 59,908 | 79,207 | 74,039 | 61,421 | 67,608 | 79,733 | 80,189 | 104,404 | |
| Infliximab | Total acquired | |||||||||
| Rebates (€) | 3.313 | 114,842 | 269,141 | 413,595 | 391,423 | 340,626 | 561,199 | 286,529 | 746,340 | |
| Rebates (% Unit) | 0.1% | 4.8% | 10.0% | 13.4% | 15.1% | 12.7% | 19.6% | 11.7% | 30.90% | |
| Bonus (U) | 0 | 0 | 65 | 112 | 134 | 0 | 118 | 84 | 34 | |
| Dispensed units (U) in RA | 1.131 | 821 | 561 | 390 | 262 | 265 | 109 | 271 | 323 | |
| Rebates in RA (€) | 935 | 22,206 | 32,831 | 33.115 | 21.908 | 20.880 | 35.927 | 18.502 | 43.842 | |
| Golimumab | Total acquired | |||||||||
| Rebates (€) | 10,455 | 71,370 | 60,224 | 134,736 | 197,754 | 83,426 | 91,867 | 142,098 | ||
| Rebates (% Unit) | 100.0% | 37.0% | 30.3% | 25.8% | 30.2% | 17.7% | 13.7% | 17.90% | ||
| Bonus (U) | 0 | 50 | 14 | 0 | 13 | 7 | 0 | 43 | ||
| Dispensed units (U) in RA | 9 | 25 | 12 | 21 | 39 | 47 | 107 | 127 | ||
| Rebates in RA (€) | 10,455 | 13,024 | 3.950 | 6.373 | 14,662 | 7.953 | 14,205 | 20,119 | ||
| Certolizumab | Total acquired | |||||||||
| Rebates (€) | 13,064 | 25,783 | 61,679 | 72,288 | 92,400 | 87,027 | 77,024.6 | 88,822 | ||
| Rebates (% Unit) | 32.3% | 22.9% | 24.6% | 26% | 25.5% | 21% | 17.7% | 17.80% | ||
| Bonus (U) | 10 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | ||
| Dispensed units (U) in RA | 14 | 240 | 462 | 490 | 552 | 701 | 777 | 929 | ||
| Rebates in RA (€) | 13,064 | 22,742 | 55,874 | 62,803 | 69,489 | 65,387 | 61,769 | 73,741 | ||
| Rituximab | Total acquired | |||||||||
| Rebates (€) | 0 | 48,633 | 82,676 | 89,485 | 95,321 | 161,332 | 197,217 | 217,623 | 256,074 | |
| Rebates (% Unit) | 0 | 4.20% | 7.50% | 7.50% | 7.50% | 13.20% | 15.10% | 15.0% | 17.40% | |
| Bonus (U) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dispensed units (U) in RA | 163 | 136 | 112 | 96 | 107 | 148 | 165 | 186 | 237 | |
| Rebates in RA (€) | 0 | 6.916 | 8.760 | 8.893 | 9.817 | 24,616 | 30,271 | 34,245 | 47,876 | |
| Abatacept | Total acquired | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| IV(mg) | IV(mg) | IV(mg) | IV(mg) | IV(mg) | IV(mg) | SC (units) | IV(mg) | SC (units) | ||
| Rebates (€) | 0 | 1.567 | 7.861 | 22,331 | 9.399 | 8.792 | 984 | 10,238 | 9.640 | |
| Rebates (% Unit) | 0.00% | 2.80% | 7.50% | 16.40% | 11.40% | 7.50% | 7.50% | 7.50% | 10.00% | |
| Bonus (U) | 0 | 0 | 4.500 | 4.500 | 2.500 | 0 | 0 | 0 | 12 | |
| Dispensed units (U) in RA | 58,250 | 39,250 | 63,250 | 76,000 | 56,750 | 72,250 | 60 | 62,500 | 416 | |
| Rebates in RA (€) | 0 | 1.567 | 7.078 | 19,908 | 8.603 | 5.105 | 984 | 6.630 | 9.348 | |
| Tocilizumab | Total acquired | |||||||||
| Rebates (€) | – | 6.680 | 13,971 | 28,766 | 73,108 | 74,536 | – | 54,698 | 20,045 | |
| Rebates (% Unit) | – | 5.60% | 7.50% | 9.30% | 21.20% | 20.90% | – | 16.10% | 22.10% | |
| Bonus (U) | – | 0 | 0 | 0 | 0 | 0 | – | 0 | 8 | |
| Dispensed units (U) in RA | – | 27,200 | 70,520 | 101,480 | 135,040 | 152,680 | – | 122,840 | 368 | |
| Rebates in RA (€) | – | 4.285 | 9.498 | 17,778 | 56,081 | 55,211 | – | 37,081 | 20,049 | |
| Abatacept | Total acquired | 2016 | 2017 | Oral drugs | Total acquired | 2017 | |||
|---|---|---|---|---|---|---|---|---|---|
| IV(mg) | SC (units) | IV(mg) | SC (units) | Baricitinib | Tofacitinib | ||||
| Rebates (€) | 9.027 | 27,504 | 18,736 | 31,083 | Rebates (€) | 580 € | 152.203 € | ||
| Rebates (% Unit) | 7.50% | 19.40% | 14.20% | 19.20% | Rebates (% Unit) | 10.30% | 19.80% | ||
| Bonus (U) | 0 | 76 | 0 | 80 | Bonus (U) | 0 | 0 | ||
| Dispensed units (U) in RA | 54,500 | 562 | 75,750 | 601 | Dispensed units (U) in RA | 4 | 4 | ||
| Rebates in RA (€) | 5.704 | 24,228 | 14,979 | 25,486 | Rebates in RA (€) | 0 | 0 | ||
| Tocilizumab | Total acquired | ||||||||
| Rebates (€) | 51,188 | 46,757 | 60,088 | 49,436 | |||||
| Rebates (% Unit) | 14.50% | 21.30% | 17.30% | 15.90% | |||||
| Bonus (U) | 0 | 72 | 0 | 0 | |||||
| Dispensed units (U) in RA | 80,960 | 843 | 110,760 | 1177 | |||||
| Rebates in RA (€) | 23,080 | 44,040 | 33,984 | 47,191 | |||||
U: Dispensed or Bonus Units.
RA: Rheumatoid arthritis.
Disease activity decreased annually in patients with optimized regimes when compared with patients without optimized regimes (P<.001) (Table 4a).
Clinical characteristics of patients according to the optimizations of their treatments.
| 2013 | P(*) | 2017 | P(*) | |
|---|---|---|---|---|
| DAS28 optimized group | 2.77(0.97) | <.001 | 2.64(0.96) | <.001 |
| DAS28 not optimized group | 4.00(1.25) | 3.61(1.34) | ||
| SDAI optimized group | 6.11(6.02) | <.001 | 5.42(4.93) | <.001 |
| SDAI not optimized group | 16.96(12.38) | 13.06(10.64) | ||
| CRP-C optimized group | 3.38 (5.5) | .004 | 4.70(7.93) | .244 |
| CRP-C not optimized group | 9.44 (17.39) | 7.65(15.54) | ||
| ESR optimized group | 17.62 (11.93) | .553 | 19.31(14.22) | .197 |
| ESR not optimized group | 19.68 (13.93) | 22.63(17.47) |
As Fig. 2 shows, active patients and percentage using optimized regimes from 2009 to 2017, reached 51.5% and 35.2% of patients with optimization by 2013 and 2017 respectively. The optimized therapies per drug and annually was analyzed (Table 4b).
Number of active patients and % patients with optimized therapies per drug.
| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | Active pat | Opt (%) | |
| Etn | 87 | 2.3% | 85 | 3.5% | 81 | 18.5% | 83 | 28.9% | 78 | 59.0% | 76 | 51.3% | 78 | 50.0% | 83 | 57.8% | 93 | 39.8% |
| Ada | 71 | 0.0% | 69 | 2.9% | 64 | 21.9% | 56 | 37.5% | 39 | 76.9% | 36 | 69.4% | 38 | 52.6% | 36 | 41.7% | 27 | 55.6% |
| Ctz | 3 | 0.0% | 14 | 0.0% | 18 | 11.1% | 26 | 34.6% | 31 | 29.0% | 33 | 36.4% | 43 | 34.9% | 44 | 29.5% | ||
| Goli | 2 | 0.0% | 1 | 0.0% | 2 | 0.0% | 4 | 50.0% | 7 | 14.3% | 11 | 36.4% | 13 | 30.8% | ||||
| Tcz SC | 2 | 0.0% | 16 | 31.3% | 27 | 25.9% | 29 | 31.0% | ||||||||||
| Aba SC | 2 | 0.0% | 14 | 0.0% | 14 | 14.3% | 13 | 7.7% | ||||||||||
| Ifx | 63 | 12.7% | 47 | 14.9% | 38 | 44.7% | 32 | 59.4% | 25 | 60.0% | 25 | 52.0% | 20 | 55.0% | 15 | 73.3% | 14 | 57.1% |
| Bios Ifx | 0 | 0.0% | 4 | 0.0% | 11 | 0.0% | 10 | 20.0% | ||||||||||
| Rtx | 32 | 0.0% | 36 | 0.0% | 24 | 0.0% | 20 | 0.0% | 22 | 13.6% | 31 | 9.7% | 37 | 24.3% | 42 | 19.0% | 43 | 34.9% |
| Tcz IV | 10 | 0.0% | 18 | 0.0% | 26 | 0.0% | 33 | 42.4% | 33 | 54.5% | 27 | 66.7% | 18 | 55.6% | 25 | 32.0% | ||
| Aba IV | 10 | 20.0% | 3 | 66.7% | 11 | 9.1% | 12 | 8.3% | 6 | 33.3% | 11 | 18.2% | 11 | 45.5% | 10 | 40.0% | 14 | 28.6% |
| Oral drugs | 5 | 0.0% | ||||||||||||||||
| Total | 263 | 4.6% | 253 | 5.5% | 252 | 18.7% | 248 | 27.0% | 231 | 51.5% | 251 | 44.2% | 285 | 42.1% | 310 | 40.0% | 330 | 35.2% |
Data are expressed as mean (SD) for continuous variables and frequencies (percentage) for categorical variables.
DAS28: Disease Activity Score 28; SDAI: Simplified Disease Activity Index.
CRP-C: C-Reactive Protein; ESR Erithrocite Sedimentation Rate.
Statistical signification P<.05; (*) T-Test Mann–Whitney U.
Active pat: number of active patients per drug.
Opt (%): Percentage of patients with optimized therapies per drug.
Etn: Etanercept; Ada: Adalimumab; Ctz: Certolizumab; Goli: Golimumab; Tcz: Tocilizumab; Aba: Abatacept; Ifx: Infliximab; Bios Ifx: Biosimilar Ifx; Rtx: Rituximab; Oral drugs: Bariticinib and Tofacitinib.
Costs evolution according to the factors studied (results in 2017 are shown in Table 5). Thus, costs savings related to therapy optimization (830,000€), costs savings by monitoring drug and anti-drug antibody (ADA) serum levels in 2017 represented a 73.87% (613,101€).
Calculation of different factors that have an impact on costs in 2017.
| Annus | Theoretical | Theoretical cost | Average | Theoretical | Annual | Saved | Rebates, | Saved Optimized |
|---|---|---|---|---|---|---|---|---|
| Drugs | Unit per annus | (Unit or mg) | Dispensed pat | Annual cost € (A) | Cost (€)(B) | Cost (€)(A-B) | Discount €(C) | Regimes €(A-B-C) |
| RA 2017 | ||||||||
| Certolizumab | 30 | 442 | 44.48 | 589.804,80 | 335.283 | 254.521,80 | 73.441,00 | 181.080,80 |
| Etanercept | 2.600,00 | 4.28 | 86.16 | 958.788,48 | 579.680 | 379.108,48 | 104.113,00 | 274.995,48 |
| Adalimumab | 26 | 480.54 | 30.73 | 383.941,85 | 215.512 | 168.429,85 | 46.500,00 | 121.929,85 |
| Rituximab | 8 | 1.261,77 | 29.51 | 297.878,66 | 242.484 | 55.394,66 | 47.876,00 | 7.518,66 |
| Abatacept SC | 52 | 218.59 | 12.86 | 146.175,50 | 102.692 | 43.483,50 | 25.486,00 | 17.997,50 |
| Abatacept IV | 9.100,00 | 1.37 | 10.21 | 127.288,07 | 90.261 | 37.027,07 | 14.979,00 | 22.048,07 |
| Infliximab+BIOSIM | 1.499,40 | 4.18 | 27.68 | 173.484,18 | 92.414 | 81.070,18 | 43.842,00 | 37.228,18 |
| Tocilizumab IV | 7.280,00 | 1.74 | 20.92 | 264.997,82 | 158.076 | 106.921,82 | 33.984,00 | 72.937,82 |
| Tocilizumab SC | 52 | 243.91 | 28.9 | 366.547,95 | 240.921 | 125.626,95 | 47.191,00 | 78.435,95 |
| Golimumab | 13 | 921.63 | 11.38 | 136.345,94 | 96.171 | 40.174,94 | 20.119,00 | 20.055,94 |
| Baricitinib (envase) | 13 | 706.06 | 0.33 | 3.029,00 | 2.508 | 521 | 580 | −59 |
| Tofacitinib (envase) | 13 | 706.06 | 0.42 | 3.855,09 | 7.600 | −3.744,91 | 424 | −4.168,91 |
| Total 2017 | 303.58 | 3.452.137,34 | 2.163.602 | 1.288.535,34 | 458.535,00 | 830.000,34 | ||
Theoretical annual cost: Theoretical unit per annus×Theoretical cost (unit or mg)×Average-dispensed-patient.
Average dispensed pat: average-dispensed-patient.
Saved cost (€): Theoretical annual cost−Annual cost.
Saved optimized regimes: Theoretical annual cost−Annual cost−Rebates and discounts.
Moreover, costs savings by drugs monitoring were 88.08% (322,882€) in 2011, 75.38% (797,906€) in 2013 and a 79.19% (730,810€) in 2015.
Moreover, we found that from 2009 to 2017 the total savings increased (Table 6). The greatest contribution to economic savings was therapy optimization (24.93%). Savings associated with official discounts and negotiated rebates (13.77%) in 2017 (Table 6).
Quantification of influential factor that affect on treatment costs in rheumatoid arthritis.
| Rheumatoid arthritis | 2009 | % | 2011 | % | 2013 | % | 2015 | % | 2017 | % |
|---|---|---|---|---|---|---|---|---|---|---|
| Annual cost (€) (AC) | 10.798,00 € | 95.00% | 9.547,00 | 77.51% | 7.491,00 | 59.55% | 7.242,00 | 61.23% | 7.116,00 | 61.29% |
| Theoretical annual cost (€)(TAC) | 11.367 € | 100.00% | 12.317 € | 100.00% | 12.579 € | 100.00% | 11.828 € | 100.00% | 11.610 € | 100.00% |
| Difference (€): (TAC) – (AC) | 568.51 € | 5.00% | 2.769,78 € | 22.49% | 5.087,96 € | 40.45% | 4.585,65 € | 38.77% | 4.493,95 € | 38.71% |
| Total saved cost (€): | 132.268,16 € | 0.58% | 574.193,00 | 22.49% | 1.062.529,00 | 40.45% | 1.049.073,00 | 38.77% | 1.288.535,34 | 38.71% |
| * Rebates+bonus+offitial discount (€) | 17.193,50 € | 0.08% | 254.837,19 | 9.98% | 264.622,48 | 10.07% | 318.262,83 | 11.76% | 458.535,00 | 13.77% |
| - Royal Decret Law (€) | 0.00 € | 0.00% | 191.500,79 | 7.50% | 197.016,76 | 7.50% | 202.938,51 | 7.50% | 249.666,02 | 7.50% |
| - Negotiated Rebates and Bonus (€) | 17.193,50 | 0.08% | 63.336,40 | 2.48% | 67.605,72 | 2.57% | 115.324,32 | 4.26% | 208.868,98 | 6.27% |
| * Saved by optimized regimes (€) | 115.074,00 € | 0.50% | 322.882,00 | 12.51% | 797.907,00 | 30.37% | 730.810,00 | 27.01% | 830.000,34 | 24.93% |
The results obtained are in line with an article that we recently published in patient with Spondyloarthritis.16 Over the study period there was a marked decrease in annual cost per-average-patient diagnosed with RA (incremental difference: −34.9%), however average-dispensed-patient trend increased. Also annual cost per drug decreased during 2009–2017.
In Spain, the Royal Decree Law 4/2010 implementation in June 2010 lead to decreased the prices of all medications by 7.5%19 this fact was associated with cost reduction from 2010 to 2011. Therapy optimization, use of biosimilar TNFi, and official discounts or negotiated rebates that lowered prices in some biologics were other factors associated to the cost reduction for 2011–2017.16
Different published studies have analyzed the economic impact of biological therapies in RA. Gómez-DeRueda et al.,20 in a study conducted from 2013 to 2015, concluded that Ifx (€10,717) had the lowest cost per patient per year under the established practice, followed by Etn (€11,015) and Ada (€11,977). Our study differs in that the costs of Ifx, Etn, and Ada were lower (41.3%, 28.5%, and 41.7%, respectively), compared with the aforementioned study in 2015. Mariatena et al.,21 in a study conducted in 2013, concluded that Ifx (€10,073) had the lowest cost per patient per year under the established practice, followed by Toci (€10,798), Eta (€11,056), and Ada (€11,512). Our study differs in that the costs of Ifx, Toci, Etn, and Ada were lower (46.1%, 22.3%, 33.1% and 37.0%, respectively), compared with the aforementioned study in 2013. Toci, Eta and Ada doses in the first study were optimized empirically and they were reduced a 13.3%, 6.9% and 10.7% for Toci, Eta and Ada, respectively. Ramírez-Herraiz et al.22 concluded that mean doses used were significantly lower with Eta than with Ada and Ifx and they used 81.0%, 93.02% and 135.73% of recommended dose for Eta, Ada and Ifx, respectively. In this study, BD were optimized empirically, controlling for disease activity. Thus patient-year cost in 2011 were €9594, €11,962 and €10,094 for Eta, Ada and Ifx, respectively. Our study differs in that the costs of Ada and Ifx were lower (15.4%, 25.6% respectively), and costs of Eta was higher (3.4%). Finally, Ivorra et al.23 published annual costs per patient and per drug referred to 2013 and our data for the same period showed that these therapies were cheaper 54.3% for Ifx, 43.6% in Ada, 40.1% in Tcz, 37.6% in Eta, 36.0% in Aba IV, 32.1% in Ctz and 19.8% in Goli, that reported in the aforementioned study.
Although in most of these studies the treatments were empirically optimized, our results showed marked differences in RA. This could be explained by the fact that the monitoring of drugs (Etn, Ifx, Ada, Toci) helps the clinician to optimize treatments earlier, with greater safety, and lower doses and wider dosing intervals regarding empirical optimization.
According to the EULAR recommendations,7 tapering of a biological drug can be considered in patients that achieve persistent remission. REDOSER project established criteria for reducing doses of biological therapies for RA, both extending the dosing interval and/or reducing the dose. In addition, serum drug levels and ADAs in serum, when available, can help to clinicians to optimize biological therapy and the clinical monitoring.24
We observed that patients with optimized regimes increased from 12 (4.6%) to 116 (35.2%) patients (2009–2017). Monitoring of Ifx, Etn and Ada using serum levels is used by clinicians in clinical practice in our center from 201114–16; serum levels for monitoring Toci began in 2014 and Goli and Rtx began in 2015, and were available in usual practice in 2017, and for their optimization, rheumatologists have stablished clinical protocols.
Our results show that optimization of biological therapies leads to a marked costs reduction. Moreover, other authors proved that dosing regimen optimization of biologicals does not mean an increase in disease activity parameters, no differences with patients under full dose regimens were found.16,25
In parallel with the beginning of the optimization of treatments, costs decreased. Ada and Ifx annual costs decreased mainly in 2011 and 2012 and Etn in 2013.
When analyzing savings related to therapy optimizations, we detected that the main factor contributing to these savings was optimization by drug serum levels monitorization that were around 80%, respect saving related to empirically optimizations, for analyzed years as we described in result section.
The majority of drugs that contributed to cost savings by optimization were Ada, Etn, Ifx and Tcz group over Goli and Ctz, coinciding with the percentage of optimized regimes for these drugs, in which Goli and Ctz were optimized in a lower percentage than first group probably they joined later.
The presence in the market of many drugs for a pathology produces an economic competition.26 However, bonus units and discounts can then reduce the expenditure on medicines. In our hospital, we have observed that introduction of Goli, Ctz, and Aba or Tcz SC was accompanied by significant invoice discounts of between 15.9% and 37% in different years; and bonus units gradually rose during the study period.
It is known that when a biosimilar is released there is an increased access and a lower health cost burden. According to the law in Spain, when a biosimilar is marketed the original have to decrease its price to the same level of the biosimilar.27,28 Over the study period the European Medicines Agency approved biosimilars of Ifx and Etn in 2013 and 2015 respectively, which led to an increase in discounts for Ifx and Eta.
Original Ifx rebate in our study (24.6%) are in line with the reduction in the price of infliximab published articles,29 however biosimilar Ifx retabe obtained (43.1%) exceed published data.30
Taking together all factors influencing annual RA cost per patient we observed that when the annual cost decreased slightly, increased the number of treated patients and the total saved costs. Our results show that the greatest saving contributions were biological therapy were optimizations, followed by official discounts and negotiated rebates.
Taking into account our results future strategies leading toward the implementation of therapeutic drug monitoring based on scientific evidence31 should be promoted in order to reduce costs and maintaining disease control at the same time.
There were several study limitations. Farmatools does not provide definitive reports of economic and clinical results in order to make a posterior statistical analysis. We have to do a data treatment before use them. Moreover, the annual theoretical cost of Ifx could be overestimated because we considered an estimated average weight of 70kg for all patients treated. Moreover, the saved by optimized regimes could be overestimated because units not dispensed by the possible lack of adherence to treatment are not included. Finally, costs from 2009 to 2017 were not adjusted.
The most important strength of our study is the very long analysis period and the large sample size, which allowed us to analyze and to quantify influential factors in decreasing cost per patient and to prove that optimization was the strategy that most influenced this decline.
Also, annual cost per average-dispensed-patient allows us to compare our data with other hospitals in Spain.
ConclusionOur study proves that the greatest contribution to economic savings in biological therapy in rheumatoid arthritis was biological therapy optimization by monitoring drug and ADA serum levels when comparing with official discounts, negotiated rebates.
Financial disclosureNo financial support.
Conflict of interestNo conflicts of interest have been declared.