To describe skin involvement (SI) in patients with systemic lupus erythematosus (SLE) at onset and during follow-up of the disease and to determine factors associated with SI at lupus diagnosis.
Materials and methodsRetrospective, observational, and descriptive study, from a single centre in patients diagnosed with SLE (ACR 1982-97 or SLICC 2012 criteria). The modified Gilliam classification for SI was used. Descriptive statistics and bivariate and multivariate analysis were performed to evaluate the factors associated with SI at diagnosis of the disease.
Results149 patients were included, 91.3% women with a median age at diagnosis of 33 years. SI at onset of the disease occurred in 125 patients (83.9%), followed by joint involvement in 120 cases (80.5%). Non-specific skin lesions were more frequent than specific lesions, 92.8% versus 66.4%, respectively. In the bivariate analysis, a longer delay to diagnosis, the presence of joint involvement, a lower presence of thrombocytopenia, and a higher SLEDAI-2K score were associated with the presence of SI at onset of the disease. In the multivariate analysis, the variable that remained independently associated was joint involvement (OR 2.8%–95% CI 1.1–7.5, p: .04). During follow-up, 4/24 patients who had not presented SI at diagnosis and 51/125 patients who had, had at least one new skin flare (range: 1–5 outbreaks).
ConclusionsOur study demonstrates the high frequency of skin involvement in SLE, both diagnostically and evolutionarily, and confirms previously reported data regarding the existence of a skin-articular phenotype.
Describir el compromiso cutáneo (CC) en pacientes con lupus eritematoso sistémico (LES) al inicio de la enfermedad y durante el seguimiento. Determinar factores asociados a dicho compromiso al comienzo de la enfermedad.
Materiales y métodosEstudio retrospectivo, observacional y descriptivo, de centro único, en pacientes con diagnóstico de LES (ACR 97 o SLICC 2012). Se utilizó la clasificación de Gilliam modificada para el CC. Se realizó estadística descriptiva; y análisis bivariado y multivariado para evaluar los factores asociados al compromiso cutáneo.
ResultadosSe incluyeron 149 pacientes, 91.3% mujeres con una mediana de edad al diagnóstico 33 años. El CC al inicio de la enfermedad ocurrió en 125 pacientes (83.9%), seguido por compromiso articular en 120 (80.5%). Las lesiones no específicas fueron más frecuentes que las específicas, 92.8% versus 66.4%, respectivamente. En el análisis bivariado, la mayor demora al diagnóstico, la presencia de compromiso articular, menor presencia de trombocitopenia y mayor puntuación de SLEDAI-2 K se asociaron con la presencia de CC al inicio de la enfermedad. En el análisis multivariado, la variable que se mantuvo asociada de manera independiente fue el compromiso articular (OR 2.8, IC 95% 1.1–7.5, p: 0.04). Durante el seguimiento, 4/24 pacientes que no habían presentado CC al diagnóstico y 51/125 pacientes que sí lo presentaron, tuvieron al menos un nuevo episodio cutáneo (rango: 1–5 brotes).
ConclusionesNuestro estudio demuestra la alta frecuencia de pacientes que presenta compromiso cutáneo en el LES, tanto en el diagnóstico como evolutivamente, y confirma los datos reportados previamente en cuanto a la existencia de un fenotipo cutáneo-articular.
Systemic lupus erythematosus (SLE) is a multiorgan autoimmune disease with a global incidence that varies from 1 to 10 cases per 100,000 inhabitants/year.1
Within the broad spectrum of clinical manifestations of SLE, musculoskeletal involvement is reported the most frequently (80%–95%), followed by skin involvement in 60%–85% of cases.2–4 The latter is the first sign of the disease in 23%–28% of cases.5,6
Skin involvement in SLE has been classified for many years by Gilliam and Sontheimer7 —according to the absence or presence of a histological pattern (interphase dermatitis)— in skin lesions that are not specific to lupus erythematosus (LE) and skin lesions that are specific to LE, respectively. The specific lesions are subdivided into cutaneous lupus erythematosus (CLE) that is acute (ACLE), subacute (SCLE) and chronic (CCLE). This original classification has been modified over time, giving rise to what is now known as the Modified Gilliam Classification,8 which is used in the majority of clinical studies.9,10
The group of non-specific lesions includes a large number of manifestations, among which the following ones stand out: vascular lesions (leukocytoclastic vasculitis, livedo reticularis, vasculitis, Raynaud’s phenomenon and erythromelalgia), non-scarring alopecia, oral lesions and ulcers on the lower limbs.8
An interesting aspect of skin involvement is that associations have been observed between certain types of the same and other clinical variables, different degrees of systemic activity and the presence of antibodies.7,11,12
This study aims to describe skin involvement in patients with SLE at the onset of the disease as well as during its evolution, evaluating factors associated with this involvement at the start and describing the treatments used.
Patients and methodsA retrospective, observational and descriptive study was performed based on the review of clinical histories of patients with SLE attended in the Rheumatology Department of San Martín de La Plata HIGA from 2000 to 2019. Patients over the age of 18 years were included, with a short-term onset of the disease (<12 months) and diagnosed SLE in our hospital, who fulfilled the modified ACR 1997 classification criteria13 or SLICC 201214 for SLE. Patients who had been diagnosed SLE in another hospital were excluded, as in this case it was not possible to obtain objective data about the onset of the disease. Patients with another associated connective tissue disease (systemic sclerosis, rheumatoid arthritis, Sjögren’s syndrome, idiopathic inflammatory myopathy, spondyloarthritis and systemic vasculitis) were also excluded, as were those with a primary skin disease such as psoriasis, pemphigus or vitiligo, and patients with drug-induced lupus.
The sociodemographic data, clinical domains, comorbidities and laboratory parameters of the patients (haemogram, immunological profile, complement—C3 and C4—, erythro sedimentation rate [ESR] and C-reactive protein [CRP]) at the onset of the disease were obtained. The immunological profile included antinuclear antibodies (ANA) detected by immunofluorescence (IF), anti-dsDNA, anti-Ro, anti-La, anti-RNP, anti-Smith (Sm), anti-cardiolipin (aCL) and anti-beta-2 glycoprotein I (B2GPI) antibodies detected by ELISA and lupus anticoagulant (LA) using the techniques proposed by the International Society of Thrombosis and Haemostasis (ISTH).15
The patients were then classified in two groups: those with and without skin involvement at the onset of the disease. The types of skin involvement were classified according to the modified Gilliam classification.5
The cutaneous events which occurred in both groups during the follow-up were then recorded. Lesions of the ACLE subtype and non-specific lesions were recorded as new events when the type of outbreak was different from the previous one or if it was the same lesion when it occurred during a period of remission of at least 4 weeks. The SCLE and CCLE types were considered to be unique events during the follow-up, unless the new episode presented in another location or with other characteristics.
The degree of systemic activity was determined at the onset of the disease using the SLE 2000 (SLEDAI-2K) disease activity scale.16
Ethical considerationsThis study was approved by the Ethics Committee of our hospital following the recommendations of the Helsinki Declaration (1964). As the study is based on a review of clinical histories, no informed consent was considered to be necessary, ensuring that data were confidential. Protocol number HSMLP2022/0069.
Statistical analysisDescriptive statistical techniques were applied using the average and standard deviation (SD) or the median and percentile 25−75 (p25−75) depending on the distribution of the continuous variables; frequency and percentage (%) were used for dichotomous variables.
Comparisons between the groups (patients with and without skin lesions at the onset of the disease) were performed using the chi-squared (χ2) test or Fisher’s exact test for dichotomous variables, and the Student’s t-test or Mann-Whitney test was used for continuous variables. A logistic regression was performed to evaluate the factors which were independently associated with the presence of skin involvement at the onset of SLE.
Results149 patients were included, of whom 136 (91.3%) were women, with a median age at diagnosis of 33 years (22−45.5 years), a delay in diagnosis of 5 months (2−12 months) and a median follow-up time of 45 months (14−72 months). All of the patients had positive ANA. Of the activity parameters at the onset of the disease, anti-dsDNA was positive in 70/138 (50.7%), C3 was low in 74/142 (52.1%) and C4 was low in 95/142 (66.9%). Table 1 shows the demographic data, comorbidities and immunological profile of all the patients. The median SLEDAI-2K score at diagnosis was 9 points (6–14 points).
Demographic data, comorbidities and antibody profile of all the patients.
| n = 149 | |
|---|---|
| Demographic data | |
| Female sex, n (%) | 136 (91.3) |
| Age at diagnosis in years, median (p25−75) | 33 (22−45.5) |
| Diagnostic delay in months, median (p25−75) | 5 (2−12) |
| Follow-up time in months, median (p25−75) | 45 (14−72) |
| Comorbidities | |
| Hypothyroidism, n (%) | 26 (17.4) |
| Hypertension, n (%) | 17 (11.4) |
| Dyslipidaemia, n (%) | 6 (4) |
| Diabetes, n (%) | 4 (2.7) |
| Smoking, n (%) | 14 (9.4) |
| Antibody profile | |
| ANA, n (%) | 149 (100) |
| Anti-Smith, n (%) | 59/142 (41.5) |
| Anti-Ro, n (%) | 53/142 (37.3) |
| Anti-La, n (%) | 22/141 (15.6) |
| Anti-RNP, n (%) | 50/138 (36.2) |
| aCL, n (%) | 35/107 (32.7) |
| LA, n (%) | 29/97 (29.9) |
| B2GPI, n (%) | 18/100 (18) |
aCL: Anti-cardiolipin; LA: Lupus anticoagulant; ANA: Antinuclear antibodies; B2GPI: Anti-beta-2 glycoprotein I.
Of the clinical manifestations at the onset of the disease, skin involvement was the most frequent and was present in 125 patients (83.9%), followed by joint involvement in 120 cases (80.5%), constitutional involvement in 68 (45.6%), lymphopenia in 49 (32.9%), renal involvement in 40 (26.8%), serositis in 35 (23.5%), leukopenia in 34 (22.8%), thrombocytopenia in 31 (20.8%), haemolytic anaemia in 17 (11.4%), nervous system involvement in 16 (10.7%), pulmonary involvement in 2 (1.3%) and gastrointestinal involvement in 1 patient (.7%).
Table 2 shows the different types of skin involvement at onset. Non-specific lesions were more frequent than specific ones, at 92.8% versus 66.4%, respectively. Seventy-four patients (59.2%) had combinations of both types of lesions. Forty-two of the patients only had non-specific manifestations, while 9 only had specific ones. The most frequent specific lesion was the acute subtype, in 79/125 patients (63.2%), followed by the chronic type in 6/125 patients (4.8%) and sub-acute in 2/125 (patients 1.6%). Of the non-specific lesions, alopecia was found in 77/125 patients (61.6%), Raynaud’s phenomenon in 48/125 patients (38.4%), and mouth ulcers in 47/125 patients (37.6%). Livedo reticularis, vasculopathy, leukocytoclastic vasculitis, lower limb ulcers, urticaria, thrombophlebitis and periungual telangiectasia were less frequent. All of the cases of leukocytoclastic vasculitis, SCLE and CCLE were diagnosed by biopsy. The diagnosis was clinical in the other patients. The histological findings are not described, as that was not one of the purposes of this study.
Types and frequency of skin involvement at disease onset.
| Cutaneous manifestations | n = 125 (100%) |
|---|---|
| Specific LE skin lesions | 83 (66.4) |
| ACLE | 79 (63.2) |
| o Malar rash | 73 (58.4) |
| o Generalized erythema | 13 (10.4) |
| SCLE | 2 (1.6) |
| CCLE | 6 (4.8) |
| o Classic discoid | 5 (4) |
| o Panniculitis | 1 (.8) |
| Skin lesions not specific to LE | 116 (92.8) |
| Leukocytoclastic vasculitis | 5 (4) |
| Vasculopathy | 7 (5.6) |
| Reticular livedo | 18 (14.4) |
| Raynaud’s phenomenon | 48 (38.4) |
| Mucosal ulcers | 47 (37.6) |
| Alopecia | 77 (61.6) |
| Urticaria | 1 (.8) |
| Lower limb ulcers | 2 (1.6) |
| Thrombophlebitis | 1 (.8) |
| Periungual telangiectasia | 1 (.8) |
EL: Erythematous lupus; ACLE: Acute cutaneous lupus erythematous; CCLE: Chronic cutaneous lupus erythematous; SCLE: Subacute cutaneous lupus erythematous.
Bivariate analysis (Table 3) found that a longer delay to diagnosis, the presence of joint involvement, reduced presence of thrombocytopenia and a higher SLEDAI-2K score were associated with the presence of skin involvement at the onset of the disease. No differences were found in terms of age at diagnosis, smoking, ANA, anti-Ro, anti-La, anti-Sm, anti-RNP, anti-dsDNA, hypocomplementaemia, aCL, AL, B2GPI, RF, ERS, CRP, serositis, constitutional, renal or nervous system involvement, haemolytic anaemia, leukopenia or lymphopenia (P > .05). In multivariate analysis (Table 3), the variable that remained associated independently was joint involvement (OR: 2.8; CI 95%: 1.1−7.5; P = .04), while no association was maintained with a delay in diagnosis or thrombocytopenia.
Factors associated with skin involvement at disease onset. Bivariate analysis and logistic regression.
| Variables | With skin involvement (n = 125) | Without skin involvement (n = 24) | P | Multivariate analysis | |
|---|---|---|---|---|---|
| OR (CI 95%) | P | ||||
| Delay in diagnosis in months, median (p25−75) | 5.5 (3−12) | 2 (1−3.5) | .04 | 1 (0.96−1) | .72 |
| Joint involvement, n (%) | 105 (84) | 15 (62.5) | .015 | 2.8 (1.1−7.5) | .04 |
| Thrombocytopenia, n (%) | 22 (18) | 9 (37.5) | .028 | 0.4 (0.15−1) | .64 |
| Median SLEDAI-2K score (p25−75) | 9 (6−15) | 6 (15.5−11.5) | .023 | – | – |
aCL: Anti-cardiolipin; LA: lupus anticoagulant; ANA: Antinuclear antibodies; B2GPI: Anti-beta-2 glycoprotein I; ESR: Erythro sedimentation rate; RF: Rheumatoid factor; CRP: C reactive protein; APS: Antiphospholipid syndrome.
No statistically significant differences were found between both of the groups studied in the use of treatments for SI or for the other manifestations at onset (P > .05). The treatments used the most often were hydroxychloroquine (130/145 [89.7%]) and oral corticoids (121/145 [83.4%]), followed at far lower frequency by cyclophosphamide (27/145 [18.6%]), methotrexate (11/145 [7.6%]), azathioprine (10/144 [6.9%]) and mycophenolate (5/145 [3.4%]).
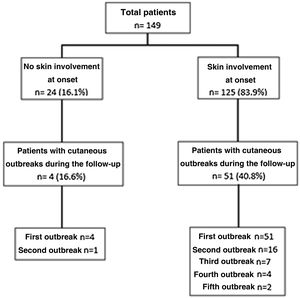
Cutaneous outbreaks occurred during follow-up in a range of from 1 to 5, and they were more frequent in patients with skin involvement at the start of the disease. Fig. 1 shows the distribution and number of skin outbreaks.
The median time taken for the first outbreak to occur after the diagnosis of SLE was 22 months (12–44 months), while ACLE (30/55 [54.5%]) was the most common specific lesion; non-specific lesions appeared in 41/55 cases (74.5%), while alopecia appeared in 21/55 cases (38.2%), oral ulcers in 17/55 (30.9%) and Raynaud’s phenomenon in 11/55 (20%). A second skin outbreak was observed in 17 patients, at a median time after diagnosis of 41 months (26–59 months), with the same percentages of specific and non-specific lesions. Seven patients had a third outbreak, at a median time of 50 months after diagnosis (33–80 months). In this case specific manifestations (85.7%) were more frequent than the non-specific ones (57.1%). A fourth and fifth outbreak were found in 4 and 2 patients, respectively, after an average time of 98 months (SD: ±36) and 117 months (SD: ±71), respectively. ACLE in both cases was the most common lesion. In the total number of 85 skin outbreaks recorded, prior to this event the patients had been under treatment for SLE in 69 (81.2%) cases with hydroxychloroquine, while 35 (41.2%) were being treated with glucocorticoids at doses higher than 7.5 mg/day and 33 (38.8%) with a dose lower than 7.5 mg/day. 26 patients (30.6%) were being treated with azathioprine, while 11 (13%) were receiving mycophenolate, 10 (11.7%) were receiving methotrexate and 6 (7%) cyclophosphamide, while only one patient was being treated with belimumab.
DiscussionThis study of patients with SLE found that skin involvement is the most frequent form at the onset of the disease, followed by joint involvement, which was found to have a statistically significant association.
A possible explanation for the higher rate of SI at the start could be that the patients included in our cohort had to have a short-term commencement of the disease, which may have led to increased attention by doctors and a search by them for this involvement in these patients.
In connection with the type of lesions, and in agreement with the data found by Gronhagen et al.,9 we observed that non-specific LE lesions predominated, even at the start of the disease. This is probably because they were associated with active states,17–19 as this is usually the case at the onset of SLE. Consistently with this finding, we observed that more than 50% of patients had positive anti-DNA antibodies and hypocomplementaemia, and that the median SLEDAI-2K score was 9 points.
ACLE was the predominant subtype of the specific skin lesions at the onset of the disease, at higher percentages of from 30% to 50% than was the case in previous descriptions.5,7,11,20 The high incidence that was observed of malar rash coincides with descriptions in the literature, as it is typically one of the first manifestations of SLE associated with systemic activity.3,4
SCLE and CCLE were less common in our study than has been reported.6,11,17,21 This finding may be associated with the fact that both subtypes are seen more commonly as isolated cutaneous entities, and they are associated with low systemic activity of the disease,2,3,9,11 which may make it less probable that cases will be referred to a rheumatology department. On the other hand, a small percentage of CCLE cases progress to SLE,3,4,22 and in our cohort of systemic lupus this would explain its low prevalence. Moreover, both subtypes have been seen more often in patients with late-onset SLE (>50 years), and this age group did not predominate in our study.5
Alopecia was the non-specific lesion found in the highest percentage of cases, as occurs in other studies, which report a frequency of from 40% to 83% in patients with SLE.9,17 This manifestation usually indicates that the disease is active,5 and it is generally associated with other cutaneous manifestations,12 as occurred in our work. The prevalence of Raynaud’s phenomenon and oral ulcers agrees with previous reports.2,9,23
Although in the cohort as a whole there was little delay in diagnosis because of the inclusion criteria, the patients with skin involvement at the onset were associated with a longer delay until diagnosis, with joint involvement and with less presence of thrombocytopenia and a higher SLEDAI-2K score; nevertheless, in the multivariate analysis only joint involvement was still independently associated. A similar result was found in a study by Ghosh et al.,18 which evaluated the clinical and immunological profiles of 55 patients with SLE and skin involvement at the debut of the disease. They found that joint involvement was the systemic manifestation associated with it the most often. Two studies that evaluated clinical patterns in SLE by analysing groups found the same association. The first of these studies, by Font et al.,20 was of a cohort of 600 patients with SLE. It found a clinical superimposition between skin and joint involvement, and although they also found that this was the case for kidney involvement, we did not find the latter in our study. The second study by To et al.24 used a conglomerates procedure to find three clinical groups, of which one was characterized by the presence of skin and joint involvement, with a low prevalence of serositis, haematological manifestations and kidney involvement. Together with the previous studies, a recent study by Zhou et al.4 of the progression from CLE to SLE described a higher frequency of joint involvement (arthralgia and arthritis) in those patients with lupus that had a cutaneous debut that then progressed to systemic involvement. The association between these two forms of involvement, which were not generally severe, has classically been linked to a group of patients with less severe organic involvement and a better prognosis.10,20
During the follow-up 55 (36.9%) patients had at least one cutaneous outbreak, and the majority of them had the same involvement at diagnosis. During the different outbreaks the types of skin involvement with the highest percentages were ACLE and non-specific skin lesions, which are usually associated with disease relapses. We found that at the moment of an outbreak the majority of patients were under treatment with hydroxychloroquine and/or corticoids. The literature contains studies of refractory cutaneous manifestations in reaction to antimalarial drugs25 that require a combination of these agents, and in some cases this lack of response was associated with smoking.26 Although this analysis was not included in the objectives of our study, as we pointed out above the majority of outbreaks during follow-up occurred in patients with skin involvement at onset, and of these patients a higher but not significant percentage were smokers.
Among the limitations of this study, we can mention the type of retrospective design and, in some cases, the lack of data in connection with the relevant variables during a period of time around each new skin outbreak. We are therefore unable to evaluate the factors associated with the said outbreaks during the follow-up. Finally, we underline that the CLASI cutaneous evaluation score was not used, which, among other characteristics, makes it possible to analyse acute and chronic manifestations, or those intrinsic to irreversible damage.
The fact that this study was carried out on real-life patients in in a single tertiary referral hospital with a long-term follow-up after the onset of the disease can be said to be a strong point.
To conclude, our study shows the impact of skin involvement in patients with SLE, both at diagnosis and during its evolution. It also confirms the data reported previous in terms of the existence of a skin-joint phenotype, which would require medical education strategies to ensure early diagnosis and suitable management. There is a need for prospective cohort studies to determine the incidence and not only the prevalence of SI in SLE, better establishing the causal relationship between the associated factors and relapses.
FinancingThis study received no specific grants from public or commercial sector agencies or not-for-profit entities.
Conflict of interestsThe authors have no conflict of interests to declare.