To determine the efficacy and safety of denosumab in osteoporosis.
MethodsA systematic search was performed in MEDLINE, EMBASE, and The Cochrane Central Register of Controlled Trials (1950 to July 2010), meeting abstracts (2009–2010), trial registries, and reference lists. The selection criteria were as follows: (population) osteoporosis patients of any age; (intervention) treatment with denosumab; (outcome) efficacy and safety; (study design) randomized clinical trials (RCTs); no language restrictions. Two reviewers independently screened titles and abstracts and subsequently extracted data from the selected studies including quality items, and on outcomes of interest. A meta-analysis was performed for safety issues.
ResultsA total of 25 studies were included. Denosumab reduces the risk of new radiographic vertebral fracture in a 68% compared with placebo (p<0.001) and increases bone mineral density (BMD) at lumbar spine, total hip, and one-third radius more than alendronate and placebo. A single subcutaneous dose of denosumab resulted in a dose-dependent, rapid, profound, and sustained decrease bone turnover markers (BTMs). Denosumab was in general well tolerated. A meta-analysis has shown an increase in the incidence of urinary infections (p=0.012) and eczema (p<0.001) in the patients treated with denosumab. Meta-analysis of efficacy was complicated due to the study features.
ConclusionsDenosumab given subcutaneously twice yearly is associated with a reduction in the risk of vertebral, nonvertebral, and hip fractures in women with osteoporosis. Denosumab is associated with greater and sustained increases in BMD and reductions in BTMs compared with placebo and/or alendronate and with a risk of urinary infections and eczema.
Determinar la eficacia y la seguridad de denosumab en la osteoporosis.
MétodosSe realizó una búsqueda sistemática en MEDLINE, EMBASE y el Registro Central Cochrane de ensayos controlados (de 1950 a Julio de 2010), resúmenes de congresos (2009-2010), registros de ensayos y listas de referencias. Los criterios de selección fueron los siguientes: (población) pacientes con osteoporosis de cualquier edad; (intervención) tratamiento con denosumab; (desenlace) eficacia y seguridad; (diseño del estudio) ensayos clínicos con selección aleatoria; sin restricciones de idioma. Dos revisores independientes revisaron títulos y resúmenes y posteriormente extrajeron los datos de los estudios seleccionados, incluyendo elementos de calidad y desenlaces de interés. Se realizó un metanaálisis con los datos de seguridad.
ResultadosSe incluyeron 25 estudios. El denosumab reduce el riesgo de fracturas radiográficas nuevas en un 68% en comparación con el placebo (p<0,001); incrementa la de densidad mineral ósea (DMO) en la columna lumbar, la cadera total y el tercio distal del radio, más que el alendronato y el placebo. Una dosis subcutánea única de denosumab provocó una disminución rápida, profunda, sostenida y dosis-dependiente de los marcadores de remodelado óseo (MRO). El denosumab fue bien tolerado en general. El metananálisis mostró un aumento en la incidencia de infecciones urinarias (p=0,012) y eczema (p<0,001) en los pacientes tratados con denosumab. No se pudo realizar metaanálisis de eficacia debido a la heterogeneidad de los estudios.
ConclusionesEl denosumab administrado por vía subcutánea, 2 veces al año, se asocia con una reducción en el riesgo de fracturas vertebrales, no vertebrales y de cadera, en mujeres con osteoporosis. El denosumab se asocia con un incremento mayor y sostenido en la DMO, y una reducción en los MRO, en comparación con el placebo y el alendronato, así como con un riesgo aumentado de infecciones urinarias y eczema.
Bone remodeling throughout life is a delicate balance between bone formation and resorption. Multiple factors, including the presence of hormones, growth factors, and cytokines influence the rate of bone loss.1 Osteoporosis is a disease characterized by decreased bone mass, microarchitectural deterioration of the skeleton and impaired bone strength which results from increased bone resorption relative to formation. Anti-resorptive drugs significantly reduce bone turnover, providing an increase in BMD and a reduction in risk of fracture.2
Recent advances have identified the Receptor Activator for Nuclear Factor κB Ligand (RANKL) as a critical mediator of bone remodeling. RANKL is essential for the formation, function, and survival of the osteoclasts. It binds to its cognate receptor RANK on the surface of precursors and mature osteoclasts, and stimulates these cells to mature and resorb bone. The physiological inhibitor of RANKL is osteoprotegerin (OPG), a soluble receptor that competes with RANK for binding to RANKL, thus neutralizing RANKL effects.3 Multiple preclinical models were used to study the effects of inhibiting RANKL showing that it leads to improved bone geometry and increased bone density and strength.4,5
Denosumab is a novel antiresorptive agent that also inhibits osteclast-mediated bone resorption but works through a different pathway than bisphosphonates. Denosumab is a fully human monoclonal antibody (IgG2) that inhibits RANKL with high specificity, mimicking the effects of OPG on RANKL.6 In recent years, a number of clinical trials evaluating the efficacy and safety of denosumab for the treatment of osteoporosis in humans have been published. Our primary objective was to determine the efficacy and safety of denosumab for the treatment of osteoporosis in humans in a systematic literature review.
MethodsAs a part of the Spanish Society of Rheumatology Consensus of osteoporosis, a systematic literature review was performed to examine the efficacy and safety of denosumab in osteoporosis.
Data sources and searchesWe systematically searched MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials up to July 2010 using a comprehensive search strategy that combined MeSH terms and free text for “Denosumab”, “Osteoporosis”, “Efficacy”, “Safety” and “Clinical Trials”. Table 1 shows the terms used in Medline to capture studies. We also handsearched abstracts from 2009 and 2010 scientific meetings of the American College of Rheumatology (ACR), the American Society for Bone and Mineral Research (ASBMR), the European Congress on Osteoporosis and Osteoarthritis (ECCEO), the International Osteoporosis Foundation (IOF) and the European League against Rheumatism (EULAR), as well as reference lists of all relevant studies, reviews, and letters, to identify additional studies. No language restrictions were applied.
Medline search strategy.
| # | Search strategy |
| 1 | (((((((((((“OP”[Mesh] OR Osteoporoses OR OP, Post-Traumatic OR OP, Post Traumatic OR Post-Traumatic Osteoporoses OR Post-Traumatic OP OR OP, Senile OR Osteoporoses, Senile OR Senile Osteoporoses OR Senile OP OR OP, Age-Related OR OP, Age Related OR Bone Loss, Age-Related OR Age-Related Bone Loss OR Age-Related Bone Losses OR Bone Loss, Age Related OR Bone Losses, Age-Related OR Age-Related OP OR Age Related OP OR Age-Related Osteoporoses OR Osteoporoses, Age-Related)) OR (“OP, Postmenopausal”[Mesh] OR Perimenopausal Bone Loss OR Bone Loss, Postmenopausal OR Bone Losses, Postmenopausal OR Postmenopausal Bone Losses OR OP, Post-Menopausal OR Osteoporoses, Post-Menopausal OR OP, Post Menopausal OR Post-Menopausal Osteoporoses OR Post-Menopausal OP OR Postmenopausal OP OR Osteoporoses, Postmenopausal OR Postmenopausal Osteoporoses OR Bone Loss, Perimenopausal OR Bone Losses, Perimenopausal OR Perimenopausal Bone Losses OR Postmenopausal Bone Loss)) OR (“Female Athlete Triad Syndrome”[Mesh] OR Female Athlete Triad)) OR (“Decalcification, Pathologic”[Mesh] OR Decalcification, Pathological OR Pathological Decalcification OR Pathologic Decalcification OR Corticosteroid Induced OP OR glucocorticoid induced OP OR Idiopathic OP OR Involutional OP OR Juvenile OP OR Primary OP OR Secondary OP OR Bone Fragility Endocrine OP OR Osteoporotic Decalcification)) OR (“Bone Density”[Mesh] OR Bone Densities OR Density, Bone OR Bone Mineral Density OR Bone Mineral Densities OR Density, Bone Mineral OR Bone Mineral Content OR Bone Mineral Contents OR BMD)) OR (“Fractures, Bone”[Mesh] OR Broken Bones OR Bone, Broken OR Bones, Broken OR Broken Bone OR Bone Fractures OR Bone Fracture OR Fracture, Bone)) OR (((((((((((((Bone mineral density[All Fields])) OR (low bone mass)) OR (low bone mass density)) OR (low bone mineral density)) OR (low bone mass in premenopausal women with depression)) OR (low bone mass premenopausal women)) OR (low bone)) OR (low bone density)) OR (postmenopausal bone loss)) OR (bone loss OP)) OR (bone loss postmenopausal)) OR (bone loss)))) |
| 2 | (((((“denosumab”[Substance Name] OR AMG 162 OR Prolia)) OR (“Antibodies, Monoclonal”[Mesh] OR Monoclonal Antibodies)) OR (“RANK Ligand/adverse effects”[Mesh] OR “RANK Ligand/antagonists and inhibitors”[Mesh] OR “RANK Ligand/pharmacology”[Mesh] OR “RANK Ligand/therapeutic use”[Mesh])) OR (“RANK Ligand”[Mesh] OR Osteoclast Differentiation Factor OR Differentiation Factor, Osteoclast OR Osteoprotegerin Ligand OR RANKL Protein OR Receptor Activator of Nuclear Factor-kappa B Ligand OR Receptor Activator of Nuclear Factor kappa B Ligand OR TNF Superfamily, Member 11 OR TRANCE Protein OR Tumor Necrosis Factor Ligand Superfamily Member 11 OR Tumor Necrosis Factor-Related Activation-Induced Cytokine OR Tumor Necrosis Factor Related Activation Induced Cytokine OR OPGL Protein OR Receptor Activator of Nuclear Factor-kappaB Ligand OR Receptor Activator of Nuclear Factor kappaB Ligand))) |
| 3 | (((((((((((((((((((((((“adverse effects”[Subheading] OR side effects OR undesirable effects OR injurious effects)) OR (“Safety”[Mesh] OR Safeties)) OR (“Drug Toxicity”[Mesh] OR Drug Toxicities ORToxicities, Drug OR Toxicity, Drug OR Drug Safety OR Safety, Drug OR Adverse Drug Reaction OR Adverse Drug Reactions OR Drug Reaction, Adverse OR Drug Reactions, Adverse OR Reaction, Adverse Drug OR Reactions, Adverse Drug OR Adverse Drug Event OR Adverse Drug Events OR Drug Event, Adverse OR Drug Events, Adverse OR Event, Adverse Drug OR Events, Adverse Drug)) OR (“toxicity”[Subheading] OR toxic potential OR margin of safety)) OR (drug fatality)) OR (‘drug mortality’ OR ‘fatal adverse drug reaction’ OR ‘fatal adverse reaction’ OR ‘fatal side effect’)) OR (drug mortality OR fatal adverse drug reaction OR fatal adverse reaction OR fatal side effect)) OR (“poisoning”[Subheading] OR poisonous effects)) OR (“Drug Hypersensitivity”[Mesh] OR Drug Hypersensitivities OR Hypersensitivities, Drug OR Drug Allergy OR Allergies, Drug OR Drug Allergies OR Hypersensitivity, Drug OR Allergy, Drug)) OR (‘drug sensitivity’ OR ‘drug sensitivity test’ OR ‘drug subsensitivity’ OR ‘drug susceptibility’ OR ‘parasitic sensitivity tests’ OR ‘susceptibility, drug’)) OR (drug sensitivity OR drug sensitivity test OR drug subsensitivity OR drug susceptibility OR parasitic sensitivity tests OR susceptibility, drug)) OR (sensitivity drug)) OR (“Drug Interactions”[Mesh] OR Drug Interaction OR Interaction, Drug OR Interactions, Drug)) OR (“drug effects”[Subheading] OR pharmacologic effects OR effect of drugs)) OR (“Adverse Drug Reaction Reporting Systems”[Mesh] OR Drug Reaction Reporting Systems, Adverse)) OR (’adverse drug reaction’ OR ‘adverse drug effect’ OR ‘adverse drug eventor adverse effect’ OR ‘adverse reaction’ OR ‘adverse reaction, drug’ OR ‘drug adverse effect’ OR ‘drug adverse reaction’ OR ‘drug reaction, adverse’ OR ‘drug side effect’)) OR (‘adverse drug reaction’ OR ‘adverse drug effect’ OR “adverse drug eventor” OR “adverse effect” OR ‘adverse reaction’ OR ‘adverse reaction, drug’ OR ‘drug adverse effect’ OR ‘drug adverse reaction’ OR ‘drug reaction, adverse’ OR ‘drug side effect’)) OR (adverse drug reaction OR adverse drug effect OR “adverse drug eventor” OR “adverse effect” OR adverse reaction OR adverse reaction, drug OR drug adverse effect OR drug adverse reaction OR drug reaction, adverse OR drug side effect)) OR (“drug carcinogenicity” OR ‘carcinogenicity, drug induced’)) OR (“drug carcinogenicity” OR carcinogenicity, drug induced)) OR (“drug cytotoxicity” OR “cytotoxicity, drug”)) OR (“Treatment Outcome”[Mesh] OR Outcome, Treatment OR Rehabilitation Outcome OR Outcome, Rehabilitation OR Treatment Effectiveness OR Effectiveness, Treatment OR Treatment Efficacy OR Efficacy, Treatment))) |
| 4 | ((((((((((“Clinical Trial”[Publication Type] OR “Clinical Trial, Phase I”[Publication Type]) OR Clinical Trial, Phase 1 OR “Clinical Trial, Phase II”[Publication Type]) AND Clinical Trial, Phase 2 OR “Clinical Trial, Phase III”[Publication Type]) OR Clinical Trial, Phase 3 OR “Clinical Trial, Phase IV”[Publication Type]) OR Clinical Trial, Phase 4 OR “Controlled Clinical Trial”[Publication Type]) OR “Multicenter Study”[Publication Type]) OR “Randomized Controlled Trial”[Publication Type])) OR ((((((((“Clinical Trials as Topic”[Mesh] OR Clinical Trial as Topic)) OR (“Clinical Trials, Phase I as Topic”[Mesh] OR Clinical Trials, Phase I OR Phase 1 Clinical Trials OR Phase I Clinical Trials OR Clinical Trials, Phase 1 OR Evaluation Studies, FDA Phase I OR Evaluation Studies, FDA Phase 1 OR Microdosing Trials, Human OR Human Microdosing Trial OR Microdosing Trial, Human OR Trial, Human Microdosing OR Trials, Human Microdosing OR Human Microdosing Trials OR Drug Evaluation, FDA Phase I as Topic OR Drug Evaluation, FDA Phase I OR Drug Evaluation, FDA Phase 1)) OR (“Clinical Trials, Phase II as Topic”[Mesh] AND *Drug Evaluation, FDA Phase II as Topic OR Drug Evaluation, FDA Phase 2 as Topic OR Evaluation Studies, FDA Phase II as Topic OR Evaluation Studies, FDA Phase 2 as Topic)) OR (“Clinical Trials, Phase III as Topic”[Mesh] OR Clinical Trials, Phase 3 as Topic OR Evaluation Studies, FDA Phase III as Topic OR Drug Evaluation, FDA Phase III as Topic OR Drug Evaluation, FDA Phase 3 as Topic OR Evaluation Studies, FDA Phase 3 as Topic)) OR (“Clinical Trials, Phase IV as Topic”[Mesh] OR Clinical Trials, Phase 4 as Topic OR Drug Evaluation, FDA Phase IV as Topic OR Evaluation Studies, FDA Phase 4 as Topic OR Drug Evaluation, FDA Phase 4 as Topic OR Evaluation Studies, FDA Phase IV as Topic)) OR (“Randomized Controlled Trials as Topic”[Mesh] OR Controlled Clinical Trials, Randomized OR Clinical Trials, Randomized OR Trials, Randomized Clinical)) OR (“Multicenter Studies as Topic”[Mesh] OR Multicentre Studies as Topic OR Multicenter Trials OR Multicenter Trial OR Trial, Multicenter OR Trials, Multicenter OR Multicentre Trials OR Multicentre Trial OR Trial, Multicentre OR Trials, Multicentre))) OR ((clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms])) |
| 5 | #1 AND #2 AND #3 AND #4 |
| 6 | Limit #e to humans |
Clinical trials that evaluated the efficacy and/or safety of denosumab for the treatment of osteoporosis were eligible for inclusion. The selection criteria were predefined by protocol. In order to incorporate a study: (1) the studied population had to include patients of any age with osteoporosis; (2) at least one of the study groups had to have received treatment with denosumab; and (3) outcome should be a measure of efficacy (such as reduction of fracture risk, changes in BMD, changes on serum bone biomarkers or bone microarchitecture) or safety. Studies including patients with oncologic disease or any condition different from osteoporosis, studies on animals and basic science research were excluded. For the purposes of this review, we considered RCTs with their extension studies and subanalysis.
Two reviewers (LS, JAM) independently screened the titles and abstracts of the citations captured by the search strategy. This process was done in 20min sessions. On a limited number of articles in which the reviewers disagreed, a third reviewer (EL) decided if the article should be included. Subsequently, selected articles were reviewed in detail. Articles that did not fulfill all the inclusion criteria were excluded from the systematic review.
Data extraction and quality assessmentThe two reviewers collected the data of the included studies, including number of patients and their characteristics, the comparator group, doses of denosumab, duration of follow-up, study quality and relevant outcomes using ad hoc standard forms. The assessment of study quality was based on the Jadad scale for clinical trials.7
Data synthesis and analysisEvidence tables were produced. Some of the results are expressed as RR: risk ratio (RR) and HZ: hazard ratio (HR). Meta-analysis was only planned in case of homogeneity. Results were combined by using random-effects models, and statistical heterogeneity was quantified by using the I2 statistic. In the studies with 3 arms of treatment (denosumab, alendronate, and placebo) we assumed that denosumab had the same risk of adverse events. Besides, for some of the meta-analyses we also assumed that the risk of adverse events was the same irrespective of the duration of the treatments. All analyses, confidence intervals, and graphics were performed with Stata 10 (Stata Corporation, College Station, TX 77845, USA).
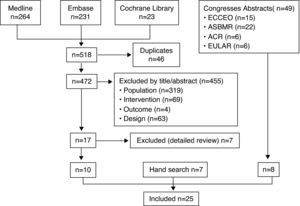
ResultsA flowchart summarizing the search results is exposed in Fig. 1. The search strategy identified 518 potentially relevant articles of which 10 fulfilled the inclusion criteria.8–17 The excluded articles18–24 and the reasons for exclusion are depicted in Table 2. In addition, 7 additional articles were identified by hand search,25–31 as well as 8 congresses abstracts.32–39Table 3 shows the main characteristics of the 25 included studies.8–17,25–39 Most of them were high quality RCT, which included more than 10,000 postmenopausal women, with a mean age range from 59 years to 72 years. Only 1 RCT14 evaluated the efficacy of denosumab in terms of reduction of risk of new fractures and the rest evaluated changes in BTMs or BMD. All the studies had been supported by Amgen.
Articles retrieved by the search strategies and result of selection and appraisal process. Abbreviations: American College of Rheumatology (ACR), the American Society for Bone and Mineral Research (ASBMR), the European Congress on Osteoporosis and Osteoarthritis (ECCEO), the International Osteoporosis Foundation (IOF) and the European League against Rheumatism (EULAR).
Excluded studies and reason for exclusion.
| Article | Reason for exclusion |
| No authors listed 2006 (24) | Editorial |
| No authors listed 2008 (25) | Treatment guideline |
| No authors listed 2009 (26) | Editorial |
| Cummings 2009 (27) | Editorial |
| Ecker-Sclipf 2010 (28) | Editorial |
| Favus 2006 (29) | Editorial |
| Lewiecki 2009 (30) | Review |
Characteristics of the included studies.
| Study | Participants | Intervention | Outcomes | Qualitya |
| Bekker (2004)9Phase I double blind placebo controlled RCT6–9m follow-up | n=49 healthy postmenopausal women, mean age range 54–63yr | • Denosumab 0.01mg/kg→0.03mg/kg→0.1mg/kg→0.3mg/kg→1mg/kg→3mg/kg• Placebo | • % change in urinary NTX• % change in serum NTX• % change in BALP• Adverse events | Oxford 1bJadad 5 |
| McClung (2006)10Phase II double blind placebo controlled RCT with an open-label arm1yr follow-up | n=412 postmenopausal women, mean age 62yr, with low BMD (T-score −1.8 to −4 at lumbar spine or −1.8 to −3.5 at proximal femur) | • Denosumab 6mg/3m; 14mg/3m; 30mg/3m; 60mg/6m; 100mg/6m; 210mg/6m• Alendronate 70mg/w• Placebo | • % change in BMD at lumbar spine, femoral neck, total hip, 1/3 radius, and total body (minus head)• % change in BTMs• Adverse events | Oxford 2b |
| Lewiecki (2007)15Phase II double blind placebo controlled RCT with an open label arm2yr follow-up | n=412 postmenopausal women, mean age 62 yr, with low BMD (T-score −1.8 to −4 at lumbar spine or −1.8 to −3.5 at proximal femur) | • Denosumab 6mg/3m; 14mg/3m; 30mg/3m; 60mg/6m; 100mg/6m; 210mg/6m• Alendronate 70mg/w• Placebo | % change in BMD at lumbar spine, total hip, 1/3 radius, and total body (minus head)% change in BTMsAdverse events | Oxford 2b |
| Beck (2008)8Post hoc analysis of phase II RCT151yr follow-up | n=116 postmenopausal women, mean age 62yr, with low BMD (lumbar spine T score −1.8 to −4 or −1.8 to −3.5 proximal femur) | • Denosumab 60mg/6m• Alendronate 70mg/w• Placebo | % change in hip BMDcross-sectional geometry parameters | Oxford 2b |
| Miller (2008)16Phase III extension study152yr follow-up | n=307 postmenopausal women, mean age 62 yr, with low BMD (T-score −1.8 to −4.0 at lumbar spine or −1.8 to −3.5 at proximal femur) | • Denosumab 6mg/6m• Denosumab cessation 1styr→denosumab 6mg/6m the 2ndyr• Placebo | % change in BMD at lumbar spine, total hip, 1/3 radius, and total body% change in BTMsAdverse events | Oxford 2b |
| Miller (2011)29Ongoing 4-yr, open-label, extension study of a phase II RCT156yr follow-up | n=178 postmenopausal women with low BMD (T-score −1.8 to −4.0 at lumbar spine or −1.8 to −3.5 at proximal femur) | • Denosumab 60mg/6m | % change in lumbar spine, hip and 1/3 radius BMD% change in BTMsAdverse events | Oxford 2b |
| Bone (2008)11DEFEND studyPhase III double blind placebo controlled RCT2yr follow-up | n=332 postmenopausal women, mean age 59yr, with lumbar spine BMD T-scores between −1 and −2.5 | • Denosumab 60mg/6m• Placebo | % change in lumbar spine BMD by DEXA% change in volumetric BMD of the distal radius by QCT% change in total hip, 1/3 radius, total body BMD by DEXAHip structural analysis% change in BTMsAdverse events | Oxford 1bJadad 4 |
| Genant (2010)26Post hoc analysis of DEFEND study2yr follow-up | n=332 postmenopausal women, mean age 59yr, with BMD T-scores between −1 and −2.5 | • Denosumab 60mg/6m• Placebo | % change in volumetric BMD, volumetric bone mineral content, cortical thickness, volume, circumference, and density-weighted PMI along distal radiusAdverse events | Oxford 1bJadad 4 |
| Bone (2011)31Off-treatment extension of DEFEND study2yr follow-up | n=256 postmenopausal women, mean age 59yr with a mean lumbar spine T score of −1.61 | • No treatment | • % change in lumbar spine, hip and 1/3 radius BMD• % change in BTMs• Adverse events | Oxford 2b |
| Brown (2009)12DECIDE studyPhase III double blind RCT1yr follow-up | n=1189 postmenopausal women, mean age 64yr, with a T-score ≤−2 at the lumbar spine or total hip | • Denosumab 60mg/6m• Alendronate 70mg/w+subcutaneous placebo injections/6m | % change in BMD in lumbar spine, total hip, femoral neck, throcanter, 1/3 radius% change in BTMsAdverse events | Oxford 1bJadad 5 |
| Kendler (2010)13STAND studyPhase III double-blind, double-dummy RCT1yr follow-up | n=504 postmenopausal women, mean age 67yr, with a BMD T-score between −2 and −4 on alendronate therapy for at least 6m | • Denosumab 60mg/6m• Alendronate 70mg/w | • % change in BMD at lumbar spine and hip• Change in BTMs• Adverse events | Oxford 1bJadad 5 |
| Kendler (2011)27DAPS studyRandomized, open-label, crossover study2yr follow-up | n=250 postmenopausal women, mean age 65yr, with low BMD | • Denosumab 60mg/6m• Alendronate 70mg/w | • Treatment adherence• Preference and satisfaction with treatment regimen• Adverse events | Oxford 2a |
| Cummings (2009)14FREEDOM studyPhase III placebo controlled RCT3yr follow-up | n=7808 women, mean age 72yr, with BMD T-score <−2.5 but not <−4 at the lumbar spine or total hip | • Denosumab 60mg/6m• Placebo | • New vertebral fracture• Nonvertebral and hip fractures• Adverse events | Oxford 1bJadad 4 |
| Eastell (2010)25FREEDOM subgroup analysis3yr follow-up | n=160 women, mean age 59yr, with BMD T-score between −2.5 and −4 at the lumbar spine or total hip | • Denosumab 60mg/6m• Placebo | • % patients with BTMs (CTX, P1NP, TACP-5b and BALP) below the premenopausal reference interval• Adverse events | Oxford 1bJadad 4 |
| Rizzoli (2010)33FREEDOM subgroup analysis3yr follow-up | Subgroups of age, race, body mass index, creatinine clearance, region, prior use of osteoporosis medication, femoral neck T score, prevalent vertebral fracture and prior nonvertebral fracture | • Denosumab 60mg/6m• Placebo | • New and worsening vertebral fracture• Severe and moderate new vertebral fracture | Oxford 1bJadad 4 |
| Boonen (2010)34FREEDOM subgroup analysis | n=5667 women at a higher risk for fracture at the hip (age ≥75yr) or vertebrae (≥2 prevalent vertebral fractures, moderate/severe prevalent vertebral fractures, or both) | • Denosumab 60mg/6m• Placebo | • New vertebral fracture• Nonvertebral and hip fractures | Oxford 1bJadad 4 |
| Boonen (2011)30FREEDOM subgroup analysis3yr follow-up | Women with multiple and/or moderate or severe prevalent vertebral fractures (n=759), aged ≥75yr (n=2471), and/or femoral neck BMD T-score ≤−2.5 (n=2790) | • Denosumab 60mg/6m• Placebo | • New vertebral fracture• Hip fractures | Oxford 1bJadad 4 |
| Keaveney (2010)36FREEDOM pos hoc analysis3yr follow-up | n=99 women with BMD T-score <−2.5 but not <−4 at the lumbar spine or total hip | • Denosumab 60mg/6m• Placebo | • Femoral strength• L2 vertebral strength | Oxford 1bJadad 4 |
| Silverman (2010)32FREEDOM study3yr follow-up | n=7808 women, mean age 72yr, with BMD T-score <−2.5 but not <−4 at the lumbar spine or total hip | • Denosumab 60mg/6m• Placebo | • Health related quality of life | Oxford 1bJadad 4 |
| Genant (2010)38FREEDOM study3yr follow-up | n=81 women, mean age 75yr, mean BMD T-score of the total hip −1.85 | • Denosumab 60mg/6m• Placebo | • Integral, cortical, subcortical and trabecular BMD• Integral, cortical, subcortical and trabecular BMC | Oxford 1bJadad 4 |
| Reid (2010)28Substudy of STAND and FREEDOM trials1–3yr follow-up | Patients from:• STAND study (n=39 women)• FREEDOM study (n=103 women) | • STAND (12m):∘ Denosumab∘ Alendronate• FREEDOM (36m):∘ Denosumab∘ Placebo | • Bone histomorphometry | Oxford 2b |
| Papapoulus (2010)37FREEDOM extension study1yr follow-up | n=4550 women | • Denosumab 60mg/6m | • % change in BMD at lumbar spine and hip• Change in BTMs• Adverse events | Oxford 2b |
| Seeman (2010)17XTREME-CT studyPhase II double blind RCT1yr follow-up | n=247 postmenopausal women, mean age 60yr | • Denosumab 60mg/6m• Alendronate 70mg/w• Placebo | • Morphologic changes (HR-pQCT at the distal radius and distal tibia, QCT at the distal radius)• Change in BTMs | Oxford 1bJadad 4 |
| Wagman (2010)39Substudy of DEFEND and XTREME-CT trials3yr follow-up | n=5 women, mean age 59yr | • No treatment | • Bone histomorphometry• Change in BTMs | Oxford 4 |
Abbreviations: mg, milligram; m, month; yr, year; BMD, bone mineral density; m, month; w, week; BALP, bone specific alkaline phosphatase; BMD, bone mineral density; BTMs, bone turnover markers; DEXA, dual-energy X-ray absorptiometry; CTX, C-telopeptide; NTX, N-telopeptide; PINP, N-terminal propeptide of type 1 procollagen; PMI, polar moment of inertia; QCT, quantitative computed tomography; TRAP-5b, tartrate-resistant acid phosphatase-5b; HR-pQCT, high-resolution peripheral quantitative computed tomography.
Bekker9 in a double-blind, placebo-controlled RCT, analyzed the effect of single dose escalation effect of denosumab, up to 3.0mg/kg, which resulted in a dose-dependent, rapid (within 12h), profound (up to 84%), and sustained (up to 6 months) decrease in urinary N-telopeptide (uNTX). At 6 months, denosumab compared with placebo, showed a greater decrease in the urinary NTX/creatinine (81% vs 10%) and in serum NTX (56% vs 2%). Bone-specific alkaline phosphatase (BALP) levels decreased remarkably after the first month, and intact parathyroid hormone (iPTH) levels increased up to 3-fold after 4 days in denosumab group, but returned to baseline levels during the follow-up.
A 2-year double blind, placebo-controlled, dose-ranging RCT with an open-label arm compared in 412 postmenopausal women with low BMD, the efficacy and safety of denosumab 14, or 30mg/3 months or denosumab 14, 60, 100, or 210mg/6 months, with alendronate 70mg/week and placebo.10 The first year denosumab increased BMD at the lumbar spine of 3–6.7% (4.6% with alendronate, −0.8% with placebo), at the total hip of 1.9–3.6% (2.1% with alendronate, −0.6% with placebo), and at the distal third of the radius of 0.4–1.3% (−0.5% with alendronate, −2% with placebo). Reductions in serum C-telopeptide (sCTX) were higher compared with placebo (p<0.001). At 24 months, compared with placebo, all doses of denosumab significantly increased BMD at all skeletal sites, and compared with alendronate, denosumab was associated with similar or greater increases in BMD, with the exception of the 14mg/6 months dose, in which the change in lumbar spine BMD was less (p=0.020). Compared with placebo, significant reductions (p<0.001) in sCTX and urine N-telopeptide/creatinine were observed for all doses of denosumab except the 14mg/6 months group, and reductions in BALP levels were also higher (p<0.002). Moreover, reductions in sCTX by alendronate were less than those observed with the higher doses of denosumab, whereas reductions in BALP were similar with alendronate and denosumab.15 In a post hoc analysis, hip scans were performed using dual-energy X-ray absorptiometry (DEXA) at baseline, 12, and 24 months in 116 patients to evaluate BMD and cross-sectional geometry parameters at the narrowest segment of the femoral neck, the intertrochanter, and the proximal shaft. These analyses showed that at 12 and 24 months, denosumab and alendronate improved these parameters compared with placebo. Denosumab effects were greater than alendronate at the intertrochanteric and shaft sites.8 After that, a 2-year extension study16 which included 307 patients showed that continuous denosumab treatment for 4 years, compared with placebo, was associated with significant increases in BMD at all skeletal sites (0.001), at the lumbar spine (9.4–11.8%), at total hip (4–6.1%), and with a sustained reduction of BTMs. Discontinuation of denosumab led to a BMD decrease of 6.6% (lumbar spine), 5.3% (total hip) within the first 12 months. Retreatment increased lumbar spine BMD by 9% from original baseline values. Levels of BTM increased upon discontinuation and decreased with retreatment. Those on continuous denosumab followed 2 additional years of treatment that led to further gains in BMD interval.29 From the extension study baseline, mean BMD increased at the lumbar spine by 2.9%, total hip by 1.1%, 1/3 radius by 1%, and femoral neck by 1.2%. Six years of continuous treatment was associated with mean BMD changes from parent study baseline of 13.3, 6.1, and 1.9% for the lumbar spine, total hip, and 1/3 radius, respectively, and 5.6% for femoral neck. At year 6, sCTX remained below parent study baseline with a median reduction of 54.8% compared with baseline.
In the DEFEND trial, a 2-year double-blind, placebo-controlled RCT11 332 postmenopausal women with osteopenia received denosumab 60mg/6 months or placebo. Compared with placebo, denosumab significantly increased BMD at lumbar spine (6.5 vs −0.6%), at the hip, 1/3 radius, and total body (p<0.001), increased distal radius volumetric BMD (p<0.010), improved hip structural analysis parameters, and significantly suppressed sCTX, tartrate-resistant acid phosphatase-5b (TRAP-5b), and intact N-terminal propeptide of type 1 procollagen (PINP). Besides, denosumab significantly increased volumetric bone mineral content (BMC) along the radius (proximal, distal, and ultradistal sections) and bone strength.26 In a 2 year off-treatment period of DEFEND study,31 BMD decreased at all sites, but those patients who previously had received denosumab maintained a higher BMD compared with those on placebo. After denosumab discontinuation, BTM increased above baseline within 3 months (sCTX) or 6 months (PINP), peaked at 30 months (sCTX) or 36 months (PINP), and returned to baseline by month 48. BTM did not significantly change in the placebo group.
The DECIDE trial, a phase III, 1 year double-blind RCT, compared denosumab (60mg/6 months) with oral alendronate (70mg/week) in postmenopausal women with low BMD. Denosumab, at the total hip, significantly increased BMD compared with alendronate (3.5% vs 2.6%, p<0.001). Furthermore, significantly greater increases in BMD were observed with denosumab treatment at all measured skeletal sites (treatment difference: 0.6% femoral neck; 1.0% trochanter; 1.1% lumbar spine; 0.6%1/3 radius). Denosumab also led to significantly greater reduction of BTM.12
The STAND study was a 1-year double-blind, double-dummy RCT that included 504 postmenopausal women (BMD T-score between −2.0 and −4.0) who had been on alendronate for at least 6 months. Subjects received alendronate 70mg/week for 1 month and then were randomly assigned to continue on alendronate or denosumab 60mg/6 months. In denosumab group, total hip BMD increased by 1.90% vs 1.05% in the alendronate group (p<0.001). Similar results were found at the lumbar spine, femoral neck, and 1/3 radius (all p<0.0130). Median sCTX levels remained close to baseline in the alendronate group and significantly decreased in denosumab patients.13
In a 2-year, randomized, crossover study,27 DAPS study, adherence, preference, and satisfaction in 250 patients on denosumab 60mg/6 months or alendronate 70mg/week were analyzed. Adherence in the first 12 months was 76.6% for alendronate and 87.3% for denosumab. Significantly more patients preferred and were more satisfied with the 6-month injection than with the weekly tablet (p<0.001).
The FREEDOM study, a 3-year phase III placebo controlled RCT14 enrolled 7868 women with osteoporosis and evaluated the effect of denosumab 60mg/6 months. As compared with placebo, denosumab reduced the risk of new radiographic vertebral fracture, cumulative incidence of 2.3% (7.2% placebo), RR=0.32 (95% CI 0.26–0.41), a relative decrease of 68%; reduced the risk of hip fracture, cumulative incidence of 0.7% (1.2% with placebo), HR=0.60 (95% CI 0.37–0.97), a relative decrease of 40%; reduced the risk of nonvertebral fracture, cumulative incidence of 6.5% (8% with placebo), HR=0.80 (95% CI 0.67–0.95), a relative decrease of 20%. Denosumab was also associated with a relative increase in BMD of 9.2% at the lumbar spine and 6% at the total hip, and a decreased in sCTX levels by 72%. Levels of PINP were also lower compared with placebo. The estimated number needed to treat (NNT) to prevent a clinical vertebral fracture with denosumab was 62 (95% CI 46.3–93.8) and for a radiologic vertebral fracture was 22 (95% CI 18.3–27.5), 230 for hip fracture.
A substudy25 of the FREEDOM trial evaluated 160 women in whom 1 month post-injection, sCTX levels in denosumab group decreased to levels below the premenopausal reference interval. The percentage of subjects with sCTX below this interval before each subsequent injection decreased from 79% to 51%. Besides, sCTX and PINP remained below the premenopausal reference interval at all time points in 46% and 31% denosumab subjects. With denosumab, but not placebo, there were significant correlations between sCTX reduction and BMD increase (r-value: −0.24 to −0.44). Another subanalysis by subgroups of age, race, body mass index, creatinine clearance, region, prior use of osteoporosis medication, femoral neck T score, prevalent vertebral fracture, and prior nonvertebral fracture found, compared with placebo a reduced risk of new and worsening vertebral fractures as well as severe and moderate new vertebral fractures but did not find differences between subgroups in the efficacy of denosumab in reducing new vertebral fractures,33 neither in a subgroup of patients at higher fracture risk.34
Similarly, in another post hoc analysis,30 denosumab, compared with placebo significantly reduced the risk of new vertebral fractures in women with multiple and/or severe prevalent vertebral fractures (16.6% placebo vs 7.5% denosumab), the risk of hip fractures in subjects aged ≥75 years (2.3% vs 0.9%) or with a baseline femoral neck BMD T-score ≤−2.5 (2.8% vs 1.4%).
More subanalyses showed that denosumab significantly increased femoral strength compared with baseline by 5.4% at 12 months, which continued over time reaching 8.4% at 36 months. In contrast, placebo decreased femoral strength at 36 months (−5.4%). The same trends were seen at the spine but changes were superior: at 36 months, vertebral strength increased by 18.1% with denosumab and decreased by −4.1% with placebo.36 With regard to the health-related quality of life, statistically significant differences between groups were not found.32 An exploratory analysis38 evaluated the magnitude of changes from baseline and from placebo in denosumab subjects with hip Quantitative Computed Tomography in 81 patients. Over 36 months, the improvements from baseline in integral hip BMD reached 6.3% and 4.8% for BMC with denosumab, but decreased with placebo. The differences between groups were highly significant (p<0.001) at 12, 24, and 36 months for integral, cortical, and trabecular BMD and BMC, except for cortical BMD at 12 months (p=0.066). Along with patients from the STAND study, 142 Iliac crest bone biopsies were collected at 24 and/or 36 months.28 In the FREEDOM study, median eroded surface was reduced by >80% and osteoclasts were absent from >50% of biopsies in the denosumab group. Double labeling in trabecular bone was observed in 94% of placebo bones, and in 19% of those treated with denosumab. Median bone formation rate was reduced by 97%. In the STAND trial, indices of bone turnover tended to be lower in the denosumab group, compared with alendronate. Double labeling in trabecular bone was seen in 20% of the denosumab biopsies and in 90% of alendronate samples.
The open-label extension of FREEDOM is evaluating the long-term (10 years) efficacy and safety of denosumab in 4550 patients.37 During the first year, in the denosumab group lumbar spine BMD increased an extra 2% to a total of 12.1%, and total hip BMD an additional 0.8% to a total of 6.5%. Reductions in BTM continued, and 31 osteoporotic nonvertebral fractures were reported.
The XTREME-CT study17 was a phase II double-blind RCT which compared morphologic changes of denosumab 60mg/6 months, alendronate 70mg/week and or placebo for 12 months. Morphologic changes were assessed using high-resolution peripheral quantitative computed tomography at the distal radius and distal tibia. Alendronate prevented the decline (−0.6% to 2.4%, p=0.051 to <0.001 vs placebo), and denosumab prevented the decline or improved these variables (0.3–3.4%, p<0.001 vs placebo). Changes in total and cortical BMD were greater with denosumab than with alendronate (p≤0.024). Similar changes in these parameters were observed at the tibia.
A small cohort of patients from the DEFEND and XTREME-CT studies discontinued denosumab treatment for 12–36 months.39 Bone histomorphometry results were compared with results from placebo-treated subjects in the bone biopsy substudy. BTMs were similar to pretreatment values, and 100% of biopsy specimens had evidence of tetracycline labels.
Safety of denosumabDenosumab was in general safe and well tolerated. The most common adverse events with denosumab were urinary tract infection, upper respiratory tract infection, and sciatica.
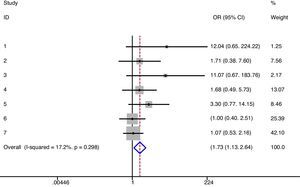
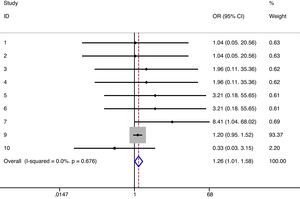
As exposed in the methods section, when possible meta-analyses were performed (Table 4). We did not find differences in the risk of any type infections between denosumab (6mg/6 months) and alendronate/placebo (irrespectively of the follow-up). There was no heterogeneity either.12–14,16 The same results were obtained when data regarding 12-month follow-up were analyzed [RR=1.13 (95% CI 0.93–1.38)] (I2=0%, p=0.329),12,13 in a direct comparison between denosumab and alendronate [RR=1.11 (95% CI 0.92–1.34)] (I2=0%, p=0.461),12,13,16 and specifically for upper respiratory infections.10,11,15,16 On the other hand, we detected a significant increase in the risk of urinary infections in patients treated with denosumab (Fig. 2). However, heterogeneity among studies was also found (17%) not statistically significant though.10,11,15,16 Moreover, there was a slight increase in the risk of severe infections with denosumab (Fig. 3) and no heterogeneity.10,11,13–16 But when we performed a meta-analysis of severe infections during the first 12 months of follow-up, these differences disappeared [RR=0.59 (95% CI 0.14–2.40)] (I2=0%, p=0.761).10,13
Safety of denosumab. Results of the meta-analysis.
| RR | 95% CI | p value | I2 (%), p value | Studies | |
| All infections | 0.97 | 0.89–1.05 | 0.441 | 0%, 0.429 | 18–20,22 |
| Upper respiratory infections | 0.11 | 0.83–1.49 | 0.472 | 0%, 0.860 | 16,17,21,22 |
| Urinary infections | 1.73 | 1.13–2.64 | 0.012 | 17%, 0.298 | 16,17,21,22 |
| Severe infections | 1.26 | 1.01–1.58 | 0.041 | 0%, 0.676 | 16,17,19–22 |
| Cancer | 1.15 | 0.93–1.41 | 0.190 | 0%, 0.920 | 16,17,19–21 |
| Cardiovascular events | 1.01 | 0.82–1.23 | 0.982 | 34%, 0.198 | 16,20,21 |
| Eczema | 1.91 | 1.43–2.55 | <0.001 | 0%, 0.661 | 16,17,20 |
| Pain in extremity | 1.01 | 0.76–1.32 | 0.991 | 0%, 0.930 | 16,17,19–23 |
Abbreviations: RR, risk ratio; CI, confidence interval; I2, heterogeneity statistic.
Regarding the risk of cancer, cardiovascular events, and pain in an extremity when we compared denosumab with alendronate/placebo (independently of the time of follow-up) we did not find differences,10,11,13–17 see Table 4.
And finally, a significant increase in the risk of eczema was found when we compared denosumab (6mg/6 months) with alendronate/placebo (irrespectively of the follow-up) without heterogeneity among the included studies (I2=0%, p=0.661).10,11,14
DiscussionDenosumab is a new drug in the treatment of osteoporosis. The rationale of denosumab emerges from recent research on the pivotal role of RANKL and osteoprotegrin in the control of osteoclastic proliferation and differentiation.3 Denosumab is a human monoclonal antibody that binds to RANKL with high affinity and specificity, and thereby mimics the action of osteoprotegrin through neutralization of RANKL and the inhibition of the osteoclastogenesis.23 According to recent clinical studies, denosumab seems to be effective and safe in the treatment of postmenopausal osteoporosis.
Therefore, the aim of this systematic literature review was to analyze the efficacy and safety of denosumab in osteoporosis. For the purpose of this review we included RCT in which the comparator was placebo or alendronate as we considered this as the best way to perform the review.Overall, denosumab was effective and safe in the treatment of osteoporosis. It decreased BTMs and increased BMD in both lumbar spine and hip.
But, interestingly, only one of the selected studies considered the incidence of new vertebral fractures as its primary endpoint.14 The rest considered fractures as an adverse event or did not evaluate this outcome. In recent years, the use of surrogate end points for antifracture efficacy of new treatments has clearly increased. Given the availability of effective drugs for osteoporosis, new placebo-controlled trials for new therapies that enroll moderate to high-risk patients are sometimes perceived as unethical in many countries. Alternatives to the current paradigm for establishing antifracture efficacy of a new drug include the design of placebo-controlled trials in subjects with low fracture risk, assuming that the results could be extrapolated to patients at high risk for fracture; or RCTs that compare the new drug with an alternative that has already demonstrated consistent and robust anti fracture efficacy. This could be achieved with either a non-inferiority or superiority design, in patients with moderate to high fracture risk. In both cases, the primary endpoint would be new fracture incidence, but the required sample sizes would be extremely large. This implies significant costs that may jeopardize the development of new therapeutic agents in osteoporosis.40
Only the FREEDOM trial fulfilled these conditions.14 In this study, denosumab reduced the risk of new radiographic vertebral fractures and also hip and other nonvertebral fractures. The estimated NNT to prevent a clinical vertebral fracture with denosumab was 62 (95% CI 46.32–93.85) and for a radiologic vertebral fracture was 22 (95% CI 18.29–27.45),14 quite similar to those reported for bisphosphonates and other antiosteoporotic drugs. On the other hand, denosumab NNT to prevent a hip fracture was higher compared to the registered for other drugs as bisphosphonates. This finding could be explained at least in part due to population differences and fracture risk factors. We could not calculate the NNT for age subgroups although in the FREEDOM study it was communicated that its efficacy is similar in higher fracture risk subgroups.
However, as mentioned above, the efficacy of denosumab increasing BMD was demonstrated in several trials,8,10–13,15,16,26 which was higher compared with alendronate and placebo. Results were presented as a percentage of change in the BMD or, in some cases, as the difference in the percentage of change between the study groups. This prevented us for performing a meta-analysis. BMD is the most frequently used intermediate outcome in osteoporosis. Prospective studies have shown that women with low BMD are at an increased risk of clinical fractures.41,42 Some other studies with denosumab43,44 have concluded that relatively large gains in total hip BMD might indicate a greater reduction in risk of nonvertebral fracture. However, from a clinical perspective, this outcome is an imperfect proxy for true clinical endpoint. Furthermore, the benefit of treatment with different agents correlates with different degrees of differences in BMD.45
Denosumab also reduced bone biomarkers very effectively, which is dose-dependent and higher compared with other therapies.9,10,12,25 The assessment of biochemical markers is the most sensitive method for monitoring acute changes in bone metabolism. Several studies have shown a good correlation46,47 between predicted and measured BMD for different groups of patients. Although the role of bone biomarkers in the follow-up of osteoporotic patients has not fully established yet, they are extensively used. However, the variability is large and the predictive ability of markers is not as certain for individual patients.
With regard to safety issues, denosumab was safe and well tolerated. The most common adverse events were urinary tract infection, upper respiratory tract infection, and sciatica. According to our meta-analysis, there was a slightly increased risk of urinary infection and eczema. There was also an increased risk of severe infections, but disappeared when we performed a meta-analysis during the first 12 months of follow-up. Averse events are probably related to the RANKL inhibition, which could affect the immune response. It is thought that CD4+ T helper cells require RANKL signaling for activation and priming, an important aspect of cell-mediated immunity.48 Because of the importance of RANK/RANKL in immunity, inhibition of this system could potentially make patients susceptible to infections and cancer. Although we found only a small and unclear increase of the risk of infections, particular attention should be given to any effects on the immune system in patients treated with long-term RANKL inhibition.
Osteonecrosis of the jaw (ONJ) has recently emerged as a serious adverse event of osteoporosis drugs.49 To date, no ONJ cases with denosumab have been communicated. Whether ONJ will complicate long-term RANKL inhibition is unknown, but since denosumab deeply suppresses bone turnover, this issue should be considered in its post-approval long-term use.
Another consequence of a profound inhibition of bone remodeling is the ‘frozen bone’. It leads to an increase of microfractures and bone fragility. A number of reports of unusual fragility fractures because of a dynamic bone disease after prolonged alendronate therapy have been published.50,51 Therefore, caution with prolonged RANKL inhibition should also be taken, although preliminary results are not alarming.35
Other issues like adherence and compliance are also important, since they are often low for chronic conditions, especially if they are asymptomatic like osteoporosis. Among patients receiving treatment for osteoporosis, approximately half discontinue therapy within the first six months.52 Analyses of administrative data suggest that more adherence and compliance are required to achieve antifracture efficacy.53 In the present systematic review, we have found that, after 12 months of treatment significantly more patients preferred and were more satisfied with the six-month injection than with weekly tablets. In case of elderly patients or individuals with relevant comorbidities and/or patients who require multiple medications, a twice-yearly subcutaneous injection seems appropriate.
In conclusion, denosumab has demonstrated to reduce the incidence of vertebral and nonvertebral fractures in women with osteoporosis and profoundly inhibits bone metabolism. An increase in the incidence of urinary infections and eczema has also been found. The role of this new drug in the therapeutic arsenal for osteoporosis will be fully established in the coming years.
Responsabilidades éticasProtección de personas y animalesLos autores declaran que para esta investigación no se han realizado experimentos en seres humanos ni en animales.
Confidencialidad de los datosLos autores declaran que en este artículo no aparecen datos de pacientes.
Derecho a la privacidad y consentimiento informadoLos autores declaran que en este artículo no aparecen datos de pacientes.
FundingSpanish Society of Rheumatology.
Conflicts of interestThe authors have no conflicts of interest to declare.