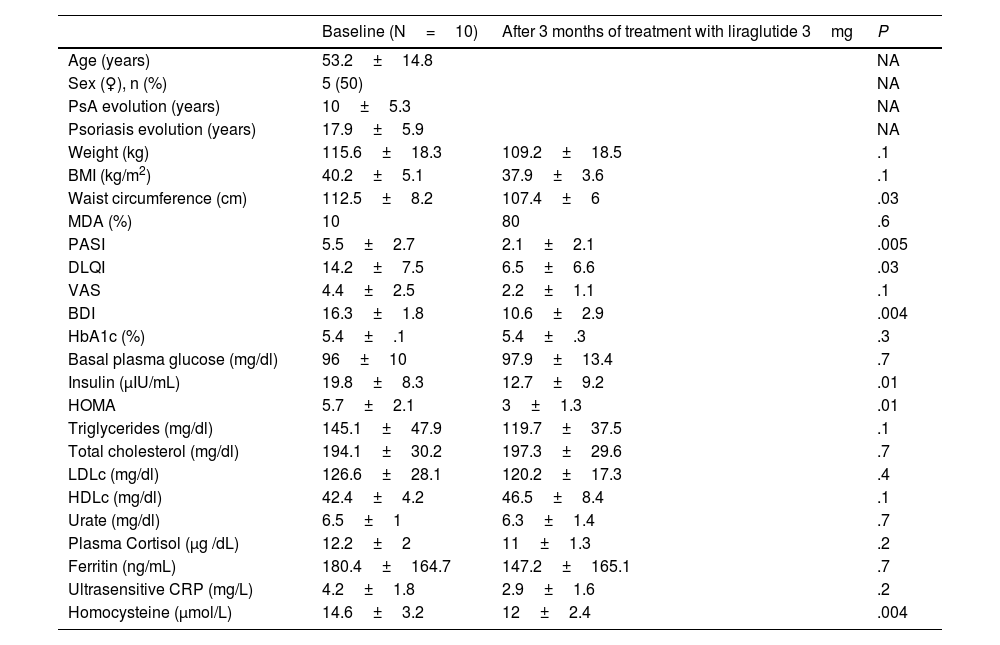
The coexistence of obesity in patients with psoriatic arthritis (PsA) is associated with increased disease activity, sub-optimal therapeutic response, and a lower likelihood of achieving minimal disease activity (MDA).1–4 This study was conducted with 10 patients with PsA and obesity (50% women, mean age 53.2±14.8 years, BMI 40.2±5.1kg/m²), and evaluated the effects after three months of treatment with liraglutide 3mg on disease activity and metabolic and psychological parameters. The results are shown in Table 1.
Changes in metabolic, dermatologic, psychological parameters and MDA after three months of treatment with liraglutide 3mg in patients with PsA and obesity.
| Baseline (N=10) | After 3 months of treatment with liraglutide 3mg | P | |
|---|---|---|---|
| Age (years) | 53.2±14.8 | NA | |
| Sex (♀), n (%) | 5 (50) | NA | |
| PsA evolution (years) | 10±5.3 | NA | |
| Psoriasis evolution (years) | 17.9±5.9 | NA | |
| Weight (kg) | 115.6±18.3 | 109.2±18.5 | .1 |
| BMI (kg/m2) | 40.2±5.1 | 37.9±3.6 | .1 |
| Waist circumference (cm) | 112.5±8.2 | 107.4±6 | .03 |
| MDA (%) | 10 | 80 | .6 |
| PASI | 5.5±2.7 | 2.1±2.1 | .005 |
| DLQI | 14.2±7.5 | 6.5±6.6 | .03 |
| VAS | 4.4±2.5 | 2.2±1.1 | .1 |
| BDI | 16.3±1.8 | 10.6±2.9 | .004 |
| HbA1c (%) | 5.4±.1 | 5.4±.3 | .3 |
| Basal plasma glucose (mg/dl) | 96±10 | 97.9±13.4 | .7 |
| Insulin (μIU/mL) | 19.8±8.3 | 12.7±9.2 | .01 |
| HOMA | 5.7±2.1 | 3±1.3 | .01 |
| Triglycerides (mg/dl) | 145.1±47.9 | 119.7±37.5 | .1 |
| Total cholesterol (mg/dl) | 194.1±30.2 | 197.3±29.6 | .7 |
| LDLc (mg/dl) | 126.6±28.1 | 120.2±17.3 | .4 |
| HDLc (mg/dl) | 42.4±4.2 | 46.5±8.4 | .1 |
| Urate (mg/dl) | 6.5±1 | 6.3±1.4 | .7 |
| Plasma Cortisol (μg /dL) | 12.2±2 | 11±1.3 | .2 |
| Ferritin (ng/mL) | 180.4±164.7 | 147.2±165.1 | .7 |
| Ultrasensitive CRP (mg/L) | 4.2±1.8 | 2.9±1.6 | .2 |
| Homocysteine (μmol/L) | 14.6±3.2 | 12±2.4 | .004 |
Data are expressed as mean±SD or absolute numbers and %.
BDI: Beck Depression Inventory; BMI: body mass index; DLQI: Dermatology Life Quality Scale; MDA: minimal disease activity; NA: not applicable; PASI: Psoriasis Severity Index; PsA: Psoriatic arthritis; VAS: Visual Analogue Pain Scale.
With the introduction of biological therapies, the management of PsA has evolved dramatically, outcomes on quality of life and control of metabolic comorbidities are still not comparable to those observed in isolated psoriasis.1–3 Obesity is one of the most frequently associated risk factors for PsA and is associated with increased disease activity and a poorer therapeutic prognosis, decreasing the possibility of achieving MDA.2–4 It has been seen that weight loss in patients with PsA prior to the start of treatment with TNFα inhibitors increases the chances of MDA.5,6 Moreover, obesity increases cardiovascular risk, the main cause of morbidity and mortality in these patients.1,2
Treatment with liraglutide, a GLP-1 analogue (aGLP1), has shown benefits not only in weight reduction, but also in the improvement of metabolic comorbidities and dermatological lesions in patients with psoriasis and obesity, which could be mediated by anti-inflammatory effects independent of weight loss.7,8 In our study, we observed a significant improvement in MDA and a reduction in the severity of PASI and DLQI, as well as in inflammatory markers, suggesting a potential direct benefit of liraglutide on the underlying inflammation of PsA.
These findings emphasize the need for a holistic therapeutic approach in PsA, which not only addresses the joint and skin manifestations, but also the metabolic comorbidities, since these lead to an increased risk of developing cardiovascular disease, the main cause of mortality in these patients.1,2 GLP-1 inhibitors have been shown to reduce cardiovascular events in patients with obesity.9,10
Although the study has limitations, including a small sample size and the absence of a control group, it is the first to specifically evaluate the effects of liraglutide in patients with PsA and obesity, and provides promising preliminary data. Furthermore, despite the short duration of follow-up, although it is another limitation, these short-term results already indicate a significant improvement in the parameters evaluated.
It is important to consider the limited access to anti-obesity treatments such as liraglutide in most health systems, including ours, which could generate a selection bias towards patients with a higher socioeconomic level and leave population sectors with a lower socioeconomic level, where the prevalence of obesity is higher, without therapeutic possibilities. For this reason, it is imperative to provide scientific evidence such as this to emphasize the importance of including effective and long-lasting weight management strategies and other metabolic comorbidities as an integral part of the treatment of PsA, to improve quality of life and reduce cardiovascular risk.
To conclude, our study suggests that liraglutide is a safe and effective intervention for patients with PsA and obesity, improving not only metabolic parameters, but also exerting a beneficial effect on the control of disease activity and skin lesions. Further studies with a larger sample size, controlled and with a long follow-up are required to confirm these findings, as well as to explore the underlying mechanisms of the observed benefits.