To describe the experience of treatment with baricitinib (BARI) and/or tocilizumab (TCZ), in monotherapy or combined, in patients admitted for interstitial pneumonia secondary to COVID19, and for 30 days after discharge.
MethodsMedical records of patients admitted with COVID19 and IP with PaO2/FiO2<300, treated with BARI and/or TCZ, and compared with patients who did not, were retrospectively reviewed.
ResultsSixty patients were included; 43 (72%) are males, mean age 67 (SD: 14) years (<50 years: 17%; 51–70: 30%; >70: 53%), with 8.5 (SD: 1) days of symptoms. Sixteen (27%) patients required ICU (94% in <70 years). Fifteen (25%) patients died, 67% in >70 years; 11 (18%) patients died in the first 15 days of admission and 4 (7%) between days 16 to 30. Twenty-three (38%) patients received BARI, 12 (52%) monotherapy (Group 1), during 6 (SD: 2.6) days on average, none required ICU and 2 (17%) died. Thirty-one (52%) patients received TCZ, 20 (33%) as monotherapy (Group 2), 16 (52%) patients required ICU and 4 (20%) died. In the 11 (18%) patients who received BARI (2.8 [SD: 2.5] days average) and TCZ combined (Group 3), 3 (27%) required ICU and died. There were no severe side effects in BARI or TCZ patients. In the 17 (28%) patients who received neither BARI nor TCZ (Group 4), none required ICU and 6 (35%) died. Mean (SD) PaO2/FiO2 at admission between groups was respectively: 167 (82.3), 221 (114.9), 236 (82.3), 276 (83.2).
ConclusionTreatment with BARI and TCZ did not cause serious side effects. They could be considered early in patients with NI secondary to COVID19 and impaired PaO2/PaFi.
Describir la experiencia con baricitinib (BARI) y/o tocilizumab (TCZ), en monoterapia o combinados en pacientes ingresados por neumonía intersticial (NI) por COVID-19 y durante los 30 días después del alta.
MétodoSe revisaron retrospectivamente las historias clínicas de los pacientes ingresados por COVID-19 y NI, con PaO2/FiO2<300, tratados con BARI y/o TCZ y se compararon con pacientes que no los recibieron.
ResultadosSe incluyeron 60 pacientes; 43 (72%) varones, edad media 67 (DE: 14) años (< 50 años: 17%; 51-70: 30%; > 70: 53%), y 8,5 (DE: 1) días de síntomas. Dieciséis (27%) ingresaron en la unidad de cuidados intensivos (UCI) (94% < 70 años). Quince (25%) fallecieron (67% > 70 años); 11 (18%) de ellos en los primeros 15 días del ingreso y cuatro (7%) entre los días 16 y 30. Veintitrés (38%) pacientes recibieron BARI, 12 (52%) en monoterapia (Grupo 1), durante seis (DE: 2.6) días de promedio, ninguno de ellos ingresó en UCI y dos (17%) fallecieron. Treinta y un (52%) pacientes recibieron una dosis de TCZ, 20 (33%) en monoterapia (Grupo 2), 16 (52%) ingresaron en UCI y cuatro (20%) fallecieron. Entre los 11 (18%) pacientes que recibieron BARI (2,8 [DE: 2,5] días de promedio) y TCZ combinados (Grupo 3), tres (27%) ingresaron en UCI y fallecieron. No hubo efectos secundarios graves entre los que recibieron BARI y/o TCZ. Entre los 17 (28%) pacientes que no recibieron ni BARI ni TCZ (Grupo 4), ninguno ingresó en UCI y seis (35%) fallecieron. La PaO2/FiO2 media (DE) al ingreso entre los grupos fue respectivamente: 167 (82,3), 221 (114,9), 236 (82,3), 276 (83,2).
ConclusiónEl tratamiento con BARI y TCZ no provocó efectos secundarios graves. Podrían considerarse precozmente en pacientes con NI secundaria a COVID-19 y deterioro de PaO2/PaFi.
Coronavirus disease 2019 (COVID-19), which started in China in December of 2019, has led to a pandemic extremely quickly, with effects on health in many countries.
When the immune response is unable to control infection, the virus activates macrophages and granulocytes, causing the progressive release of proinflammatory cytokines, especially IL6, IL1, IL10, interferon and TNF, provoking a real “cytokine storm”.1–5 In some patients, it induces the appearance of pulmonary infiltrates and rapid development of respiratory failure and severe acute respiratory syndrome (SARS), which is the main cause of mortality in these patients.6–8 Although mortality vary between countries, it has been estimated 0–3% to 1% in general population infected and 14% among hospitalized cases (95% CI 3.9–32%).9–12
Management, especially in patients with severe disease, is one of the main problems posed by this disease. So far, it has been based on observational studies or on drugs used in other viral diseases. Although there are numerous clinical trials underway, there is no evidence to recommend a specific treatment.
In the first phase of the disease, treatment with antiviral drugs (remdesivir,13 a combination of lopanivir/ritonavir,14 or interferon β1b15), or drugs that may interfere with the development of the virus (hydroxychloroquine),16 predominates. After a few days, in the second inflammatory phase, associated with the release of cytokines, the use of drugs such as tocilizumab (TCZ), with anti-IL6 action and therefore for the treatment of SARS, predominates.5,8,17
Given its high contagiousness, this virus has caused an explosion of cases, with saturation of the health system and of intensive care units (ICU). In Spain, 43% of infected patients required hospital admission and 3.9% ICU admission. Approximately 75% of patients admitted for COVID19 present positive imaging for interstitial pneumonia (IP).10,18,19
As in many other hospitals in Spain, with a large number of patients admitted for COVID19-IP, our center had to restructure most of the hospitalization areas and the activities of the entire medical staff at the times of greatest impact of the pandemic. In this context, and with the possibility of supply problems of drugs such as TCZ, in cases of SARS, possible therapeutic alternatives were sought, for their early administration or as a bridge to reduce the consumption of TCZ.
Baricitinib (BARI) is a selective JAK1/JAK2 intracellular route inhibitor drug, administered orally at a daily dose of 2–4mg, and approved for the treatment of adult patients with rheumatoid arthritis.20 Unlike other biological drugs, which are predominantly inhibitors of one cytokine, BARI inhibits multiple cytokines, such as IL-6 and interferon, among others.21–22 In addition to its anti-inflammatory effect, there are data indicating that BARI, at the doses used in rheumatology, may have antiviral action, interfering with its binding to ACE2 receptors (angiotensin-converting enzyme).23–24 This inhibits the entry of the virus into the cell and its intracellular coupling by binding to GAK (cyclin G-associated kinase), which regulates endocytosis and acts on AAK1 (Associated protein kinase 1), thus interfering with viral replication.24–25
The main objective of the study is to describe the experience of treatment with BARI and/or TCZ, in monotherapy or combined, in patients admitted for IP secondary to COVID19, and for 30 days after discharge.
MethodsThis is a retrospective observational study. The medical records of the patients admitted to our center due to IP secondary to COVID19, demonstrated by PCR (polymerase chain reaction) technique, who started treatment with BARI and/or TCZ, from March 27 to April 2, 2020 and their clinical progress until discharge, was reviewed. Survival and serious complications were reviewed after 30 days of discharge.
PatientsPatients received, at the discretion of the responsible physician, standard antiviral treatment, and anti-cytokine therapy (BARI or TCZ), in patients with the following inclusion criteria: (1) Interstitial pneumonia and (2) PaO2/FiO2 (ratio between PaO2 in mmHg and FiO2 in%) <300. Exclusion criteria: pregnancy, previous thrombosis history, HBV infection, current bacterial infection, neutrophil<1000/mm3, lymphocyte <300/mm3, platelets <50,000/mm3 and transaminases values 4-fold higher than the upper normal limit.
For analysis, they were divided into 4 groups: Group 1: patients treated with BARI, at an oral dose of 2mg or 4mg daily; Group 2: patients receiving an intravenous dose of TCZ, adjusted for weight (400mg in patients weighing <75kg or 600mg in those weighing ≥75kg); Group 3: patients who received BARI and TCZ combined and Group 4: patients who received neither BARI nor TCZ.
Data collectionGeneral patient data, comorbidities and the Charlson Comorbidity Index, which includes the estimated 10-year survival were included. During admission: symptoms (fever, cough, dyspnea, diarrhea), time of progression of symptoms; chest x-ray; laboratory data: blood count, general biochemistry, arterial blood gas, D-dimer, sedimentation rate (ESR), C-reactive protein (CRP), O2 saturation, PaO2/FiO2 (mild if <300, moderate if <200, and severe if <100),26 respiratory rate (RR); treatment and doses of drug received: remdesivir, lopanivir/ritonavir, interferon β1b, hydroxychloroquine, azithromycin, and anti-inflammatory (corticosteroids, BARI, TCZ); and outcome: admission to the ICU, hospital discharge, or death.
The study was approved by the Spanish Agency for Medicine and Health Products and (AEMPS), and the Ethics Committee of the University General Hospital of Elche (Alicante), Spain (Code: COVID19-BARI/TCZ-HMB).
Statistical analysisCategorical variables are expressed as frequencies and percentages, and continuous variables with normal distribution of data as mean and standard deviation (SD). The chi-square (χ2) test and Student's t-test were used for comparison of qualitative and quantitative variables, respectively. Statistical significance was set at p<0.05.
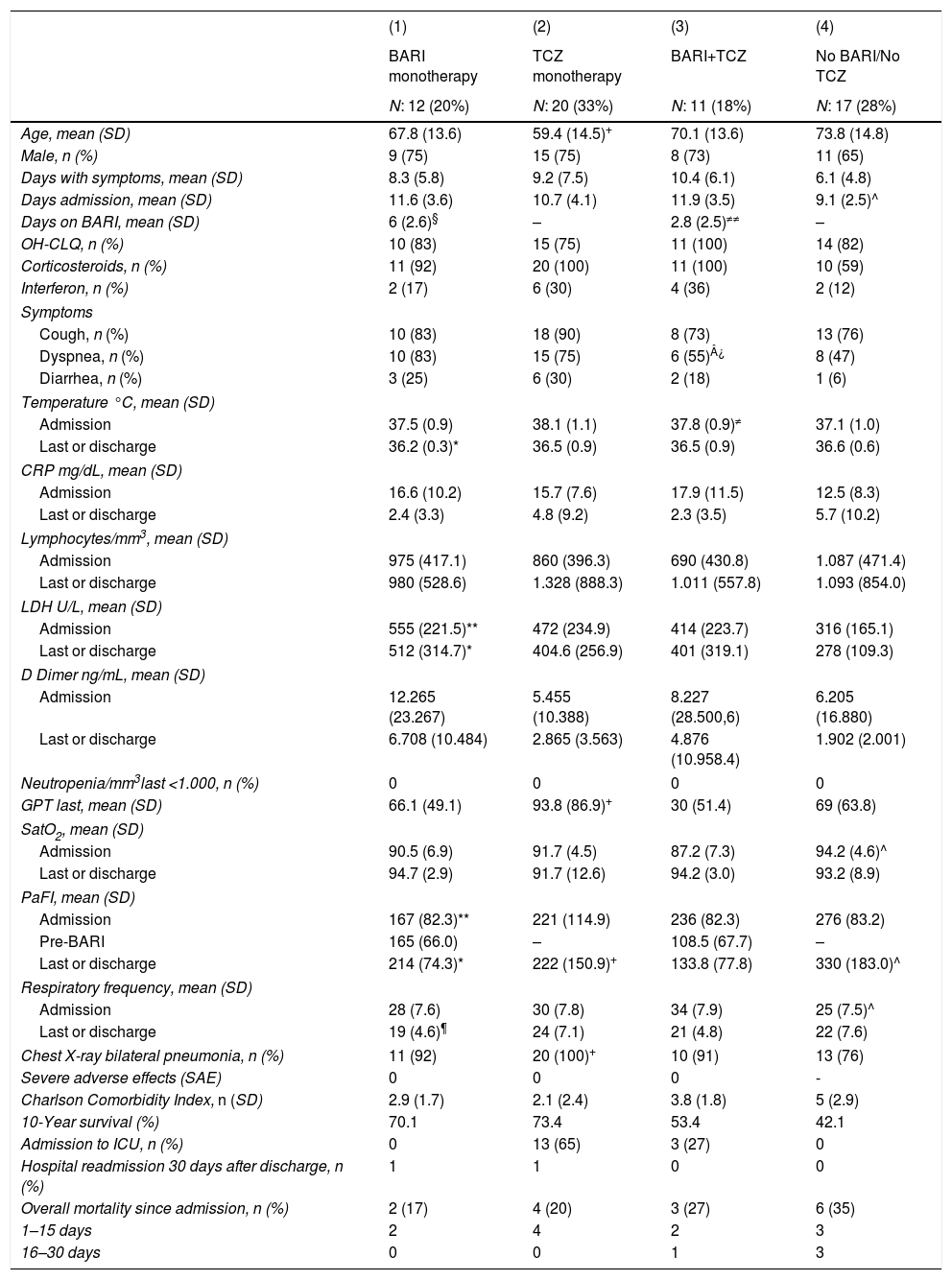
ResultsOverall population characteristicsA total of 60 patients were included, of which 43 (72%) were male, with a mean age of 67 (SD: 14) years (range: 34–91 years; <50: 17%, 50–70: 30%, >70: 53%). The mean time of symptom progression was 8.5 (SD: 1) days and mean time of hospital admission, 11 (SD: 3.7) days. Comorbidities included, high blood pressure: 24 (40%) patients, diabetes mellitus: 10 (17%), ischemic heart disease: 9 (15%), COPD: 6 (10%), cancer: 6 (10%), liver cirrhosis: 2 (4%) and 1 (2%) patient respectively, liver transplant, ankylosing spondylitis in treatment with adalimumab, hepatitis C virus, and AIDS. The Charlson Comorbidity Index score was 3.41, with estimated 10-year survival of 59.8%.
Upon admission, 40 (67%), patients had a mean temperature of 37.5°C (SD: 1.0), RR: 29 (SD: 7.6), O2 saturation: 91% (SD: 6; range: 75%-96%) and PaO2/FiO2: 233.7 (SD: 100.9; range: 77–401). Forty-nine (82%) patients presented cough, 39 (65%) had dyspnea and 13 (22%) diarrhea. The mean CRP was 15.4mg/dL (SD: 9.2), lymphocytes: 813/mm3 (SD: 448; range: 300–2200), neutrophils>1500/mm3: 100%, LDH: 434 U/L (SD: 218), D-dimer: 7560ng/mL (SD: 19,979; range: 511–107,000), GPT: 51U/L (SD: 62). In 55 (92%) patients there were radiological bilateral pulmonary infiltrate (Table 1).
Characteristics of patients admitted for COVID 19, according to treatment received: (1) BARI monotherapy. (2) TCZ monotherapy. (3) The patients have received BARI and TCZ. (4) Patients not treated with either BARI or TCZ.
| (1) | (2) | (3) | (4) | |
|---|---|---|---|---|
| BARI monotherapy | TCZ monotherapy | BARI+TCZ | No BARI/No TCZ | |
| N: 12 (20%) | N: 20 (33%) | N: 11 (18%) | N: 17 (28%) | |
| Age, mean (SD) | 67.8 (13.6) | 59.4 (14.5)+ | 70.1 (13.6) | 73.8 (14.8) |
| Male, n (%) | 9 (75) | 15 (75) | 8 (73) | 11 (65) |
| Days with symptoms, mean (SD) | 8.3 (5.8) | 9.2 (7.5) | 10.4 (6.1) | 6.1 (4.8) |
| Days admission, mean (SD) | 11.6 (3.6) | 10.7 (4.1) | 11.9 (3.5) | 9.1 (2.5)^ |
| Days on BARI, mean (SD) | 6 (2.6)§ | – | 2.8 (2.5)≠≠ | – |
| OH-CLQ, n (%) | 10 (83) | 15 (75) | 11 (100) | 14 (82) |
| Corticosteroids, n (%) | 11 (92) | 20 (100) | 11 (100) | 10 (59) |
| Interferon, n (%) | 2 (17) | 6 (30) | 4 (36) | 2 (12) |
| Symptoms | ||||
| Cough, n (%) | 10 (83) | 18 (90) | 8 (73) | 13 (76) |
| Dyspnea, n (%) | 10 (83) | 15 (75) | 6 (55)¿ | 8 (47) |
| Diarrhea, n (%) | 3 (25) | 6 (30) | 2 (18) | 1 (6) |
| Temperature °C, mean (SD) | ||||
| Admission | 37.5 (0.9) | 38.1 (1.1) | 37.8 (0.9)≠ | 37.1 (1.0) |
| Last or discharge | 36.2 (0.3)* | 36.5 (0.9) | 36.5 (0.9) | 36.6 (0.6) |
| CRP mg/dL, mean (SD) | ||||
| Admission | 16.6 (10.2) | 15.7 (7.6) | 17.9 (11.5) | 12.5 (8.3) |
| Last or discharge | 2.4 (3.3) | 4.8 (9.2) | 2.3 (3.5) | 5.7 (10.2) |
| Lymphocytes/mm3, mean (SD) | ||||
| Admission | 975 (417.1) | 860 (396.3) | 690 (430.8) | 1.087 (471.4) |
| Last or discharge | 980 (528.6) | 1.328 (888.3) | 1.011 (557.8) | 1.093 (854.0) |
| LDH U/L, mean (SD) | ||||
| Admission | 555 (221.5)** | 472 (234.9) | 414 (223.7) | 316 (165.1) |
| Last or discharge | 512 (314.7)* | 404.6 (256.9) | 401 (319.1) | 278 (109.3) |
| D Dimer ng/mL, mean (SD) | ||||
| Admission | 12.265 (23.267) | 5.455 (10.388) | 8.227 (28.500,6) | 6.205 (16.880) |
| Last or discharge | 6.708 (10.484) | 2.865 (3.563) | 4.876 (10.958.4) | 1.902 (2.001) |
| Neutropenia/mm3last <1.000, n (%) | 0 | 0 | 0 | 0 |
| GPT last, mean (SD) | 66.1 (49.1) | 93.8 (86.9)+ | 30 (51.4) | 69 (63.8) |
| SatO2, mean (SD) | ||||
| Admission | 90.5 (6.9) | 91.7 (4.5) | 87.2 (7.3) | 94.2 (4.6)^ |
| Last or discharge | 94.7 (2.9) | 91.7 (12.6) | 94.2 (3.0) | 93.2 (8.9) |
| PaFI, mean (SD) | ||||
| Admission | 167 (82.3)** | 221 (114.9) | 236 (82.3) | 276 (83.2) |
| Pre-BARI | 165 (66.0) | – | 108.5 (67.7) | – |
| Last or discharge | 214 (74.3)* | 222 (150.9)+ | 133.8 (77.8) | 330 (183.0)^ |
| Respiratory frequency, mean (SD) | ||||
| Admission | 28 (7.6) | 30 (7.8) | 34 (7.9) | 25 (7.5)^ |
| Last or discharge | 19 (4.6)¶ | 24 (7.1) | 21 (4.8) | 22 (7.6) |
| Chest X-ray bilateral pneumonia, n (%) | 11 (92) | 20 (100)+ | 10 (91) | 13 (76) |
| Severe adverse effects (SAE) | 0 | 0 | 0 | - |
| Charlson Comorbidity Index, n (SD) | 2.9 (1.7) | 2.1 (2.4) | 3.8 (1.8) | 5 (2.9) |
| 10-Year survival (%) | 70.1 | 73.4 | 53.4 | 42.1 |
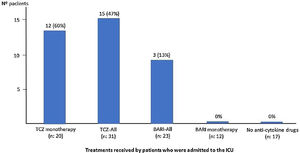
| Admission to ICU, n (%) | 0 | 13 (65) | 3 (27) | 0 |
| Hospital readmission 30 days after discharge, n (%) | 1 | 1 | 0 | 0 |
| Overall mortality since admission, n (%) | 2 (17) | 4 (20) | 3 (27) | 6 (35) |
| 1–15 days | 2 | 4 | 2 | 3 |
| 16–30 days | 0 | 0 | 1 | 3 |
BARI: baricitinib. TCZ: tocilizumab. No BARI/No TCZ: not treated with baricitinib or tocilizumab.
(1) BARI monotherapy vs (2) TCZ monotherapy: ¶p: <0.5.
(1) BARI monotherapy vs (3) No BARI-No TCZ: *p:<0.05. **p: <0.01.
(1) BARI monotherapy vs (4) BARI+TCZ: ≠p<0.05. ≠≠p<0.01.
(2) TCZ monotherapy vs (3) No BARI-No TCZ: +p: <0.05. ++p: <0.01.
(2) TCZ monotherapy vs (4) BARI-TCZ: ¿p<0.05.
(3) No BARI-No TCZ vs (4) BARI-TCZ: ^p<0.01 ^^p<0.001.
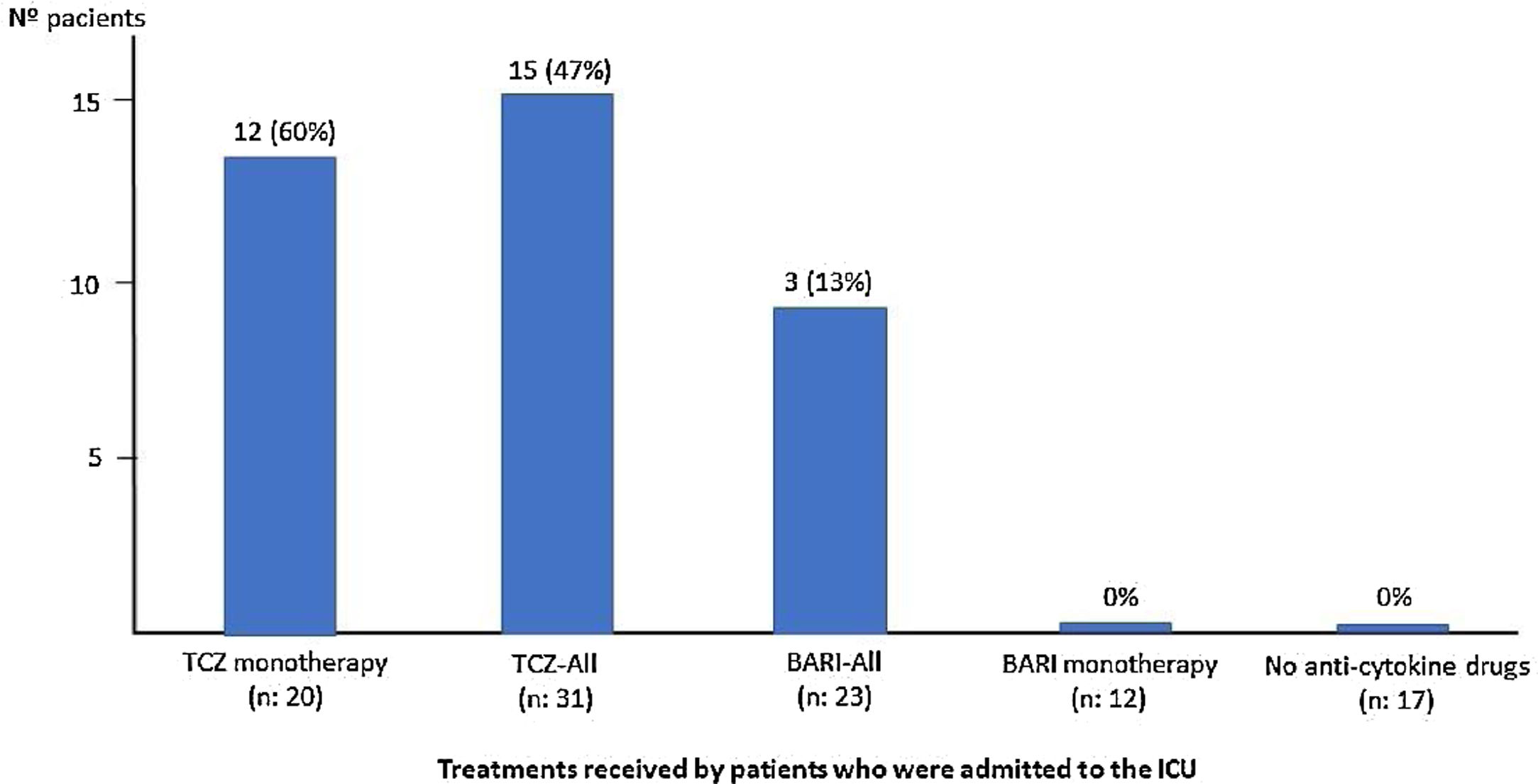
Treatments received during admission was: antiviral drugs: 28 (47%) patients, azithromycin: 53 (88%), hydroxychloroquine: 51 (85%), interferon: 14 (23%), corticosteroids: 49 (82%), of which in 42 (86%) patients as intravenous bolus of 250–500mg. Thirty one (52%) patients received TCZ, 23 patients (38%) received BARI, both as monotherapy or combined and 17 (28%) patients did not receive any of the anti-cytokine drugs (Table 1). Neutropenia, thrombotic event, or other relevant side effect was not detected among patients who received BARI and/or TCZ.
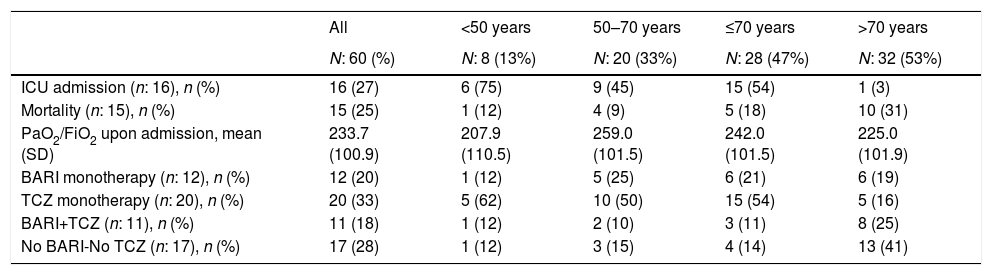
Impact of age on mortality and ICU admissionThirty-two (53%) patients were over 70 years old and 28 (47%) were under 70, 8 (14%) of them were under 50 and 20 (33%) between 51 and 70.
Fifteen (25%) patients died, 5 of them in ICU, 11 (18%) patients in the first 15 days of admission and 4 (7%) between days 16 to 30 (Table 1). Sixteen (27%) patients required ICU admission: 15 (94%) of them were under 70 years of age and 1 (6%) patient over 70. Among patients over 70 years versus those under 70, PaO2/FiO2 at admission was similar but presented numerically higher mortality (10 [31%] vs 5 [18%], p=0.59), higher percentage of patients who did not receive BARI or TCZ (13 [41%] vs 4 [14%], p=0.32) and lower percentage of ICU admission (1 [3%] vs 15 [94%], p=0.32) (Table 2).
ICU admission, mortality, PaO2/FiO2 and anticytokine drugs received in patients admitted with interstitial pneumonia.
| All | <50 years | 50–70 years | ≤70 years | >70 years | |
|---|---|---|---|---|---|
| N: 60 (%) | N: 8 (13%) | N: 20 (33%) | N: 28 (47%) | N: 32 (53%) | |
| ICU admission (n: 16), n (%) | 16 (27) | 6 (75) | 9 (45) | 15 (54) | 1 (3) |
| Mortality (n: 15), n (%) | 15 (25) | 1 (12) | 4 (9) | 5 (18) | 10 (31) |
| PaO2/FiO2 upon admission, mean (SD) | 233.7 (100.9) | 207.9 (110.5) | 259.0 (101.5) | 242.0 (101.5) | 225.0 (101.9) |
| BARI monotherapy (n: 12), n (%) | 12 (20) | 1 (12) | 5 (25) | 6 (21) | 6 (19) |
| TCZ monotherapy (n: 20), n (%) | 20 (33) | 5 (62) | 10 (50) | 15 (54) | 5 (16) |
| BARI+TCZ (n: 11), n (%) | 11 (18) | 1 (12) | 2 (10) | 3 (11) | 8 (25) |
| No BARI-No TCZ (n: 17), n (%) | 17 (28) | 1 (12) | 3 (15) | 4 (14) | 13 (41) |
ICU: Intensive Care Unit. BARI: baricitinib. TCZ: tocilizumab.
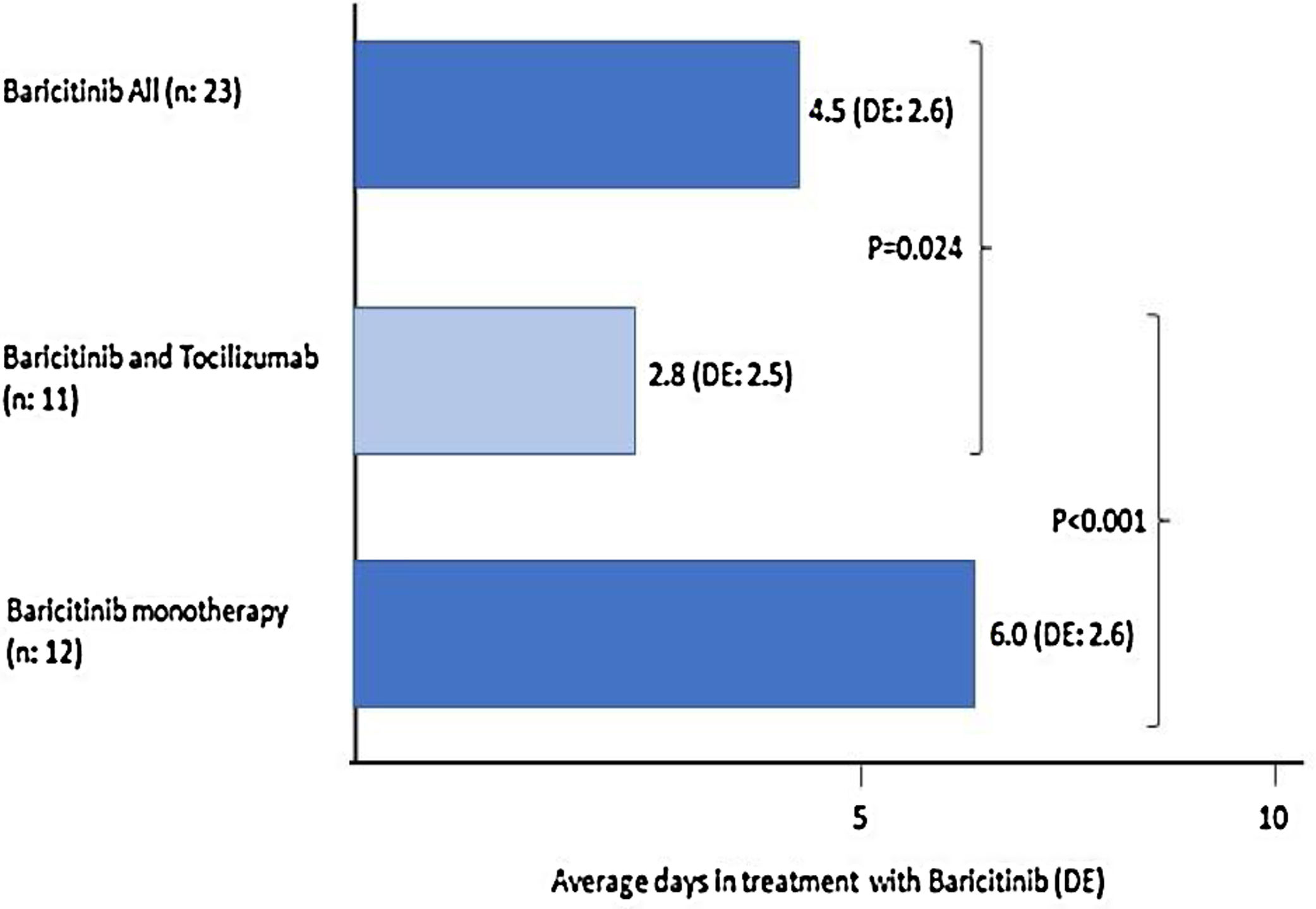
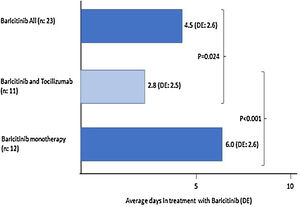
Of the 23 (38%) patients who were treated with BARI, 17 (74%) were male, mean aged of 69 (SD: 13.5) years (range: 30–85). Mean progression of symptoms: 9.3 (SD: 5.8) days and of admission 12 (SD: 3.6) days; the mean time in treatment with BARI: 4.5 (SD: 2.6) days (Fig. 1). In 9 (39%) patients the BARI dose received was 4mg and in 14 (61%) 2mg daily. Three (13%) patients received only one dose, prior to TCZ. Eight (35%) discontinued BARI at the start of TCZ and 3 (27%) continued BARI after receiving TCZ. Other treatments included antiviral: 10 (44%) patients, azithromycin: 18 (78%), hydroxychloroquine: 21 (91%), interferon: 6 (25%), and 23 (100%) patients received corticosteroids, of whom 18 (78%) received intravenous bolus of 250–500mg
When comparing the 23 patients who received BARI, to the 37 (62%) patients who did not (20 [54%] patients received TCZ), significant differences were detected at discharge or during the last evaluation, in RR (20, SD: 4.6 vs 24, SD: 7.4. p<0.05).
Twelve (52%) patients received BARI monotherapy (Group 1), during 6 (SD: 2.6) days on average (Fig. 1). The PaO2/FiO2 average prior to receiving BARI and at discharge was: 167 (82.3) vs 214 (74.3). None of the patients required ICU and 2 (17%) died, in the first 15 days of admission (Table 1).
Tocilizumab treatmentThirty-one (52%) patients received a dose of intravenous TCZ; 24 (77%) were male, mean age of 63 (SD: 14.3) years (range: 29–85), a mean time of progression of symptoms upon admission of 9.7 (SD: 6.8) days and of admission of 11 (3.8) days (Fig. 2).
Twenty (65%) patients received a dose of TCZ monotherapy (Group 2). The PaO2/FiO2 average previous receive TCZ and at discharge was: 221 (114.9) vs 222 (150.9). Thirteen (65%) patients required ICU and 4 (20%) died, in the first 15 days of admission there were no differences between the BARI and TCZ groups in monotherapy, except in the RR at discharge (Table 1).
Eleven (18%) patients received TCZ and BARI (Group 3). The PaO2/FiO2 average prior to receiving TCZ and at discharge was: 224 (103) vs 188 (136). Three (27%) patients required ICU and died, two of them in the first 15 days of admission (Table 1).
When comparing the 31 patients who received TCZ to the 29 (48%) patients who had not, the group receiving TCZ at admission was younger: 63 (SD: 14) years vs 71 (SD: 14) years (p<0.05), had higher temperature: 37.9°C (SD: 0.9) vs 37.1°C (SD: 1) (p<0.05), were treated shorter time with BARI: 2.8 (SD: 2.5) vs 6 (2.6) days (p<0.0001) in patients treated with BARI monotherapy (Fig. 1); and before starting BARI, had lower PaO2/FiO2 (108.5 [67.7] vs 165 [66.0], p=0.06).
Patients not treated with Baricitinib or TocilizumabIn the group of 17 (28%) patients who did not receive any of the anti-cytokine drugs (Group 4), 11 (65%) were male, mean age of 71.8 (SD: 14.8) years (range: 41–91; 76% >70 years old), a mean time of progression from symptoms upon admission of 6.1 (SD: 4.8) days, and of admission of 9.1 (2.5) days. The PaO2/FiO2 average at admission and at discharge was: 319 (193.8) vs 330 (183). None of the patients required ICU admission. However, 6 (35%) patients died, 3 (50%) of them in the first 15 days of admission (Table 1).
When comparing the patients of this group to patients who received BARI monotherapy, patients treated with BARI had a significantly higher LDH level upon admission (555 [SD: 221.5] vs 316 [SD: 165], p<0.01) and at discharge (512 [SD: 314.7] vs 278 [SD: 109.3]), p<0.05), and lower PaO2/FiO2 level upon admission (167 [SD: 82.3] vs 276 [SD: 83.2], p<0.01) and at discharge (214 [SD: 74.3] vs 330 [SD: 183], p<0.05). In both groups, none of the patients required admission to the ICU (Table 1).
When comparing the patients of this group with patients who received TCZ monotherapy (Table 1), patients treated with TCZ significantly presented upon admission, a higher percentage of bilateral radiological pneumonia (20 [100%], vs 13 [76%], p<0.05), higher temperature (38.1°C [SD: 1.0] vs 37.1°C [SD: 1.1], p<0.01), and higher LDH level (472 [SD: 235] vs 316 [165], p<0.05).
DiscussionCOVID19 infection causes mild illness in most patients. However, approximately 20% of patients can progress to SARS due to the appearance of IP, sepsis, or septic shock, requiring admission to an ICU in about 5% of cases.27 Since many people become infected within a short period of time, it can lead to a health care collapse.
The current treatment of patients with IP by COVID19 is a challenge for clinicians. Pharmacological treatment is not based on the results of clinical trials, but on previous experiences with other viral infections. There are studies that demonstrate a direct relationship between IL6 level and patient severity.28 Hence the interest in using drugs with anti-IL6 activity, such as TCZ, in these patients.
In general, TCZ was used after the first published efficacy data in China, due to its anti-IL6 action in the “cytokine storm”, in patients with IP and SARS.17 Given the possibility of difficulties in its supply in that time, the possible benefit of another drug with anti-IL6 action, such as BARI, administered orally, was assessed at our center at an early stage of respiratory failure due to COVID19.
Initially, BARI was introduced in our center for patients waiting to start TCZ, at a dose of 2mg daily, due to the age of the patients and the presence of high levels of D-dimer. Subsequently, since all patients received prophylactic doses of low molecular weight heparin, it was changed to 4mg daily, without finding relevant differences between both doses.
As expected, in our study patients receiving BARI and/or TCZ, presented more serious data upon admission, especially PaO2/FiO2. However, in the group who received BARI monotherapy, with the worst PaO2/FiO2 at admission, none of the patients was admitted to the ICU, as occurred in the group that was not treated with BARI and/or TCZ. Therefore, 3 patients, with PaO2/FiO2<200), received BARI before adding TCZ and continued to receive BARI at the same dose (sequential treatment), not requiring admission to the ICU. Regarding mortality, no differences were detected between all groups. On the other hand, patients who received TCZ, had the worst clinical evolution and therefore there was a higher percentage who entered the ICU, but with similar mortality. In addition, patients with poor clinical evolution or those did not respond to BARI, received TCZ, and presented a higher percentage of admission to ICU than the other groups.
These results could reinforce the idea of introducing BARI early, at the beginning of respiratory failure, measured with PaO2/FiO2, and TCZ could be add, if there is no clinical response and PaO2/FiO2 worsening, to prevent the need for ICU admission. On the other hand, a sequential treatment (BARI-TCZ-BARI) could be considered.
The length of time that the patient receives BARI and its early introduction, may be related to the possible efficacy of the drug. In patients of our study receiving BARI monotherapy, the mean treatment is 6 days compared to 2.8 days for the group receiving BARI and TCZ combined, mainly because in many patients BARI was started waiting for TCZ.
During the study, no relevant side effects related with BARI or TCZ have been recorded, even 30 days after discharge, such as thrombotic symptoms, herpes zoster, leukopenia, thrombopenia, or significant alteration of blood transaminases. This can be related to the short period of treatment received.
The age of the patients can be relevant for the prognosis. In our study, numerically relevant differences were detected between those over and under 70 years of age. In patients over 70, 3% of them were admitted to the ICU, but the mortality rate reached 31%. The mortality rate varies between series. In our study 15 (25%) patients died, all in the first 30 days after admission, but the 73% in the first 15 days. However, in the study of Chen,12 14% of 799 patients admitted died. In the registry from Spanish Society of Internal Medicine (SEMI), with 6.424 patients included, 8.5% received TCZ and 8% required ICU. The mortality rate was 21%, reaching 73% in patients over 70.29
So far, two studies from Italy have been published in patients treated with BARI for pneumonia due to COVID19. The first study, in 12 patients, although the patients were not randomized, they were compared with previous patients who did not receive BARI,30 with promising results. Patients received standard treatment and BARI daily for 2 weeks and their clinical parameters, CRP, and respiratory evaluation improved in the first and second week. No severe side effects were detected and none of the patients required ICU admission. Recently, the same group has published a multicenter study with 113 patients to which BARI was added to the standard treatment and compared with 87 patients who received standard treatment. At 2 weeks of treatment, the BARI group had significantly lower mortality and ICU admission.31
Our study has obvious limitations: it is a retrospective and observational study, with a small number of patients and a great heterogeneity among patients treated with BARI or TCZ. It presents a confusion bias due to the severity of the disease, because the most severe patients, and therefore with greater probability of admission to the ICU and mortality, received treatment with anti-IL6 drugs. However, this study aims to draw attention to the possible benefit of BARI in severe cases of IP in this disease.
In summary, the treatment for patients with IP due to COVID19 has not been fully established. However, the combination of cytokine storm and IP, especially in those older than 70 years, darkens the prognosis. We have showed our experience with BARI and TCZ in this clinical situation. Treatment with BARI and TCZ did not cause serious side effects. They could be considered early in patients with NI secondary to COVID19 and impaired PaO2/PaFi. Randomized clinical studies with these drugs, including sequential therapy are required.
Ethical approvalThis article does not contain any studies with animals performed by any of the authors. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The design of the study was approved by the AEMPS (Spanish Agency of Medicines and Medical Products) and by the Research Ethics Committee of University Hospital of Elche, Spain.
Authors’ contributionAll authors contributed to the study conception, data collection and design. analysis were performed by José Rosas. The first draft of the manuscript was written by José Rosas and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Informed consentAs it is a retrospective study, it is not necessary to obtain informed consent.
FundingThe study was supported by a research grant from the Association for Research in Rheumatology of the Marina Baixa (AIRE-MB).
Conflict of interestThe authors have no conflict of interest.
Our thanks to all the healthcare workers in our health department for their sacrifice and dedication to their patients during the pandemic.
Anesthesia: Gracia Barber Ballester, Francisco Martínez Adsuar, Agustí Martínez Tomás, Lourdes Marugán Rodríguez, Alejandra Molines Cantó, Cristina Munck Álvarez, Beatriz Navarro, Victoria Ortiz Sánchez, Henry Romero Quintana, Isabel Tarí Valls, Patricia Valls Linares, Vicente de Vera. Cardiology: Mar Erdociain Perales, Manuel Macias, Begoña Tocado Unzalu. Endocrinology: Carlos Argente Villaplana, Sara Alonso Díaz. Gastroenterology: Antonio García Herola, Laura Gómez Escolar Viejo, Belén Herreros Martínez, Cristina Quílez Ivorra, Jaime Valverde de la Osa. Internal Medicine: Josep Tomás Algado Rabasa, Concepción Amador Prous, Concepción Benito Santaleocadia, Javier Ena Muñoz, Pere Esquerdo Ramis, Ana Mª Garijo Saiz, Mª Angeles Gil Hurtado, Enrique Gómez Segado, David de Haedo Sánchez, Cristina Jauset Alcalá, Ángela Navarro Corral, Roser Navarro Soler, Fernando Martínez Salazar, Pablo Oteo López, Francisco Pasquau Liaño, Santiago Pérez Martín, Melina Pucciarelli Saccomandi, Lara Ramón Múgica, Isabel Selles Sirera. Intensive Care: Susana Almanza López, Fernando Asensio Paya, Eugenia Blasco Ciscar, José María Carrasco Barea, Pablo Fernández Arroyo, Isabel Fernández López, Manuel Marco Escoto, María Luisa Navarrete Rebollo, Ricardo Palomino León, María Jesús Prieto Bragado, Laura Ruiz Pérez, Julia Tejerina Puig, José Vaya Moscardó. Medicine Preventive: Patricia García Shimizu. Microbiology: Francisco José Arjona Zaragozí, Bárbara Gómez Alonso, Carmen Martínez Peinado. Neurology: Leticia Berenguer Ruiz, Araceli Bernal Velasco, Mª Empar Blanco Cantó, Raquel Hernández Lorido, Elías Khabbaz Cañavate. Nephrology: Carlos García Aparicio, Yussel González Galván. Pharmacy: Mari Luz Boquera Ferrer, Alberto Martí Llorca, Amparo Raga Beser, Gregorio Sanz Tamargo, Elisa Soler Giner. Pneumology: Patricia Ferrer Ferrer, Mónica Llombart Cantó, Adela Martínez Sanchís, Brian Vila Auli. Rheumatology: José Antonio Bernal Vidal, Ana Pons Bas, José Rosas, Gregorio Santos Soler, José Miguel Senabre Gallego.