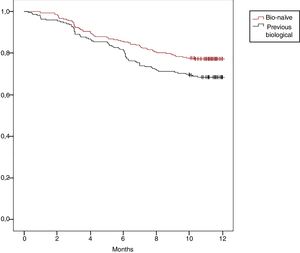
Regarding with our previous RENACER (REgistro NAcional CERtolizumab) Study,1 we would like to present data in a larger population of Rheumatoid Arthritis (RA) patients on the use of Certolizumab-PEGol (CZP). CZP is a biological agent approved for RA which inhibits Tumor Necrosis Factor-alpha (TNFi). Its unique molecular structure allows its use in particular situations.2,3 Our study collected data in clinical practice from 2011 to present in 37 different sites in Spain, collecting socio-demographics, smoking status, clinical and safety data at baseline, 3-, 6- and 12-month visits. Clinical outcomes were defined by completion of EULAR Good/Moderate and DAS28 Remission. Drug survival was also assessed (Kaplan–Meier curve). A total of 501 RA patients were included: 78.6% women, mean age 53.6 yr (±13.2 SD), 23% were aged >65 yr; mean disease duration 7.5 yr (±7.3 SD), 27.7% having early RA (<2 yr); prior csDMARD number 1.5 (±1.1 SD); mean prior bDMARD number was 0.8 (±1.2 SD); mean exposure time to CZP was 9.8 months (±3.4 SD); concomitant steroids intake 12.6%, csDMARD 24.2% and csDMARD plus steroids 54.9%; 69.8% never smoked, 12.9% former smoker and 17.3% current smoker. A total of 135 discontinued CZP (27%). Clinical and treatment outcomes are shown in Table 1, statistically significant improvement in all parameters at 12-month visit compared to baseline was observed. 12-month EULAR Response was reached in 69.8% of patients, 64.4% when using CZP as 2nd-line treatment after 1st-bDMARD clinical failure (N=90, data not shown). 12-month DAS28 Remission was achieved in 40.5% of patients (34.4% in 2nd-line). Overall CZP 12-month survival was 73.1%. We found CZP survival rate in bio-naïve patients was higher than in those who used previous bDMARD, 77.2% vs. 68.5% (p=0.029), respectively (Fig. 1). Moreover, the use of CZP in combination to csDMARD showed better DAS28 Remission criteria rate than in monotherapy (p=0.030). Adverse events were reported in 13% of cases, mostly mild or non-life threatening (N=65; 2.4% cutaneous rash, 1% upper respiratory infection, as the most frequents). During the study we observed the following discontinuation reasons: 68 patients (50%) showed secondary inefficacy, 36 (26%) intolerance, 15 (11%) primary inefficacy and 16 (12%) others.
Baseline and follow-up activity, serological and treatment data.
| Baseline | 3-month | 6-month | 12-month | p value | |
|---|---|---|---|---|---|
| TJC | 9.3±6.2 | 5.0±5.5 | 3.9±4.7 | 3.6±4.9 | <0.001 |
| SJC | 6.7±5.1 | 3.4±4.2 | 2.4±3.5 | 2.1±3.3 | <0.001 |
| RF (positive/negative) | 53.8%/46.2% | 53.2%/46.8% | 52.3%/47.7% | 49.8%/50.2% | 0.165 |
| CCP antibodies (positive/negative) | 68.8%/31.2% | 63.6%/36.4% | 67.2%/32.8% | 62.6%/37.4% | 0.300 |
| ESR | 29.2±23.1 | 23.4±20.6 | 22.8±21.3 | 21.3±20.0 | <0.001 |
| CRP (mg/L) | 6.9±15.8 | 4.9±11.1 | 4.3±10.7 | 4.3±10.5 | <0.001 |
| Mean Steroids Dose (mg) | 7.9±6.2 | 6.0±5.5 | 5.1±5.2 | 4.4±4.8 | <0.001 |
| DAS28 | 4.7±1.1 | 3.7±1.3 | 3.4±1.3 | 3.2±1.3 | <0.001 |
| DAS28 Remission (%) | – | 22.9 | 31.3 | 40.5 | – |
| EULAR Good/Moderate Response (%) | – | 54.8 | 64.8 | 69.7 | – |
Abbreviations: tender joint count (TJC); swollen joint count (SJC); rheumatoid factor (RF); anti-cyclic citrullinated peptide antibodies (CCP); erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); Disease Activity Score 28-joints (DAS28); European League Against Rheumatology (EULAR); milligrams (mg); percentage (%).
Since our first publication, two other studies assessing patients treated with CZP have been developed in Italy and Sweden. The Italian study in 278 RA patients found similar EULAR Response rates at one year from CZP onset (66%). The authors also found that clinical response at 3-month visit predicted low disease activity at 12-month assessment (68% as 1st-line treatment).4 The Swedish registry collected data from 945 RA patients that received CZP (57% as 1st-line treatment, 23% as 2nd-line). The authors found a 71% EULAR Response achievement at 6-month visit and better 30-month survival drug on those TNFi-naïve.5 In another clinical trial, a total of 38.1% ACR-50 completion at 12-month assessment was shown, with positive predictive value for those patients who showed a >1.2 points reduction in DAS28 from baseline.6 We conclude that CZP use in severe RA patients should be specially recommended in bio-naïve patients and in those who failed to one TNFi over those who failed to two or more TNFi, with reasonable safety profile. Further studies are recommended in order to establish CZP long-term survival.