Palindromic rheumatism (PR) is characterized by repetitive, afebrile episodes of acute arthritis and peri-arthritis. The aim of this study was considering the long-term outcomes of patients with PR who were treated with tight control strategy using Disease-modifying anti-rheumatic drugs (DMARDs).
MethodsWe reviewed the charts of 106 patients diagnosed with PR who were referred to the Connective Tissue Diseases Research Center (CTDRC). We recruited all the patients diagnosed with PR according to the criteria of Hannonen. They visited the CTDRC clinic regularly and were treated with hydroxychloroquine and low dose prednisolone because of active episodes of PR. In cases that the attacks did not come under control in 3–6 months, methotrexate was added or replaced and the dose was increased up to 25mg/week. In resistant cases, sulfasalazine was added, followed by the addition of leflunomide and then azathioprine. Disease outcome was evaluated by getting complete or partial remission and prevention of disease evolution to rheumatoid arthritis (RA) or other inflammatory connective tissue diseases.
ResultsThis study included 92 patients with PR who were treated with DMARDs. Attacks were controlled completely or partially in 76 (82.6%) patients. Medications free remission was obtained in 16.3% of the patients. RA developed in 8.7% of the patients. By multivariate logistic regression analysis, age ≤40 at disease presentation, non-adherence to therapy and PIP joints involvement were the only factors which independently predicted the risk of treatment failure.
ConclusionsTight control strategy by using DMARDs may control PR and prevent disease progression to RA.
El reumatismo palindrómico (PR) se caracteriza por episodios repetitivos y afebriles de artritis aguda y periartritis. El objetivo de este estudio fue considerar los resultados a largo plazo de los pacientes con PR que fueron tratados con una estrategia de control estricta utilizando fármacos antirreumáticos modificadores de la enfermedad (DMARD).
MétodosRevisamos los cuadros de 106 pacientes diagnosticados con PR que fueron remitidos al Centro de Investigación de Enfermedades de Tejido Conectivo (CTDRC). Reclutamos a todos los pacientes diagnosticados con PR según los criterios de Hannonen. Visitaron la clínica de CTDRC regularmente y fueron tratados con hidroxicloroquina y prednisolona a dosis bajas debido a episodios activos de PR. En los casos en que los ataques no se controlaron en 3 a 6 meses, se agregó o reemplazó metotrexato y la dosis se aumentó hasta 25mg/semana. En casos resistentes, se añadió sulfasalazina, seguido de la adición de leflunomida y luego azatioprina. El resultado de la enfermedad se evaluó obteniendo la remisión completa o parcial y la prevención de la evolución de la enfermedad a la artritis reumatoide (AR) u otras enfermedades inflamatorias del tejido conectivo.
ResultadosEste estudio incluyó 92 pacientes con PR que fueron tratados con DMARD. Los ataques fueron controlados total o parcialmente en 76 (82,6%) pacientes. La remisión libre de medicamentos se obtuvo en el 16,3% de los pacientes. La AR se desarrolló en el 8,7% de los pacientes. Mediante el análisis de regresión logística multivariante, la edad ≤40 en la presentación de la enfermedad, la no adhesión al tratamiento y la afectación de las articulaciones PIP fueron los únicos factores que predijeron de forma independiente el riesgo de fracaso del tratamiento.
ConclusionesUna estrategia de control estricta mediante el uso de DMARD puede controlar la RP y prevenir la progresión de la enfermedad a AR.
Palindromic rheumatism (PR) is a clinical syndrome characterized by repetitive, afebrile episodes of acute arthritis and peri-arthritis, lasting from a few hours to several days with variable frequency and producing no permanent tissue damage. Arthritis attacks usually are monoarticular and may appear in any joint, but proximal interphalangeal (PIP) joints, metacarpophalangeal (MCP) joints, wrists, and knees are most commonly affected.1,2 PR, in addition to reducing the quality of life, may also be transformed into chronic inflammatory connective tissue diseases. Several case series with long-term follow-up showed that 28–67% of PR cases evolve into rheumatoid arthritis (RA) and other inflammatory connective tissue diseases.3–11
Despite the relatively high frequency of this disease and significant risk of developing RA, no controlled clinical trials have been performed and no consensus exists on the best therapeutic strategy for PR.12,13 Patients may be treated with non-steroidal anti-inflammatory drugs (NSAIDs) or steroids during attacks.12,13 Disease-modifying anti-rheumatic drugs (DMARDs) like hydroxychloroquine (HCQ), D-penicillamine, gold salts and sulfasalazine (SSZ) have been used for prophylaxis of attacks and prevention of disease evolution to RA but have not been evaluated systematically.12
The aim of this study was considering the long-term outcomes of patients with PR who were treated with tight control strategy using DMARDs.
Materials and methodsWe reviewed the charts of 106 patients who were referred to the Connective Tissue Diseases Research Center (CTDRC) with diagnosis of PR from October 2005 to November 2017. We recruited all the patients diagnosed with PR according to the criteria of Hannonen (Box 1)14 and were treated with DMARDs because of active episodes of PR. The disease activity was evaluated by phone call in patients who had not been visited in the last 6 months. In cases where the phone call was not possible, the patient was excluded from the study. Written informed consent was obtained from all the patients and the study protocol was approved by the Ethics Committee of Tabriz University of Medical Sciences. The study protocol was in compliance with the Helsinki declaration.
Data relating to the demographic characteristics, clinical manifestations, laboratory findings, therapies and adherence to therapy were extracted from medical notes. Adherence to treatment was evaluated by the 5-item version of the Compliance Questionnaire for Rheumatology (CQR5) questionnaire.15 Rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibodies (anti-CCP) were measured in all patients at the first visit. Disease outcome was evaluated by getting complete or partial remission and prevention of disease evolution to RA or other inflammatory connective tissue diseases. Complete remission was defined as complete stopping of the attacks for 12 weeks. Partial remission was defined as at least 50% reduction in the frequency of attacks for 12 weeks. For the purpose of this analysis, treatment failure was defined as decreasing less than 50% in the frequency of attacks (persistent PR) or conversion of PR to RA. Based on the CTDRC protocol treatment with DMARDs was performed in all the patients with attacks that impaired their quality of life. The treatment was started with the hydroxychloroquine (HCQ) 5mg/kg/d and low dose prednisolone (5–10mg/d). If remission was attained, the dose of prednisolone was tapered 1.25mg/d every 8–16 weeks and then discontinued. In cases that the attacks did not come under control in 3–6 months or that the patient did not tolerate the HCQ, methotrexate (MTX) 10mg/week was added or replaced and the dose was increased up to 25mg/week. In resistant cases, sulfasalazine (SSZ) 1500–2000mg/d was added, followed by the addition of leflunomide 20mg/d and then azathioprine 2–2.5mg/kg/d. All the patients were visited and their responses to the treatment were evaluated every 8–16 weeks.
Statistical analysis was performed using SPSS version 16.0 software (SPSS Inc., Chicago, IL). Distribution of the data was assessed using Kolmogorov–Smirnov test. T-test was used to compare the quantitative data, and chi squared test was used to compare the qualitative data. P-value less than 0.05 was considered significant. We carried out multivariate analyses with a logistic regression model with disease remission as the main outcome variable to calculate odds ratios with 95% confidence intervals (OR, 95% CI). Models were adjusted for age, gender, body mass index (BMI), smoking status, disease duration before treatment, frequency of attacks, duration of attacks, number of involved joints in each attack, involved joints, seropositivity for RF or anti-CCP and adherence to therapy. P-value less than 0.05 was considered significant.
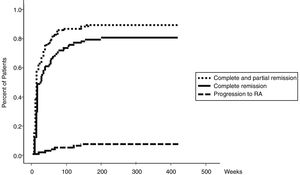
ResultsOne hundred and six patients diagnosed with PR were considered for eligibility, 14 patients were excluded (6 patients were not treated with DMARDs and 8 patients were lost the follow up) and finally 92 patients were included in this study. Demographic, clinical and laboratory characteristics of the participants at the baseline are presented in Table 1. Attacks were controlled completely or partially in 76 (82.6%) patients (Table 2). Prednisolone dose was decreased from 7.4 to 3.1mg/d. Medications’ free remission was obtained in 16.3% of the patients. RA was developed in 8.7% of the cases (Table 2). Table 3 presents clinical and paraclinical characteristics of PR patients with and without response to treatment. All of the cases of RA were developed within 3 years after the diagnosis of PR (Fig. 1). By multivariate logistic regression analysis, age ≤40 at disease presentation, non-adherence to therapy and hand PIP joints involvement were the only factors which independently predicted the risk of treatment failure. The relative risk (RR) of treatment failure were 11.2 (P=0.023, 95% CI=2.1–10.8), 14.6 (P=0.003, 95% CI=2.1–25.4) and 8.6 (P=0.044, 95% CI=1.5–34.2) for age ≤40, non-adherence to therapy and PIP joints involvement, respectively.
Demographic, clinical and paraclinical characteristics of included patients (n=92).
| Age at the time of diagnosis | 42.3±13.2 (min 18, max 76) |
| Disease duration before diagnosis (months) | 24.8±6.4 (min 6.5, max 240) |
| Female/male | 53/39 (1.4) |
| Frequency of attacks (weeks) | 3.2±1.9 (min 0.2, max 18) |
| Duration of attacks (days) | 2.6±1.5 (min 0.2, max 7) |
| Number of involved joints in each attack | 1.2±0.5 (min 1, max 4) |
| Involved structures | |
| Knees (%) | 63 (68.5) |
| MCP joints (%) | 51 (55.4) |
| Shoulders (%) | 52 (56.5) |
| Wrists (%) | 49 (53.3) |
| Hand PIP joints (%) | 45 (48.9) |
| Ankles (%) | 33 (35.9) |
| Elbows (%) | 21 (22.8) |
| MTP joints (%) | 12 (13) |
| Hips (%) | 8 (8.7) |
| Foot PIP joints | 6 (6.5) |
| Periarticular structures (%) | 10 (10.9) |
| Positive RF (%) | 39 (42.4) |
| Positive anti-CCP (%) | 58 (63) |
| Serum 25(OH)D | 28.6±8.2 |
MCP, metacarpophalangeal; PIP, proximal interphalangeal; MTP, metatarsophalangeal; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide; 25(OH)D, 25 hydroxy vitamin D.
Patients medications and outcomes of treatment (n=92).
| Medications | |
| Prednisolone (%) | 88 (95.7) |
| Hydroxychloroquine (%) | 86 (93.5) |
| Methotrexate (%) | 38 (41.3) |
| Sulfasalazine (%) | 8 (8.7) |
| Azathioprine (%) | 2 (2.2) |
| Leflunomide (%) | 1 (1.1) |
| Duration of follow-up (months) | 33.3±22.5 (min 3, max 108) |
| Disease activity status | |
| Complete remission (%) | 64 (69.6) |
| Partial remission (%) | 12 (13) |
| Active disease (%) | 8 (8.7) |
| Conversion to RA (%) | 8 (8.7) |
| Flare up of disease during treatment | 35 (38) |
| Time to complete or partial remission (weeks) | 58.1±32.1 (min 6, max 420) |
| Initial prednisolone dose | 7.4±2.5 |
| Final prednisolone dose | 3.1±2.8 |
| Prednisolone discontinuation (%) | 30 (32.6) |
| DMARDs therapy status | |
| Continuation of initial DMARD (%) | 48 (52.2) |
| Changing of initial DMARD because of in effectivity or intolerance (%) | 6 (6.5) |
| Combination therapy with DMARDs (%) | 23 (25) |
| Discontinuation of DMARDs because of remission (%) | 15 (16.3) |
| Duration of remission (months) | 22.9±17.6 (min 3, max 93) |
DMARDs, Disease-modifying antirheumatic drugs; RA, rheumatoid arthritis.
Clinical and paraclinical characteristics of palindromic rheumatism patients with and without response to treatment.
| Parameters | Response to treatment(N=76) | No response treatment(N=16) | P-value |
|---|---|---|---|
| Age ≤40 | 26 (34.2) | 9 (56.3) | 0.05 |
| Female/male | 44/32 (1.4) | 9/7 (1.3) | NS |
| BMI | 26.6±4.9 | 25.3±3.1 | NS |
| Smokers | 16 (21.1) | 4 (25) | NS |
| Disease duration before treatment (months) | 22.1±9.3 | 34.1±8.8 | NS |
| Frequency of attacks (weeks) | 3.1±1.9 | 4.3±2.1 | NS |
| Duration of attacks (days) | 2.4±1.8 | 2.8±1.3 | NS |
| Number of joints in each attack | 1.1±0.3 | 1.3±0.1 | 0.031 |
| Hand PIP joints involvement (%) | 33 (43.3) | 12 (75) | 0.035 |
| MCP joints involvement (%) | 41 (53.9) | 10 (62.5) | NS |
| Wrist involvement (%) | 39 (84.5) | 8 (91.7) | NS |
| Shoulder involvement (%) | 42 (55.3) | 9 (56.3) | NS |
| Elbow involvement (%) | 18 (23.7) | 3 (18.8) | NS |
| Knee involvement (%) | 55 (72.4) | 8 (50) | 0.05 |
| Ankle involvement (%) | 27 (35.7) | 6 (37.5) | NS |
| Positive RF | 32 (42.1) | 7 (43.8) | NS |
| Positive anti-CCP | 48 (63.2) | 10 (62.5) | NS |
| Adherents to therapy | 60 (78.9) | 5 (31.23) | 0.001 |
NS, non-significant; BMI, body mass index; PIP, proximal interphalangeal; MCP, metacarpophalangeal; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide.
The present study showed that tight control strategy by DMARDs controlled PR attacks successfully and only 8.7% and 8.7% of the patients had persistent PR or developed RA after 33.3 months, respectively. However, therapy with the initial DMARD was continued in 52.2% of the cases and in 23% of the cases combination therapy with DMARDs was performed. Steroid free and medication free remission happened in 32.6% and 16.6% of the PR patients, respectively. Patients with age ≤40 at disease presentation, PIP joints involvement and non-adherent patients had the worst outcome. There was no significant relationship between sex, BMI, smoking status, RF or anti-CCP status and response to the treatment.
Some uncontrolled studies reported the efficacy of DMARDs in controlling PR attacks and preventing disease evolution to RA. In a report on 5 patients with PR, using d-penicillamine completely controlled the attacks in 4 patients.16 In a report by Hanonen et al., gold, HCQ and SSZ were effective in controlling PR in 9 out of 16 patients, 8 out of 17 patients and 3 out of 8 patients, respectively14. In another report by Hanonen et al., gold was effective in the treatment of 26 out of 50 patients with PR.17 However, it was stopped because of side effects in 14 patients.17 Golding in a study on 14 patients with PR reported that treatment with SSZ 2g/d successfully controlled attacks in 8 patients.18 Youssef et al., in a study on 71 PR patients in which 51 patients were treated with anti-malarials, reported that 77.5% and 63% of the patients experienced reduction in frequency and duration of attacks, respectively.19 Sixteen out of 71 patients developed RA.19 Gonzalez-Lopez et al., in a retrospective study on 113 PR patients, 55% of which were treated with HCQ, reported that 32% of the cases in the HCQ group and 39% of the patients who did not receive therapy developed RA or other inflammatory connective tissue diseases.7 Treatment with HCQ significantly reduced the risk of chronic inflammatory connective tissue diseases development (hazard ratio=0.24).7 Shinjo et al. reported a case of PR associated with hypertrophic osteoarthropathy who had a good response to MTX.20 To the best of our knowledge, no studies published on the use of leflunomide, cyclosporine, mycophenolate mofetil, cyclophosphamide or biologics in the treatment of PR.
The results of our study about predictive factors of prognosis in PR were different from the previous studies. Contrary to our study, in Gonzalez-Lopez study on 127 PR patients with mean follow-up of 40 months, 34% of the patients subsequently developed a connective tissue disease.7 The hazard ratio for the development of a chronic condition in patients with a positive RF was 2.9, it was 2.4 for PIP joints involvement, 2.5 for wrist involvement, 2.2 for female sex and 1.03 for age at onset (per year).7 Koskinen et al. studied the progression of PR to RA in 60 patients with PR.10 Fifty-eight patients were treated with DMARDs.10 In a follow up of 20 years, two-thirds of the patients developed RA. Positive RF was significantly more common in those who developed RA.10 Sanmartí et al. conducted a similar study on 71 PR patients.21 In sixteen of the cases (22.5%) PR progressed to RA. Interestingly, no significant association was found between sex, anti-CCP positivity and treatment with HCQ and evolution of disease to RA.21 However, 87.5% of the patients who developed RA were RF positive; while this figure for patients with persistent PR was 48.9%.21 The difference was significant.21 Russel et al., in their study on 61 PR patients with a mean follow-up of 5.4 years, showed that in 29 cases the disease had progressed to RA.9 The positive predictive value of RF and anti-CCP to predict disease progression to RA were 60% and 71%, respectively.9 In Tamai et al. study on 28 PR patients with mean follow-up of 38 months, 11 patients developed RA.22 PIP joint involvement and positive anti-CC were the only predictors. In Chen et al. study on 84 patients with PR, having the sonographic findings of synovitis and a positive anti-CCP were significant predictors for progression of PR to RA.23 Compared to previous studies, a low probability of RA development in our study may be related to using a low dose of prednisolone and sequential DMARDs therapy in all the patients with active PR in our center and also a relatively lower follow-up duration. The different disease duration before diagnosis, different treatment strategies and different follow-up duration may explain the difference in predictive factors of disease prognosis in our study and previous studies.
The main limitations of our study were the retrospective design of the study, a relatively short follow-up duration and the scarcity of patients who were resistant to treatment or had developed RA. The strength of our study was the uniformity of the treatment strategy in all the patients.
ConclusionTight control strategy by using DMARDs may control PR and prevent disease progression to RA.
FundingThis research was funded by the Connective Tissue Diseases Research Center of Tabriz University of Medical Sciences.
Conflict of interestWe declare that we have no potential conflict of interest.
We are grateful to Dr. L. Khabbazi for editing this manuscript.