Eosinophilic granulomatosis with polyangiitis (EGPA) is a type of ANCA-associated vasculitis mostly mediated by eosinophils. Mepolizumab (MPZ) reduces the absolute number of eosinophils in the peripheral blood and tissues and has proven efficacy in the maintenance of EGPA, as shown in a randomized controlled trial. The aim of this study is to describe the use of MPZ in EGPA in real clinical practice.
MethodsThis is a descriptive, retrospective and comparative analysis of the clinical features, course, response rates, outcomes and adverse effects of patients receiving MPZ for EGPA in thirteen Spanish Rheumatology departments.
ResultsA total of 30 EGPA patients treated with MPZ were included in the analysis. Up to 19 patients needed at least one concomitant immunosuppressant. The mean follow-up was 16 months. The use of MPZ reduced the median Birmingham Vasculitis Activity Score (BVAS), as well as the mean C-reactive protein and the levels of eosinophils. In addition, the median dose of glucocorticoids (GC) required was reduced in 79.3% of the patients and was completely suspended in 57.1%. Interestingly, the median Vasculitis Damage Index (VDI) was also calculated in 27 patients (90%), and remained stable (3 [0–12] pre-MPZ and 3 [0–12] post-MPZ). None of the patients suffered severe adverse effect, local reactions or serious infections during the treatment.
ConclusionsOur data on the real practice use of MPZ in EGPA suggests that this drug has good efficacy, is safe and reduces the dependence on glucocorticoids or even their complete withdrawal. Furthermore, it appears to prevent the organic damage progression associated with this disease.
La granulomatosis eosinofílica con poliangeítis (GEPA) es un tipo de vasculitis asociada a anticuerpos anticitoplasma de neutrófilos (ANCA) principalmente medida por eosinófilos. El mepolizumab (MPZ) reduce el número absoluto de eosinófilos en sangre periférica y tejidos y ha demostrado eficacia en el mantenimiento del a GEPA, comprobado en un ensayo clínico aleatorizado. El objetivo de este estudio es describir el uso de MPZ en GEPA en práctica clínica real.
MétodosAnálisis descriptivo, retrospectivo y comparativo de los aspectos clínicos, curso, ratios de respuesta, desenlace y efectos adversos de pacientes con GEPA recibiendo MPZ en trece servicios de Reumatología españoles.
ResultadosUn total de 30 pacientes con GEPA tratados con MPZ han sido incluidos en el análisis; 19 pacientes necesitaron un inmunosupresor concomitante. El tiempo medio de seguimiento fue de 16 meses. El uso de MPZ redujo el Birmingham Vasculitis Activity Score (BVAS) medio, así como la proteína C reactiva (PCR) y los niveles de eosinófilos. Además, la dosis media de glucocorticoides (GC) se redujo en el 79,3% de los pacientes, y se suspendió por completo en el 57,1%. El daño medio medido por Vasculitsi Damage Index (VDI) se calculó en 27 pacientes (90%) y se mantuvo estable (3 [0 - 12] pre-MPZ y 3 [0 - 12] pos-MPZ). Ningún paciente sufrió algún efecto adverso grave, reacción local o infección grave durante el tratamiento.
ConclusionesNuestros datos en la práctica clínica real con el uso de MPZ en GEPA sugieren que es un fármaco con buena eficacia, seguro y que reduce la dependencia a GC e incluso permite su retirada. Además, parece prevenir el daño orgánico propio de la progresión de la enfermedad.
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome, is a type of ANCA-associated vasculitis that characteristically presents asthma, sinusitis, pulmonary infiltrates (including both alveolar and interstitial involvement), peripheral neuropathy and eosinophilic vasculitis in one or several organs, including the heart and central nervous system. The role of the eosinophil plays a crucial role in the pathogenesis of this disease, via infiltration and inflammation of the different affected tissues.
During the last decades, systemic glucocorticoids have been the main treatment for EGPA, controlling most symptoms. Nonetheless, many patients remain dependent on glucocorticoid therapy, relapses are frequent and GC-related effects as diabetes, hypertension, infections, osteoporosis and gastrointestinal bleeding are also quite common, leading to accrual organ damage. In patients with relapsing or refractory EGPA, as well as life-threatening disease, immunosuppressive agents can be used, although there is little evidence of their efficacy in EGPA and they also carry a high risk of serious infections.1
Interleukin-5 plays a central role in the proliferation, maturation, differentiation and survival of eosinophils. Furthermore, this cytokine is increased in the peripheral blood of patients with EGPA. Mepolizumab (MPZ) is an anti-interleukin-5 monoclonal antibody that binds to interleukin-5 and prevents its interaction with its receptor on the eosinophil surface. In that way, it reduces the absolute eosinophil count, improving the symptoms produced by eosinophilic disorders such as EGPA and asthma.2
As there are only a few publications about the use of mepolizumab in EGPA in real clinical practice and only few data are available regarding its impact on organ damage, the aim of our study was to assess the safety of this drug in a multicenter cohort from Rheumatology departments.
MethodsWe carried out a descriptive, retrospective and comparative analysis of clinical features, evolution, severity, outcomes and adverse effects of MPZ in a multicenter cohort of patients with EGPA (ACR 1990 criteria) older than 18 years old from thirteen different Spanish Rheumatology departments. Patients were recruited from January 2019 to January 2023. The sampling method was consecutive. The reason for starting MPZ were ineffectiveness of immunosuppressive treatments and/or need to withdraw corticosteroids. The Birmingham Vasculitis Activity Score (BVAS) and EULAR Severity Index were calculated for disease activity, Five Factor Score (FFS) for disease severity, and Vasculitis Damage Index (VDI) for damage. A physician global assessment (PGA) of treatment response measures the overall response to treatment as assessed by the physician, and it was used to evaluate disease improvement and remission after MPZ.
The mean, standard deviation and quartiles were calculated to describe the quantitative variables (C-reactive protein, prednisone dose, MPZ dose, disease duration, BVAS, FFS, VDI and PGA). Kolmogorov–Smirnov test was used to check the normality of the quantitative variables. Qualitative variables (clinic manifestations, ANCA status, use of other immunosuppressants and adverse effects) were described using absolute and relative frequency. Friedman test was used to compare the numerical variables data at different time moments and two-to-two comparisons have been made with Bonferroni correction. To compare different time moments of the variables, non-parametric Wilcoxon signed-rank test was used. Every variable was checked at baseline visit, one-month visit, three-months visit, six months visit and last visit of the patient. A p-value less than 0.05 was considered significant. The statistical program used was R Core Team 2023, version 4.3.2.
This study was approved by the Ethical Investigation Committee of the Gran Canaria University Hospital Doctor Negrín.
ResultsA total of thirty patients with EGPA treated with MPZ were eventually included. The median age at diagnosis was 52 years old (p25–p75; 42–60) and sixteen patients were men (53.3%). Regarding cardiovascular risk factors, 4 patients had hypertension (13.3%), 5 had dyslipidemia (16.7%) and 12 were or had been smokers (40%).
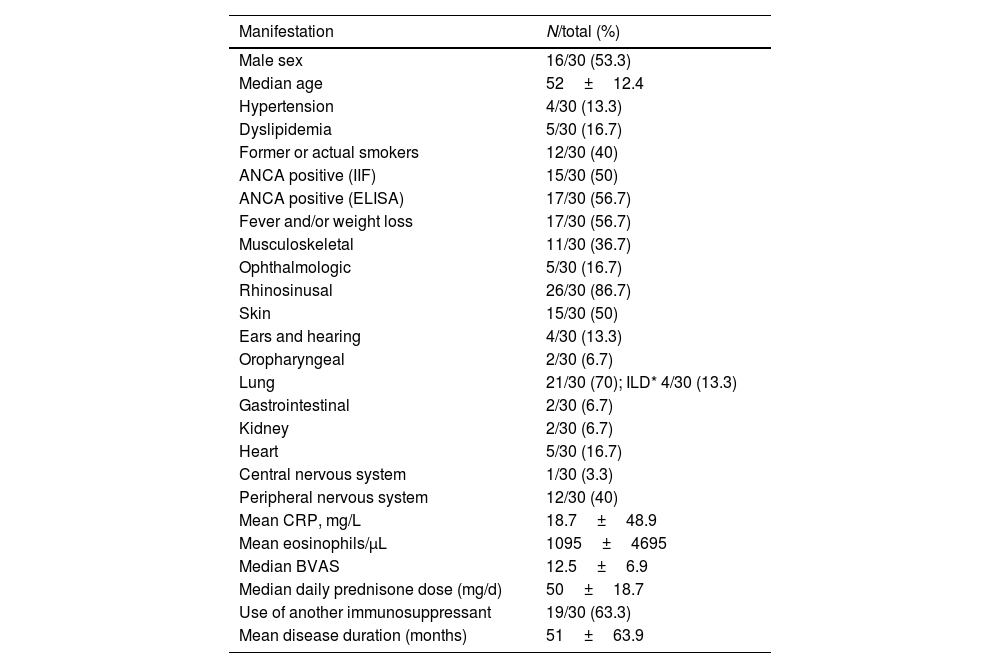
Patients characteristics before mepolizumab treatment, including clinical characteristics, are shown in Table 1; most patients had rhinosinusal manifestations (86.7%) and lung manifestations (70%); asthma was not counted as a pulmonary manifestation since all patients had it, and 4 patients (13.3%) had ILD, two of them with nonspecific interstitial pneumonia (NSIP) radiological pattern, and the other two with a radiological pattern other than NSIP and usual interstitial pneumonia, the majority with mild extension.
Patients characteristics before mepolizumab treatment.
| Manifestation | N/total (%) |
|---|---|
| Male sex | 16/30 (53.3) |
| Median age | 52±12.4 |
| Hypertension | 4/30 (13.3) |
| Dyslipidemia | 5/30 (16.7) |
| Former or actual smokers | 12/30 (40) |
| ANCA positive (IIF) | 15/30 (50) |
| ANCA positive (ELISA) | 17/30 (56.7) |
| Fever and/or weight loss | 17/30 (56.7) |
| Musculoskeletal | 11/30 (36.7) |
| Ophthalmologic | 5/30 (16.7) |
| Rhinosinusal | 26/30 (86.7) |
| Skin | 15/30 (50) |
| Ears and hearing | 4/30 (13.3) |
| Oropharyngeal | 2/30 (6.7) |
| Lung | 21/30 (70); ILD* 4/30 (13.3) |
| Gastrointestinal | 2/30 (6.7) |
| Kidney | 2/30 (6.7) |
| Heart | 5/30 (16.7) |
| Central nervous system | 1/30 (3.3) |
| Peripheral nervous system | 12/30 (40) |
| Mean CRP, mg/L | 18.7±48.9 |
| Mean eosinophils/μL | 1095±4695 |
| Median BVAS | 12.5±6.9 |
| Median daily prednisone dose (mg/d) | 50±18.7 |
| Use of another immunosuppressant | 19/30 (63.3) |
| Mean disease duration (months) | 51±63.9 |
Fifteen of 30 (50%) patients were ANCA positive as determined by indirect immunofluorescence and seventeen (56.7%) by EIA. FFS at diagnosis was available only in 23 patients, and the results were 0 in 17 (73.9%) and 1 in 6 patients (26.1%). The EULAR Severity Index (N=29) was 0 in two patients (6.9%), 1 in twelve patients (41.4%), 2 in ten patients (34.5%) and 3 in five patients (17.2%). On the other hand, median BVAS at diagnosis (N=28) was 12.5 (p25–p75; 9.5–18.3).
The median daily prednisone dose (or equivalent) at diagnosis was 50mg/day. Up to 9/30 patients (30%) required intravenous pulses of methylprednisolone and 19 patients (63.3%) needed at least an immunosuppressant (11 out of 19 cyclophosphamide).
The mean disease duration of the EGPA when MPZ was started was 51 months, and the mean follow-up during treatment MPZ was 16 months. In 13 patients (43.3%) the MPZ dose was 100mg subcutaneous once a month, while 1 patient started at 200mg/m, and 16 others started at 300mg/m (53.3%). A total of 12 patients (40%) needed a concomitant immunosuppressant drug with MPZ (10 of them were azathioprine and the other 2 mycophenolate mofetil).
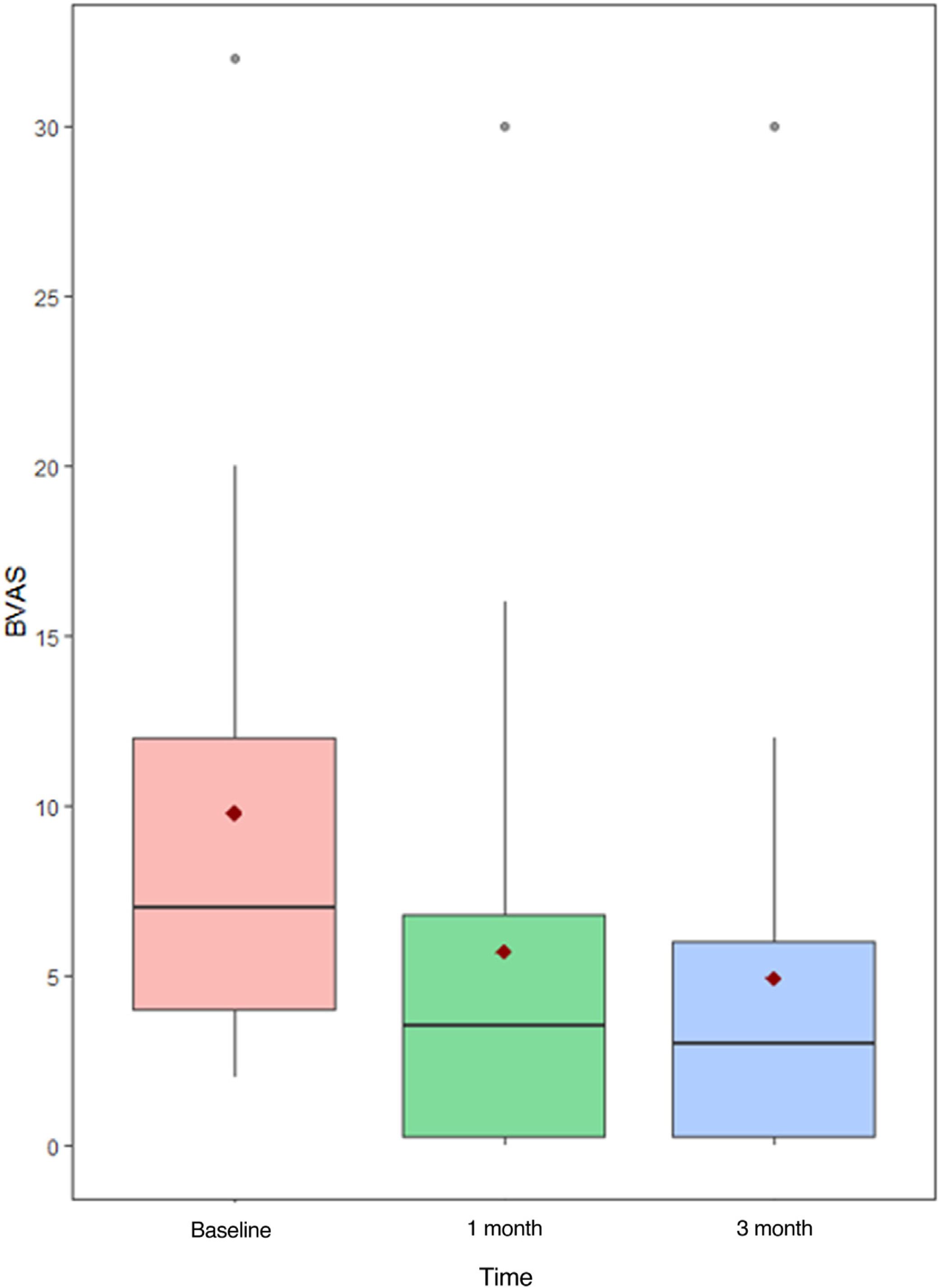
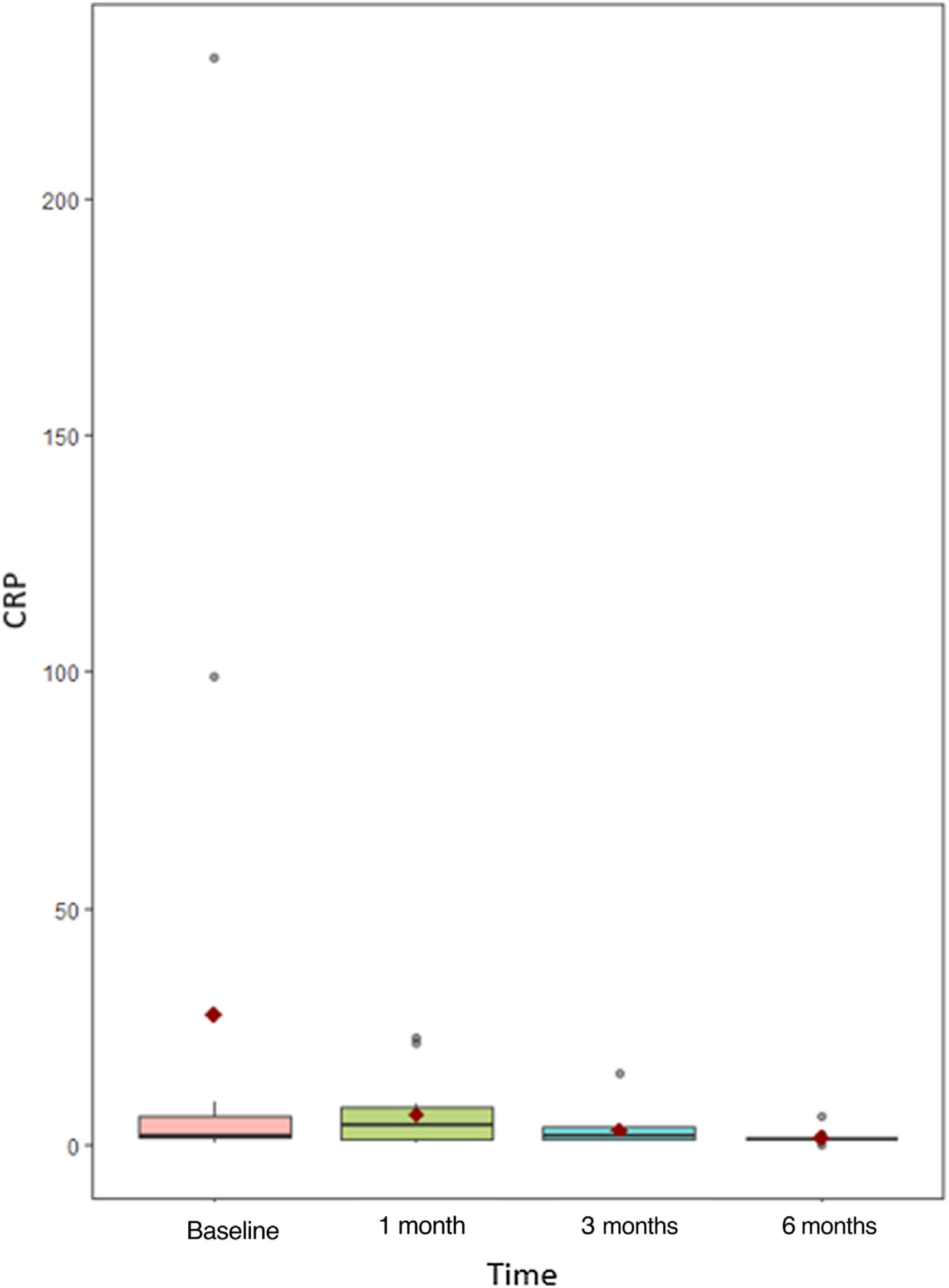
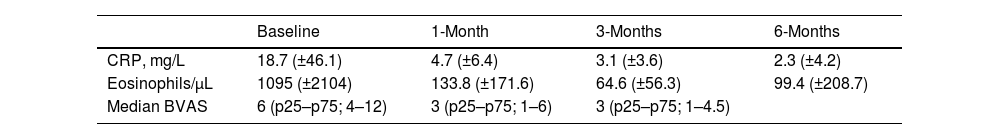
Prior to administration of MPZ, the mean daily dose of prednisone was 18.7mg/d (±16.6mg), while afterwards it fell to 2.4mg/d (±3.2). In up to 23 patients (76.7%) the daily GC dose was reduced, while and in 16 others (53.3%) complete withdrawal of GC was achieved. The median BVAS before MPZ was 6 (p25–p75; 4–12), which was reduced to 3 after just one month of treatment (Fig. 1 and Table 2) statistically significant (p<0.001) with Shapiro–Wilk normality test. The mean value of C-reactive protein (CRP) before MPZ was 18.7mg/L (±46.1), after 3 months of treatment it was reduced to 3.1mg/L (±3.6), and after 6 months to 2.3mg/dL (±4.2) (p=0.056) (Fig. 2 and Table 2).
In addition, prior to MPZ the patients had a mean value of 1095 eosinophils/μL (±2104), and after 3 months of treatment this fell to 65 eosinophils/μL (±56.3) and 99 eosinophils/μL (±208.1) after 6 months (Fig. 3 and Table 2). Twenty-six patients (86.7%) were 20% responders according to PGA, twenty-three patients (76.7%) were 50% responders and fourteen patients (46.7%) achieved clinical remission after 6 months using MPZ. Four patients were non-responders (13.3%).
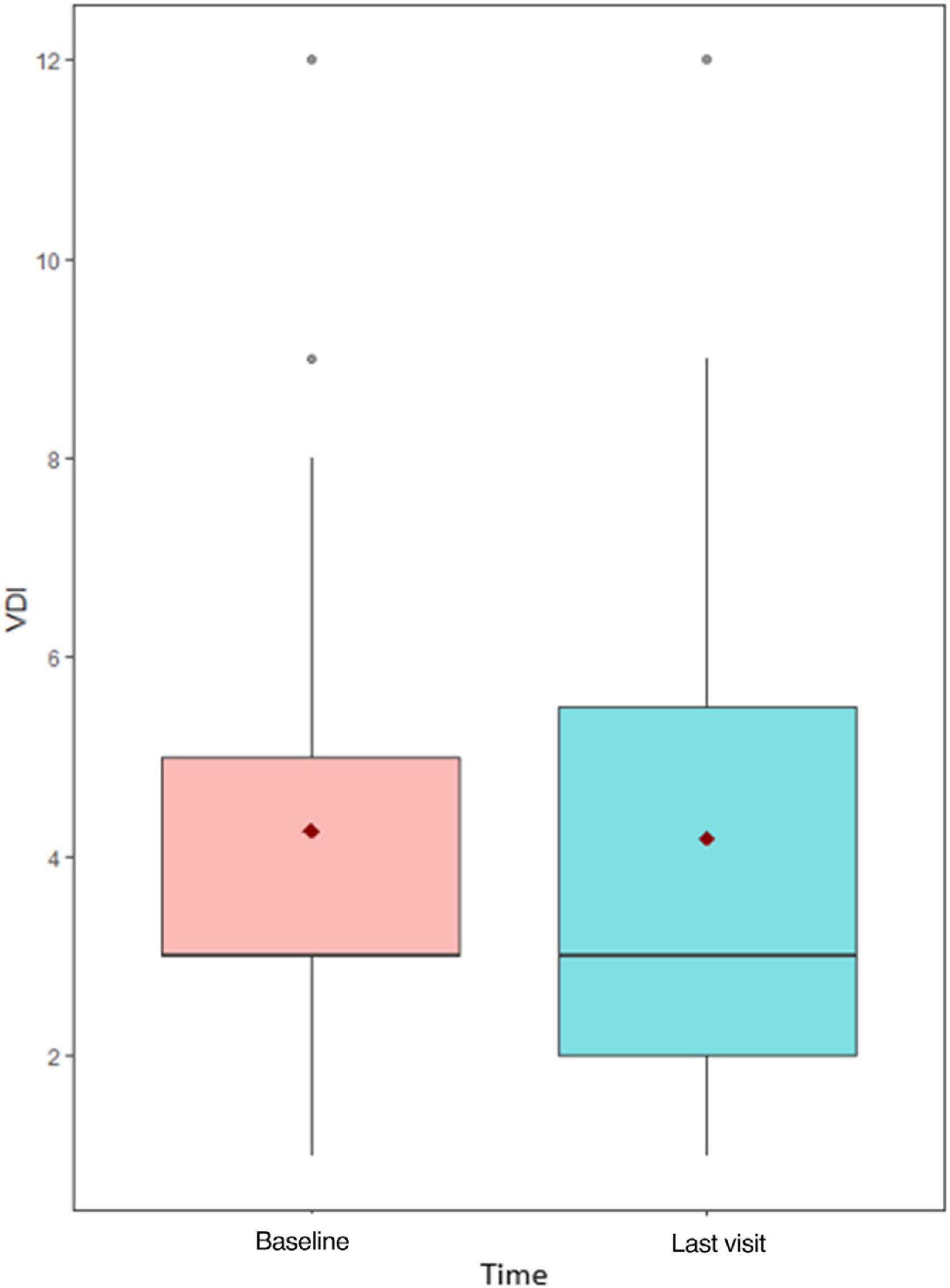
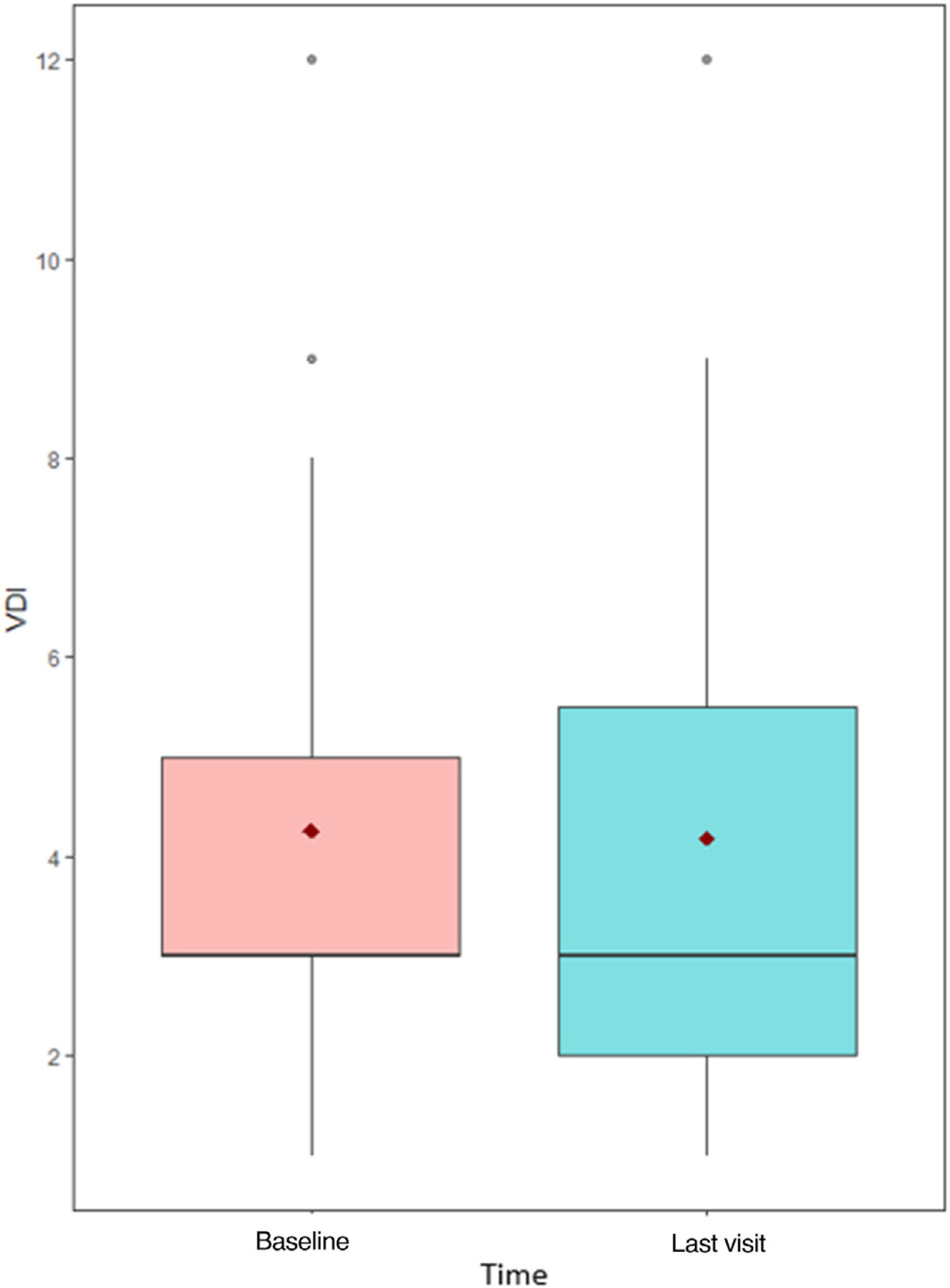
Moreover, median VDI before treatment with MPZ was 3 (p25–p75; 3–5), and afterwards, in the last visit, it continued being 3 (p25–p75; 2–5), without significant statistical differences (p=0.774) (Fig. 4). VDI progressed in only 4 patients (15.4%) (one point in 3 patients and two points in the other one).
At the last available visit, 24 patients (80%) remained on MPZ treatment. No patient suffered local reactions to MPZ injection or serious infections. One patient was diagnosed with a malignancy and another discontinued the drug due to musculoskeletal pain, switching successfully to benralizumab (a monoclonal antibody anti-IL-5 receptor). The patient with the malignancy eventually died, though no apparent relation with MPZ was found.
DiscussionManagement of EGPA remains challenging, especially in refractory or relapsing disease when GCs and immunosuppressant drugs might be toxic or ineffective.
In this retrospective analysis of real clinical practice involving Spanish patients with EGPA treated with MPZ, this drug showed substantial efficacy, allowing for a reduction or withdrawal of GCs, consistent with the randomized, double-blind, placebo-controlled trial conducted by Wechsler et al.1 Results from this trial exhibit even more clinical benefits of the treatment with MPZ when using alternative definitions of response, such as physician global assessment, to capture any clinical improvement, in a post-hoc analysis.3
The optimum dose of MPZ in EGPA has yet to be established, and some experts have found low doses adequated for achieving remission. However, Canzian et al., suggested that MPZ 300mg/m was more effective than 100mg/m, resulting in greater remission rates (82% and 76%, respectively).4 In our study, 16/30 (53.3%) patients were treated with the 300mg monthly dose (dose indicated in the technical sheet of MPZ for EGPA in Spain), although due to the low number of patients, a comparative analysis was not possible. Furthermore, the study by Canzian et al. reported a very high rate of treatment survival for MPZ without any flares, similar to the observed in our cohort, in which 80% of patients continued treatment after 14 months.4
In the study by Ueno et al, the retention rate in the MPZ group was 100% and it was considered very safe. In fact, MPZ was associated with a lower risk of bacterial and fungal infections as compared to intravenous cyclophosphamide. Moreover, at month 6, BVAS and the rate of decrease in BVAS in MPZ and cyclophosphamide group did not differ.5 In our study, the safety profile was really good, with only one patient discontinuing the drug because of musculoskeletal pain.
Steinfeld et al. achieved a 50% or greater decrease in daily oral GC even in patients who did not achieve remission. Moreover, these patients remained flare free.3 Ueno et al. also achieved a reduction of GC after starting MPZ, from 30mg/d pre-MPZ to 5mg/d, 6 months after MPZ was started.5 In our cohort, the median daily dose of oral GC was reduced from 18.7mg/d to 2.4mg/d after the introduction of MPZ. Actually, 16 patients (53.3%) were able to discontinue prednisone and all of them were also relapse free.
Interestingly, only a previous study has addressed the impact of MPZ in organ damage; in their analysis, Ueno et al. did not find differences pre-MPZ versus post-MPZ in VDI scores in a group of 16 patients treated with MPZ, after 12 months of follow-up.5 In our study, encompassing larger number of patients under treatment of MPZ, the median VDI remained also stable overtime, after 14-month follow-up overtime in MPZ, without significant statistical differences between pre-MPZ and post-MPZ measurements.
Compared with the largest European series of MPZ-treated patients with EGPA reported so far (Bettiol et al.),6 our patients used more frequently the dose of 300mg/m (53.3% vs 17%), but also, the median BVAS prior MPZ was higher in our cohort (6 vs 4). In Bettiol's series, 21.7% patients experienced adverse effects and 16 (7.9%) discontinued the treatment because of that; meanwhile in our series, adverse effects that led to MPZ discontinuation were reported only in 2 patients (6.7%). There were no adverse effects requiring hospitalization in our study, but it occurred in 6 patients of Bettiol et al. cohort (2.9%).
Fujii et al. reported adverse effects in 24% of the patients and hypersensitivity reactions in 5.3% of the patients in their series.7 In our cohort, there were no hypersensitivity reactions reported. Moreover, in their series, 5 patients (1.6%) worsened of EGPA symptoms after MPZ and none of our patients suffered a disease worsening. In the real-world pharmacovigilance study of mepolizumab in the FDA adverse event reporting system database leaded by Zou et al.,8 it was observed that females experienced a higher occurrence of negative responses to mepolizumab in comparison to males (55.76 vs 26.21%), most commonly reported adverse events were related to respiratory, thoracic and mediastinal disorders, and the median time for mepolizumab-related adverse events to occur was 109 days, with most cases happening within the initial month (N=1134; 34.75%); however, in our series the only adverse effect that occurred in the first month of treatment was intense arthromyalgia in a male patient.
In the longest assessment period to date in patients with EGPA treated with MPZ, by Ishii et al., they observed a reduction in glucocorticoids dose and also in clinical symptoms after a minimum of 144 weeks.9 38% of patients in this study reached to discontinue glucocorticoids by weeks 45–48, compared with 53.3% in our cohort. They also reported an optimal safety profile, with no adverse effects drug related.
Shiomi et al. analyzed the factors associated with mepolizumab retention in EGPA patients, and they saw that 5 years retention rate was 78.7% and it was associated with the use of immunosuppressant prior to the introduction of MPZ, but not with eosinophils counts and ANCA positivity; moreover, they observed that the VDI was significantly lower in the MPZ continuation group than in the MPZ discontinuation group. This last observation on the VDI is in line with the data from our series in which this value only increased in 4 patients (15.4%), so MPZ may be involved in reducing accumulated damage.10
The limitations of our study are those inherent to a retrospective analysis, a relatively low number of patients and the absence of control group. A larger number of patients and longer follow-up would be needed to verify the safety and damage prevention data of MPZ in EGPA.
ConclusionsSo far as we know, this is the largest Spanish cohort of patients with EGPA treated with MPZ in real practice. Our data suggests that it is an effective and safe drug that allows glucocorticoids withdrawal without relapses. Furthermore, MPZ may prevent organ damage progression as measured by VDI.
GrantsFERBT2023. The authors thank the Spanish Foundation of Rheumatology for providing medical writing/editorial assistance during the preparation of the manuscript.
We also thank the Systemic Autoimmune Disease Work Group from the Spanish Rheumatology Society (EASSER) for helping to recruit the patients.
Conflicts of interestNo author has conflicts of interest with this paper.