Olecranon bursitis (OB), characterized by inflammation and fluid collection in the olecranon bursa is a commonly encountered out-patient condition. The data is heterogeneous regarding a stepwise and standardized approach to aseptic OB treatment and the efficacy of intra-bursal corticosteroid injections (CSI). The objective of this review is to systematically evaluate the non-surgical treatment options for aseptic OB.
MethodsThis systematic review was conducted in accordance with PRISMA recommendations. The English and non-English literature search was performed in 5 medical databases to identify studies evaluating the treatment of OB. All included studies were evaluated for risk of bias (RoB) using the revised Cochrane RoB tool for randomized control trials (RCTs) and the Newcastle-Ottawa Scale (NOS) for case–control and cohort studies.
ResultsFor the final analyses, 2 RCTs and 2 observational studies were included. The RoB for the RCTs was high and both failed to demonstrate a significant difference in terms of the resolution of OB and bursal tenderness among various invasive and non-invasive treatment options. Corticosteroid injection (CSI) was associated with a significant decline in the duration of symptoms. However, it was associated with a higher number of complications including bursal infection and skin atrophy.
ConclusionBased on the available data, it appears that the clinical resolution of aseptic OB can occur with conservative methods if implemented earlier in the disease course. Although CSI is more effective than other treatments, it should be reserved for refractory cases because of a higher complication rate.
La bursitis olecraniana (BO), que se caracteriza por inflamación y acumulación de líquido en la bolsa olecraniana, es una situación muy común en el ámbito ambulatorio. Existen datos heterogéneos en cuanto al enfoque terapéutico gradual y estandarizado de la BO séptica y la eficacia de las inyecciones de corticosteroides (CSI) intrabursales. El objetivo de esta revisión es evaluar sistemáticamente las opciones terapéuticas no quirúrgicas para la BO séptica.
MétodosEsta revisión sistemática se llevó a cabo de acuerdo con las recomendaciones PRISMA. La búsqueda en la literatura inglesa y no inglesa fue realizada en 5 bases de datos médicas para identificar los estudios que evalúan el tratamiento de la BO. Se evaluó el riesgo de sesgo (RoB) en todos los estudios incluidos, utilizando la herramienta RoB Cochrane revisada para ensayos controlados aleatorizados (ECA), y la escala Newcastle-Ottawa (NOS) para estudios de casos y controles y de cohortes.
ResultadosPara los análisis finales se incluyeron 2 ECA y 2 estudios observacionales. El RoB para los ECA fue alto, no demostrando ambos estudios una diferencia significativa en términos de resolución de la BO y sensibilidad bursal entre las diversas opciones terapéuticas invasivas y no invasivas. La inyección de corticosteroides (CSI) estuvo asociada a una reducción significativa de la duración de los síntomas. Sin embargo, también estuvo asociada a un número más elevado de complicaciones, incluyendo infección bursal y atrofia cutánea.
ConclusiónSobre la base de los datos disponibles, parece que la resolución clínica de la BO séptica puede producirse con métodos conservadores si estos implementan con carácter temprano en el curso de la enfermedad. Aunque las CSI son más efectivas que otros tratamientos, deberían reservarse para casos refractarios, dada su tasa de complicación más alta.
The olecranon bursa is susceptible to repeated trauma and infection because of its superficial location and limited blood supply. Olecranon bursitis (OB) is characterized by inflammation and fluid collection in the bursal cavity. It is a commonly seen condition in outpatient settings and the majority of cases are of aseptic bursitis.1 It is usually self-limited because of the intrinsic healing abilities of the bursa. Etiologies can vary; repeated trauma, structural bony abnormalities, long-term hemodialysis, rheumatoid arthritis, and crystal arthropathies can predispose patients to OB. Clinical presentation may range from painless swelling to serious infections. Management of aseptic OB is derived from a limited evidence base. The data is heterogeneous regarding a stepwise and standardized approach to treatment, the efficacy of intra-bursal corticosteroid injections (CSI), and management largely depends on the clinician's preferences. Initial conservative treatment includes avoidance of pressure to the area, application of ice, compression bandaging, orthosis, or oral NSAIDs. Invasive methods including bursal needle aspiration with or without intra-bursal CSI have shown efficacy in resolving symptoms. Surgery including endoscopic or open bursectomy, osseous resection with or without bursectomy, and/or percutaneous suction-drainage are opted for when conservative methods fail. To our knowledge, there is only one systematic review performed by Sayegh et al. in 2014 reporting the treatment outcomes for septic and aseptic bursitis.2 The purpose of our review is to systematically evaluate the comparative non-surgical management approaches toward aseptic OB.
MethodsThis systematic review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) recommendations.3
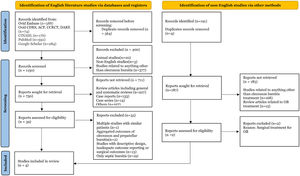
A trained medical librarian conducted searches in Embase, Cochrane Database of Systematic Reviews, ACP Journal Club, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects (all via the Ovid interface), Medline, CINAHL, and Google Scholar. The search terms included MeSH and keywords of the following: bursitis, elbow, and olecranon. The searches were run on March 12, 2021. Initially, the search was limited to English literature, however, an additional search for non-English citations was completed on May 27, 2021. The bibliography of identified studies was manually scanned to identify further studies. A sample search strategy is available in Appendix 1.
We included randomized clinical trials (RCTs) and cohort studies evaluating non-surgical treatment methods in adult patients with aseptic olecranon bursitis. Outcomes accessed were clinical resolution of aseptic olecranon bursitis and complications following each treatment. We excluded duplicate studies, case reports, reviews, meta-analyses, case series, commentary, animal studies, and cadaver studies. In addition, the studies assessing treatment outcomes without a control arm, studies not describing treatment protocol or clinical outcomes, and the studies only describing septic bursitis outcomes, or describing an aggregate treatment outcome for different types of bursitis were excluded. In multiple studies with similar patients, studies not meeting the other inclusion criteria were excluded. From the studies reporting other types of bursitis, outcomes of only olecranon bursitis were included in the review.
Data were extracted on the excel sheet and verified by another author. Parameters extracted were study characteristics, patient characteristics, treatment protocol, complications, and clinical resolution of aseptic OB following non-surgical treatment. Study characteristics included the study design, type and location of bursitis, sample size, and follow-up interval. Patient characteristics included age, gender distribution, comorbid condition, and symptom duration. Interest complications included persistent swelling or tenderness, persistent drainage, bursal infection, scar-related complications, and skin atrophy. A descriptive analysis was performed for the clinical outcomes. Patients who failed to respond by the last follow-up in a particular study were considered treatment failures.
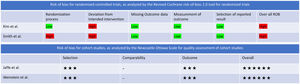
The methodological quality for RCTs was assessed using the Revised Cochrane risk-of-bias tool for RCTs and the Newcastle-Ottawa Scale (NOS) for cohort studies.4,5 For the NOS, a score of ≥6 was suggestive of higher study quality and study credibility.
ResultsLiterature search and study characteristicsThe initial search identified 1714 citations with a result of 1150 citations after removing duplicates. The non-English literature search identified 187 citations after the removal of duplicates. PRISMA flow diagram describing the inclusion process is mentioned in Fig. 1. Four studies containing 181 patients were included in the final analysis; Two were RCTs, and 2 were retrospective cohort studies.6–9 These studies were published between 1984 and 2015. All these studies evaluated only OB. The study by Jaffe et al. also included patients with septic olecranon bursitis; however, only aseptic bursitis cases were included in the systematic review.
Patient characteristicsA total of 181 patients (125 from RCTs and 56 from cohort studies) were included in the final analysis. The mean age of patients was 57 years, and 87% were male. Out of 83 patients enrolled by Kim et al., 15 patients had a history of high blood pressure, 10 had diabetes mellitus, and 41 had traumatic bursitis. Patients with bursitis combined with gout or rheumatoid arthritis and concomitant elbow pathology were excluded from the study.6 A bone spur was observed in 10/42 patients by Smith et al., 14 patients had a history of trauma and 1 had monosodium urate crystal-induced bursitis.7 Weinstein et al. evaluated 47 (100%) patients with traumatic bursitis. Patients with systemic rheumatic disease and gout were excluded.8 The duration of symptoms is mentioned in Table 1 and the follow-up duration is mentioned in Table 2.
Study and patient characteristics.
| Year of publication | Study design | Number of patients | Mean age (range) | Males | Comorbidities | Traumatic | Bone spur | Mean duration of symptoms | |
|---|---|---|---|---|---|---|---|---|---|
| Kim et al.6 | 2015 | RCT | 83 | 46 (13–81 years) | 59 (71%) | HBP- n=15DM-10 | 41 | N/A | 4 weeks (mean) |
| Smith et al.7 | 1989 | RCT | 42 | 60.5 (27–92) | 42 (100%) | Gout=1 | 14 | 10 | NA |
| Jaffe et al.9 | 1984 | Retrospective cohort study | 9 | 57 (35–68 years) | 9 (100%) | NA | 1 | NA | NA |
| Weinstein et al.8 | 1984 | Retrospective cohort study | 47 | 57.5 (59 in aspiration group and 56 in group II) | 47 (100%) | NA | 47 | NA | Median: 14 days in group I (A) and 11 days in group II (AS) |
RCT: randomized control trial; HBP: high blood pressure; DM: diabetes mellitus; NA: not available.
Clinical outcomes.
| Number of patients | Resolution at final follow-up | Other measures | Time to resolution | Follow-up duration | Relapse and recurrence | Complications | |
|---|---|---|---|---|---|---|---|
| Kim et al.6 | 83 | p-value: 0.073 (No significant difference among groups) | Pain VAS | Median weeks | 12 weeks (mean)4–140 weeks range | N/A | No infection, atrophy, persistent drainage |
| I) Compression/NSAIDs | 30 | 25 (83%) | 1.9 | 3.2 weeks | |||
| II) Aspiration+compression/NSAIDs | 26 | 17 (65%) | 1.7 | 3.1 weeks | |||
| III) Aspiration with CSI+compression/NSAIDs | 27 | 23 (85%) | 1.7p-value: 0.88 | 2.3 weeksp-value: 0.015 | |||
| Smith et al.7 | 42 | p-value>0.05 | Respirations at 6 months | Significant difference in mean bursal swelling; group I and II had a rapid decline in swelling at week 1 (p value-0.005) as compared to group III and IV | 6 months interval (total follow-up period) | NA | No skin atrophy or infection |
| I) Oral NSAIDS+CSI+compression+aspiration | 11 | 11 (100%) | Group 1 and 2 patients had only 0.1±0.3 reaspirations | ||||
| II) Oral Placebo+CSI+compression+aspiration | 10 | 9 (90%) | Group 1 and 2 patients had only 0.1±0.3 reaspirations | Persistent tenderness=1 | |||
| III) Oral NSAIDS+compression+aspiration | 10 | 9 (90%) | 1.0±1.2 [mean (±SD)] re-aspirations | Persistent tenderness=2 | |||
| IV) Oral Placebo+compression+aspiration | 11 | 10 (91%) | 0.4±0.7 [mean (±SD)] re-aspirations | Persistent tenderness=1 | |||
| Jaffe et al.9 | 22.4 months (12–29 months) | NA | |||||
| I) Aspiration+CSI+compression dressing | 4 | 2 | 2 (recurrence of painless swelling) | ||||
| II) Aspiration+PO NSAIDS+compression dressing | 5 | 3 | 0 | ||||
| Weinstein et al.8 | 31 months (mean)6–62 months (range) | ||||||
| I) Bursal aspiration alone | 22 | 21 | Slow reduction in percentage of patients | n=3 (self-limited relapse) | No infection, skin atrophy reported; chronic pain (n=2) | ||
| II) CSI+aspiration | 25 | 25 | Marked and abrupt decline in the percentage of patients with effusion at week 1 according to Modified Life Table analysis (p-value N/A) | n=2 (self-limited relapse) | Infection (3 cases), skin atrophy (5 cases), and chronic local pain (7 cases) | ||
NA: not available; VAS: visual analog scale; CSI: corticosteroid injection.
In the study by Kim et al., the compression and oral NSAIDS alone (n=30) group was compared with aspiration along with compression/NSAIDS (n=26), and patients who received both aspiration and CSI along with compression/NSAIDS (n=27). For intra-bursal injection, 1mL of 40mg/mL triamcinolone acetonide mixed with 1mL of 2% lidocaine was used. After the application of povidone, the bursal aspiration was performed at a point slightly distal to its center using a secured drape. The elbow was lightly compressed by applying an elastic bandage in all patients. Aspiration or aspiration+CSI was repeated on a weekly basis if swelling failed to resolve in the last two groups respectively. Treatment failure was defined as a persistent olecranon bursal fluid collection or swelling recurrence to the initial size at Week 4.6 In the study by Smith et al., treatment outcomes were compared among 4 groups: oral NSAIDS+CSI+compression+aspiration (n=11), oral placebo+CSI+compression+aspiration (n=10), oral NSAIDS+compression+aspiration (n=10) and oral placebo+compression+aspiration (n=11). Aspiration was performed at a point lateral to the olecranon bony projection under aseptic conditions. For intrabursal injection, 20mg methylprednisolone acetate was used. A compression dressing around the involved elbow was required by all patients for a 10-day period continuously. A persistent olecranon bursal swelling from fluid reaccumulation despite at least three re-aspirations was considered a failed treatment.7 In the study by Jaffe et al., 4 patients received aspiration+CSI+compression dressing and 5 received aspiration+oral NSAIDS+compression dressing. For intra-bursal injection, 40mg methylprednisolone acetate and 1% lidocaine were used.9 Weinstein et al. compared the clinical outcomes of traumatic bursitis where all patients (n=47) were aspirated once followed by only 25 patients injected with 20mg of intra-bursal triamcinolone hexacetonide at a mean duration of 7 days following initial aspiration.8
The clinical resolution, recurrence, and relapseDue to heterogeneity in the data in terms of comorbidities and clinical outcomes, a meta-analysis could not be performed. Kim et al. reported no significant difference in overall resolved cases (Compression/NSAIDs n=25/30 vs Aspiration+Compression/NSAIDs n=17/26 vs Aspiration with CSI+Compression/NSAIDs n=23/27; p-value: 0.073) and pain Visual Analog Scale (VAS) among the non-invasive and invasive methods.6 However, CSI after aspiration was associated with a statistically significant earlier recovery compared to compression with NSAIDS and aspiration (2.3 weeks vs 3.2 weeks vs 3.1 weeks; p-value: 0.015). Also, a longer duration of symptoms (6 weeks vs 4 weeks) was significantly associated with treatment failure (p-value: 0.08). Similarly, Smith et al. observed a significant difference in mean bursal swelling in patients treated with CSI+aspiration±NSAIDs at one week (p-value 0.005) which was sustained at weeks 3 and 6 (p-value 0.05), and less mean number of re-aspirations by 6 months (p-value: 0.025). These patients only had 0.1±0.3 re-aspirations while the NSAIDS+aspiration+compression group had a mean (±SD) of 1.0±1.2 re-aspirations and aspiration+compression+placebo group had a mean of 0.4±0.7 re-aspirations. There was no significant difference in terms of failure to respond to treatment among these 4 groups.7 Jaffe et al. noticed 50% overall resolution in the CSI+aspiration group with 50% recurrence as compared to 60% resolution with no recurrence in the aspiration and NSAIDs group. Statistical significance was not mentioned.9 Weinstein et al. observed a 100% resolution in the CSI+aspiration group after the first treatment followed by a relapse in 8% and a complete resolution in all patients by end of the study follow-up (mean 31 months). The aspiration-alone group had a 95% resolution after the first treatment followed by relapse in 13.6% of patients and resolution in 95.5% by end of the study follow-up (mean 31 months). The modified life table analysis showed that the CSI+aspiration group had rapid recovery in around 85% of patients by the first week, however, the aspiration alone group had only 25% of cases resolved in the first week.8
ComplicationsSmith et al. observed persistent tenderness in the oral NSAIDS+compression+aspiration group (20% of patients, n=2), oral placebo+CSI+compression+aspiration (10% of patients, n=1), and oral placebo+compression+aspiration group (9% of patients, n=1); however, the results were not statistically significant in groups (p>0.05).7 There were no reports of complications of infection or skin atrophy mentioned in the studies performed by Kim et al. and Smith et al.6,7 Jaffe et al. reported that 40% of patients in the aspiration+NSAIDS+compression group had persistent tenderness.9 Weinstein observed a high rate of long-term side effects (6–62 months) in the CSI+aspiration group; persistent tenderness in 28%, wound infection in 12%, and skin atrophy in 20% of patients. 9.1% of patients in the aspiration-alone group had persistent tenderness.8
Risk of bias (RoB)RoB for both the RCTs was high and even though both retrospective cohort studies scored well (6 stars each), they fared poorly in terms of comparability (Fig. 2).
DiscussionThis systematic review evaluated the non-surgical management of aseptic bursitis by analyzing 2 RCTs and 2 observational studies with a comparative arm of different approaches. Both the RCTs failed to demonstrate a statistically significant difference in terms of the resolution of OB and bursal tenderness among various invasive (aspiration±CSI) and non-invasive (NSAIDS, compression) non-surgical treatment options. It is noteworthy that both RCTs had high ROB and the study by Kim et al. was powered to detect only a 30% difference in the treatment efficacy among groups.6,7 The corticosteroid injection was associated with a significant decline in the duration of symptoms and a reduction in the mean number of re-aspirations in the RCTs. Similarly, Weinstein et al. observed a marked and abrupt reduction in the percentage of patients with effusion in the first week with CSI as compared to bursal aspiration alone.8 It is evident that co-morbidities including diabetes mellitus, hypertension, bone spur, and immunosuppressant therapy were associated with impaired healing. Interestingly, only one study reviewed that diabetes and hypertension were not associated with treatment failure.6 Rather, a longer duration of symptoms before the treatment initiation was significantly associated with a failed clinical resolution by 4 weeks. The effect of co-morbidities on bursitis resolution and complications needs to be explored in future studies. Among all the studies, Weinstein et al. followed the treated patients for a longer duration and reported that CSI was associated with a higher number of complications including bursal infection (3 cases), chronic local pain (7 cases), and skin atrophy (5 cases) which is previously reported in the literature.10 Skin atrophy and bursal infection were not reported in patients treated by Kim et al. and Smith et al.6,7 Protocolized procedures to maintain an optimal aseptic practice and using a compression dressing after injection might be potential explanations. A lateral olecranon entry might have the advantage of avoiding infiltration of the steroid preparation into subcutaneous tissue surrounding the posterior tip of the olecranon bony process, a common site for irritation or pressure from arm resting. It is noteworthy that the duration of follow-up to evaluate the safety outcomes related to treatment was shorter in studies by Kim et al. (mean 12 weeks) and Smith et al. (6 months) compared to Weinstein et al. (mean 31 months).6–8 Further evidence and high-quality studies with a large sample size are needed to address the long-term safety profile of CSI in OB. Based on this review, it appears that the clinical resolution of aseptic bursitis can occur with conservative methods if implemented earlier in the disease course. Although corticosteroid injections are more effective than other treatment modalities, they possess a risk of secondary bursal infection and should be used with caution.
This systematic review has several limitations. A meta-analysis couldn’t be performed due to inconsistency in the various study outcomes. Inadequate reporting leads to a difficult assessment of some study findings e.g., pain VAS and repeated procedures before final resolution. The statistical significance of resolved cases among different treatment groups was not reported by observational studies making the implications of results challenging. Response to different therapeutic measures can differ by the etiology of OB and traumatic versus inflammatory OB (such as due to gout or rheumatoid arthritis) can have different responses to different therapeutic measures. Jaffe et al. did not mention comorbidities, and patients with inflammatory etiology of OB were excluded by Kim et al. and Weinstein et al., and only 1 patient in the study by Smith et al. had monosodium urate-induced bursitis, therefore, further studies are needed to delineate responses to different therapeutic measures based on etiology of OB.6–9
ConclusionIn summary, this systematic review suggests that conservative methods are a safe option for aseptic bursitis treatment due to a similar rate of clinical resolution and a lower risk of complications than invasive management options. CSI should be reserved for refractory cases where bursitis does not subside with conservative management.
Conflict of interestThe authors declare they have no conflict of interest.