We present the case of a 56-year-old female patient with a history of cutaneous psoriasis. She presented with several months of progressive swelling and pain in proximal interphalangeal (PIP) joints of the right hand, accompanied by morning stiffness. Laboratory evaluations revealed: ESR 19mm/h, CRP 0.5mg/dL, anti-cyclic citrullinated peptide antibodies: 7U/mL. A doppler ultrasound of the right hand showed significant increased vascularity in PIP joint. Treatment with methotrexate was initiated and titrated to 25mg subcutaneously per week without achieving clinical improvement.
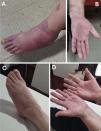
Adalimumab (Hyrimoz®) was then started, resulting in a highly favorable response. However, three months after initiating Hyrimoz®, the patient's health insurance replaced it with another adalimumab biosimilar, Amgevita®. Two months later, she developed new psoriasiform lesions on her hands and feet, along with recurrence of joint swelling and pain (Fig. 1A and B).
Given the appearance of new and severe skin lesions and worsening joint symptoms, two clinical questions emerged: Was this a paradoxical reaction to the anti-TNF agent, suggesting a need to switch therapeutic class? Or could reintroduction of the previously effective biosimilar be considered? Treatment with Hyrimoz® was resumed, leading to marked and sustained improvement after three months (Fig. 1C and D).
Psoriasis is a chronic, immune-mediated skin disorder affecting 2–3% of the global population.1 Anti-TNF agents have revolutionized the management of inflammatory diseases. However, they are associated with paradoxical reactions.2–5 Paradoxical adverse events can occur with both biologics and biosimilars.6 These reactions involve the unexpected onset or worsening of an immune-mediated condition typically treated by the same drug. While they often improve after discontinuing or switching the biologic, some cases may reflect a class effect, requiring complete withdrawal of anti-TNF therapy.7,8
In this case, the recurrence of symptoms appears to have been related specifically to the biosimilar switch rather than a class effect. Reintroduction of the original biosimilar (Hyrimoz®) led to a favorable clinical response, highlighting the importance of individualized evaluation when managing treatment with biosimilars.