Issue recommendations on practical aspects of the monitoring of levels of biological drugs that may be useful for rheumatologists.
MethodsWe conducted a systematic review of studies in which drug and anti-drug antibody levels were determined in patients with rheumatoid arthritis (RA) or spondyloarthritis (SpA) to study whether they could predict different outcomes. In light of the results of the review, a group of experts discussed under what circumstances testing biological drug levels and their antibodies could be useful. The discussion resulted in a series of clinical questions that were answered with the scientific evidence collected, and in algorithms that facilitate decision making.
ResultsIt was established that the determination of drug levels can be especially useful in two clinical situations, on treatment failure (primary or secondary) and on sustained remission. It is also reviewed which laboratory technique and timing for sample drawing are the most suitable for the measurement. Recommendations are issued on the interpretation of drug levels and on factors to be taken into account (for example, body mass index and disease modifying drugs).
ConclusionsEvidence-based algorithms and guidelines have been established to test drug levels and anti-drug antibodies in patients with RA and SpA, which can help clinical decision making.
Emitir recomendaciones sobre aspectos prácticos de la monitorización de los niveles de fármacos biológicos que puedan ser de utilidad para reumatólogos.
MétodosSe realizó una revisión sistemática de la literatura de estudios en los que se determinaron niveles de fármaco y de anticuerpos antifármaco en pacientes con artritis reumatoide o espondiloartritis para estudiar si podían predecir diferentes desenlaces. Con los resultados de la revisión un grupo de expertos discutió bajo qué circunstancias podría ser útil la solicitud de niveles de fármacos biológicos y sus anticuerpos, lo que se concretó en una serie de preguntas clínicas que fueron respondidas con la evidencia científica disponible y creándose algoritmos para facilitar la toma de decisiones.
ResultadosSe establece que la determinación de los niveles de fármaco puede ser especialmente útil en 2 situaciones clínicas, cuando hay fallo al tratamiento (primario o secundario) y en remisión mantenida. Se revisa también qué técnica de laboratorio y momento para tomar la muestra son los más adecuados para la medición, y se establecen recomendaciones sobre la interpretación de los niveles de fármaco y sobre factores a tener en cuenta (por ejemplo, índice de masa corporal y fármacos modificadores de la enfermedad).
ConclusionesSe han elaborado algoritmos y establecido posibles pautas y directrices para solicitar niveles de fármaco y de anticuerpos antifármaco en pacientes con artritis reumatoide y espondiloartritis, basados en la evidencia, que pueden ayudar a la toma de decisiones clínicas.
The biological drugs are protein macromolecules with a high molecular weight and complex pharmacokinetic and pharmacodynamic properties. These macromolecules depend on several factors such as net load, binding to the neonatal Fc receptor, Fcgamma receptor, glycosylation, PEGylation and aggregation.1 Although the principles of pharmacokinetics are consistent, the mechanisms that determine the processes of absorption, distribution, metabolism and excretion of biological drugs are very different from small molecule drugs and need to be studied in complex bioanalytical assays.1
Many factors influence the pharmacokynetics of anti-TNFs, altering the elimination of monoclonal antibodies and therefore the half-life: serum albumin, molecular weight, comorbidities, the activity itself of the underlying disease and the concomitant administration of immunosuppressants (e.g. methotrexate), as well as the formation of anti-drug antibodies (ADAs).2 Multiple factors contribute to the immunogenicity of anti-TNFs3; some depend on the drug itself: protein sequence, three-dimensional structure or post-translational modifications. Others depend on the manufacturing process that can affect both aggregation and post-translational modifications. The administration route, patient characteristics and use of concomitant medication4 will play a role in immunogenicity. This is why since the introduction of the biological therapies, anti-TNF especially, great variability has been observed between individuals in drug and ADA levels.5
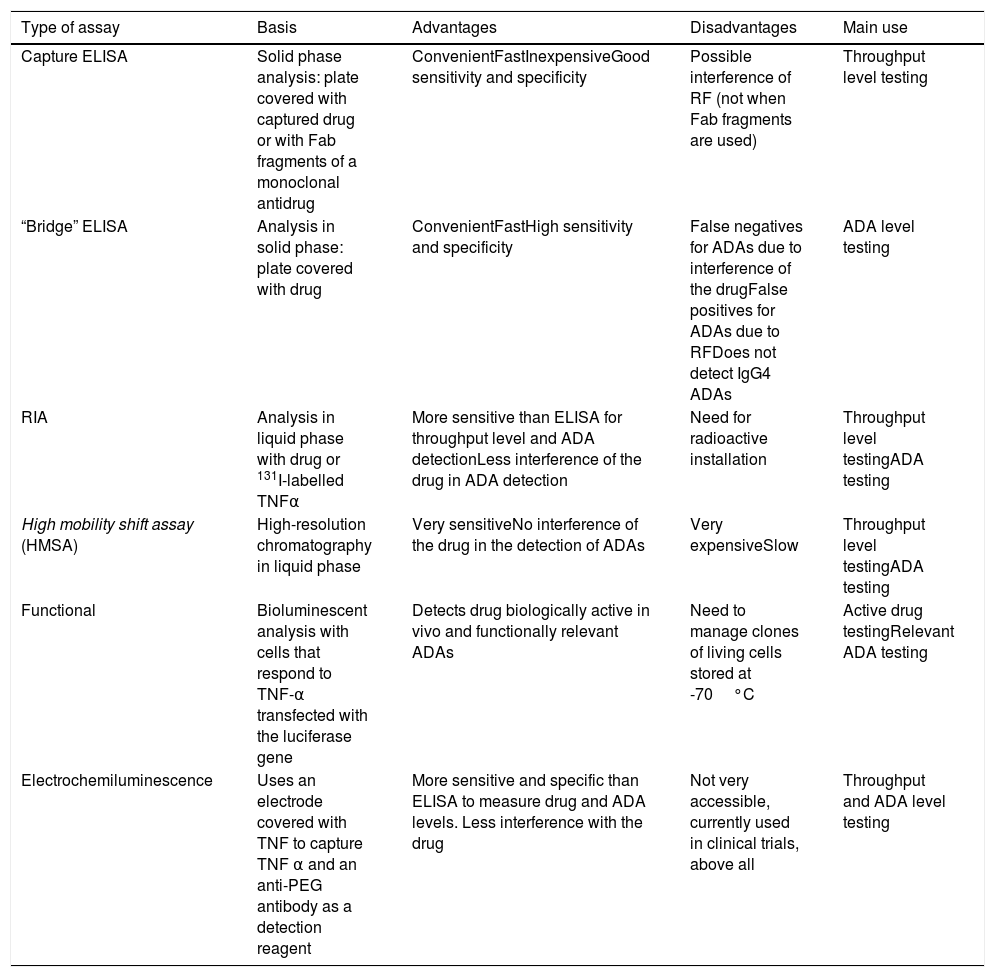
The objective must be to reach and maintain a level in the therapeutic range of the drug, in order to achieve clinical remission or low disease activity. There are several techniques for measuring biological throughput and ADA levels4 (Table 1).
Types of assay.
| Type of assay | Basis | Advantages | Disadvantages | Main use |
|---|---|---|---|---|
| Capture ELISA | Solid phase analysis: plate covered with captured drug or with Fab fragments of a monoclonal antidrug | ConvenientFastInexpensiveGood sensitivity and specificity | Possible interference of RF (not when Fab fragments are used) | Throughput level testing |
| “Bridge” ELISA | Analysis in solid phase: plate covered with drug | ConvenientFastHigh sensitivity and specificity | False negatives for ADAs due to interference of the drugFalse positives for ADAs due to RFDoes not detect IgG4 ADAs | ADA level testing |
| RIA | Analysis in liquid phase with drug or 131I-labelled TNFα | More sensitive than ELISA for throughput level and ADA detectionLess interference of the drug in ADA detection | Need for radioactive installation | Throughput level testingADA testing |
| High mobility shift assay (HMSA) | High-resolution chromatography in liquid phase | Very sensitiveNo interference of the drug in the detection of ADAs | Very expensiveSlow | Throughput level testingADA testing |
| Functional | Bioluminescent analysis with cells that respond to TNF-α transfected with the luciferase gene | Detects drug biologically active in vivo and functionally relevant ADAs | Need to manage clones of living cells stored at -70°C | Active drug testingRelevant ADA testing |
| Electrochemiluminescence | Uses an electrode covered with TNF to capture TNF α and an anti-PEG antibody as a detection reagent | More sensitive and specific than ELISA to measure drug and ADA levels. Less interference with the drug | Not very accessible, currently used in clinical trials, above all | Throughput and ADA level testing |
ADA: anti-drug antibody; ELISA: enzyme immunoassay; Fab: fragment of the monoclonal antibody molecule that binds to the antigen; RF: rheumatoid factor; RIA: radioimmunoassay; TNF-α: tumour necrosis factor α.
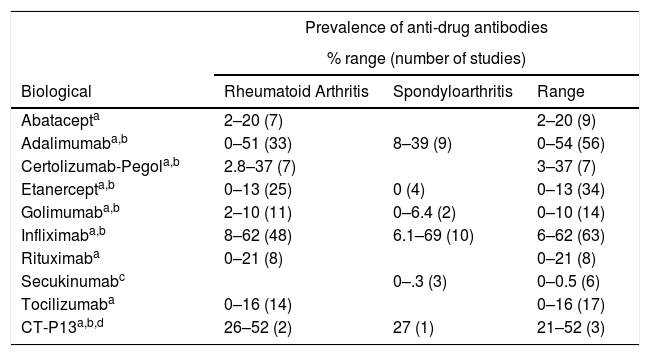
Although in clinical practice, in gastroenterology there is clear willingness to use throughput levels, in rheumatology it is not widespread practice since, in addition, testing is not available in many centres. Many rheumatologists wonder whether it is really useful to monitor the serum levels of biological drugs, who should be monitored, when, how often, using which technique, the optimum serum level of the drug and whether testing is cost-effective. A recent systematic review6 analysed different levels of ADAs found compared to the different biological agents used in rheumatic and inflammatory bowel diseases (Table 2). Prevalence found varies widely between studies; although we can extrapolate that the highest appear in studies of infliximab or its biosimilar.
Frequency of frequency of antidrug antibody formation of drugs authorised for rheumatoid arthritis and spondyloarthritis.
| Prevalence of anti-drug antibodies | |||
|---|---|---|---|
| % range (number of studies) | |||
| Biological | Rheumatoid Arthritis | Spondyloarthritis | Range |
| Abatacepta | 2–20 (7) | 2–20 (9) | |
| Adalimumaba,b | 0–51 (33) | 8–39 (9) | 0–54 (56) |
| Certolizumab-Pegola,b | 2.8–37 (7) | 3–37 (7) | |
| Etanercepta,b | 0–13 (25) | 0 (4) | 0–13 (34) |
| Golimumaba,b | 2–10 (11) | 0–6.4 (2) | 0–10 (14) |
| Infliximaba,b | 8–62 (48) | 6.1–69 (10) | 6–62 (63) |
| Rituximaba | 0–21 (8) | 0–21 (8) | |
| Secukinumabc | 0–.3 (3) | 0–0.5 (6) | |
| Tocilizumaba | 0–16 (14) | 0–16 (17) | |
| CT-P13a,b,d | 26–52 (2) | 27 (1) | 21–52 (3) |
Therefore we set ourselves the objective of issuing practical advice, based on the best available evidence, on the practical aspects of monitoring biological throughput levels that could be useful in the field of rheumatology. These indications refer to throughput levels where drug and antibody levels (TNF inhibitors – anti-TNF or TNFi – and tocilizumab) are currently measured, in both their standard and their optimised regimens, and that affect patients with rheumatoid arthritis (RA) and spondyloarthritis (SpA), including psoriatic arthritis.
MethodsA group of experts, with different a priori positions on the usefulness of throughput measurement, specified the content of the document through a list of clinical questions to be answered based on scientific evidence. With this “questions map”, the content skeleton was prepared with useful clinical questions, which had to be answered using the articles selected by the systematic review.
The panel of experts defined as “useful” any intervention that modified or helped in making a cost-effective decision.
Systematic literature searchThe following literature databases were screened: Medline, Embase and Cochrane Library from their inception until November 2016, using strategies that would enable the identification of studies that answered the following PICO questions:
- a)
Whether in patients with RA and SpA under treatment with biological therapy and in maintained remission (P) throughput levels (I) serve as predictors of relapse (O).
- b)
Whether in patients with RA and SpA under treatment with biological therapy and primary or secondary failure (P) information on throughput levels (I) modifies the therapeutic attitude according to throughput levels: change of drug or target, or intensification of dose (O).
- c)
Whether in patients with RA and SpA treated with biological therapy in combination or otherwise with methotrexate (MTX) (P) biological throughput levels (I) are associated with response (O).
For all the questions we included any type of design, experimental or observational, provided the study included at least 10 patients, and excluded animal and basic science studies.
The systematic search was followed by peer selection (MML and LC) and related articles were retrieved. Subsequently, a secondary manual search of the literature was undertaken of articles that were eventually included.
Discussion of practical aspects based on the evidenceIn a meeting of the panel with the reviewers the results of the systematic reviews were analysed and the answer to each question was discussed, consensus was sought among all the experts for drafting, and the level of evidence that supported the resulting recommendation was noted. During the meeting and subsequent interaction by email, the opinion of the experts was included on practical aspects for which no scientific evidence was found to support it. No further experts or users were consulted.
With the evidence and opinion a series of practical advice was elaborated for clinical decision-making pathways, on the usefulness of determining throughput and ADA levels and their interpretation. All the indications were drafted with the consensus of the experts.
For each suggestion the reviewers wrote a summary of the evidence and sought the opinion of the panellists, which was in line with the evidence, and the panellists added the practical aspect that could be derived from it. Discussion was open and each section was drafted by consensus until all the panellists confirmed that they were comfortable with the wording.
ResultsThe systematic reviews that constitute the first part of this paper have been published.7 The results of the discussion and practical advice are presented below, with the summary of the supporting evidence resulting from the systematic review of the literature.
In which clinical situations is the measurement of throughput levels useful?Measurement of throughput levels can be considered useful in making decisions in the event of primary or secondary biological drug failure in patients in remission, before optimising or discontinuing the biological drug, and it seems of little use in temporary suspension or used in a baseline or routine manner.
Mulleman et al. showed in 24 patients that measuring infliximab levels can modify the decision of physicians in 50% of cases and improve control of disease activity.8 Méric et al., using the same algorithm as Mulleman, observed an inverse relationship between serum concentrations of infliximab and BASDAI in SpA.9 Garcês et al. studied 105 patients with RA and treatment failure who were randomised to continue with the standard strategy or an algorithm that included the serum level of the drug.10 The group that followed the therapeutic algorithm based on throughput and ADA levels every 3 months were more likely to respond (OR=7.91, p<.001, 95% CI: 3.27–19.13) and achieve low disease activity (OR=9.77, p<.001, 95% CI: 4.69–20.37) than those who were not followed by algorithm.
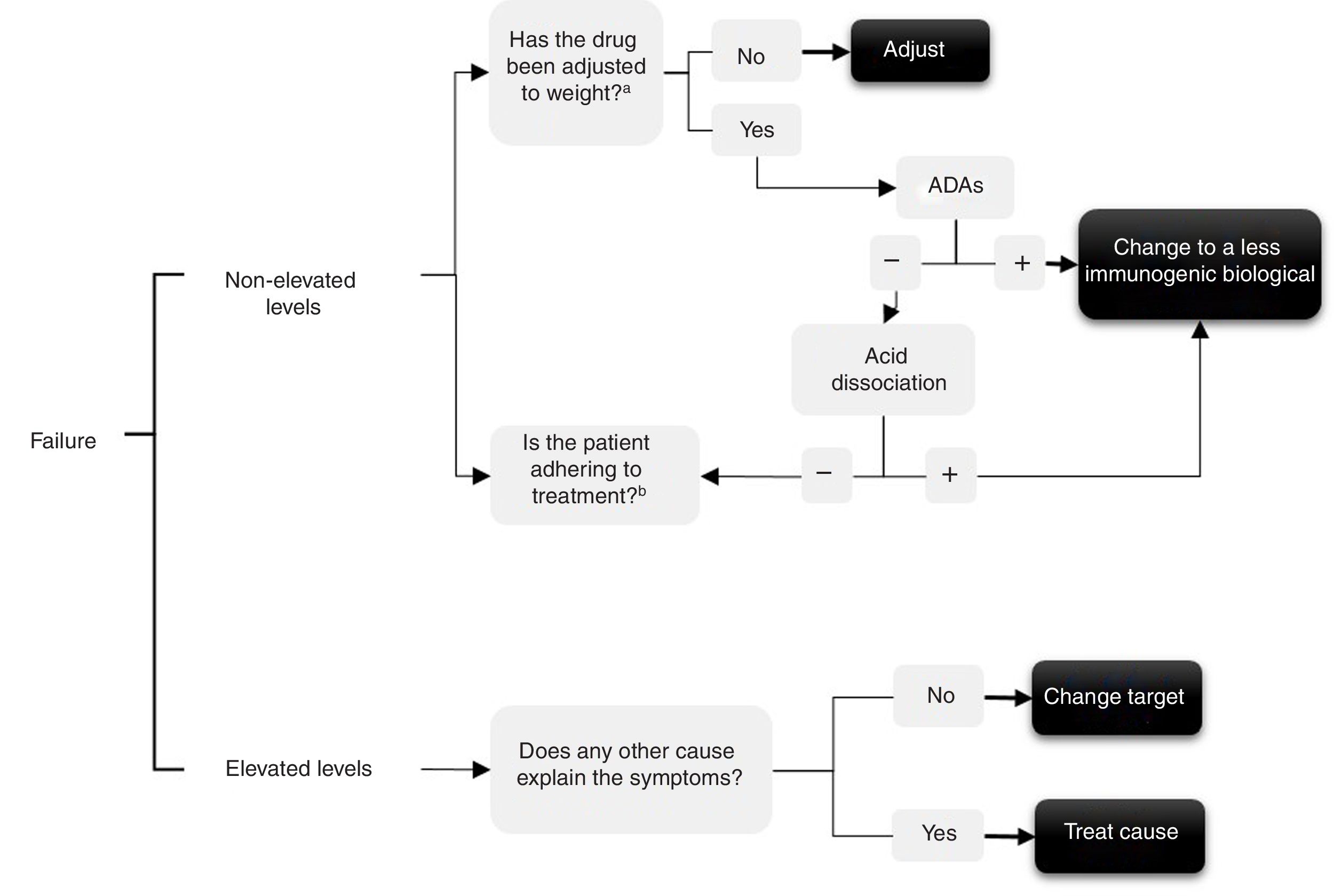
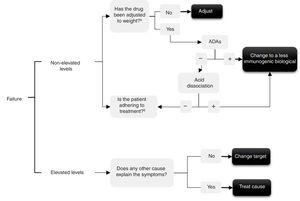
In the event of treatment failure determining levels enables the possible cause to be understood and a decision to be made. Several authors have made various interpretations for determining levels,10–13 that are summarised in the algorithm in Fig. 1. In short, if throughput levels are undetectable or in subtherapeutic range this could be due to: (1) immunogenicity, which would have to be checked by determining levels of anti-drug–antibodies – and in this case use the acid disassociation technique if there are negative ADAs, since this will demonstrate ADAs if they were present but bound to the drug14—or (2) due to a lack of treatment adherence, both to the biological and possible concomitant treatment with synthetic disease-modifying anti-rheumatic drugs (DMARDs), the cause of which would have to be checked with the patient.
Conversely, if throughput levels are high or in therapeutic range and yet the drug is not effective, 2 situations are possible: (1) the target is really not adequate, so we must switch to another drug with a different mechanism and not insist on the same family (primary failure); or (2) the cause of the current symptoms might not relate to the inflammatory activity of the disease for which the biological drug was prescribed and might be due to other causes (for example, pain produced by spinal canal stenosis in a patient for whom the anti-TNF was prescribed for SpA).
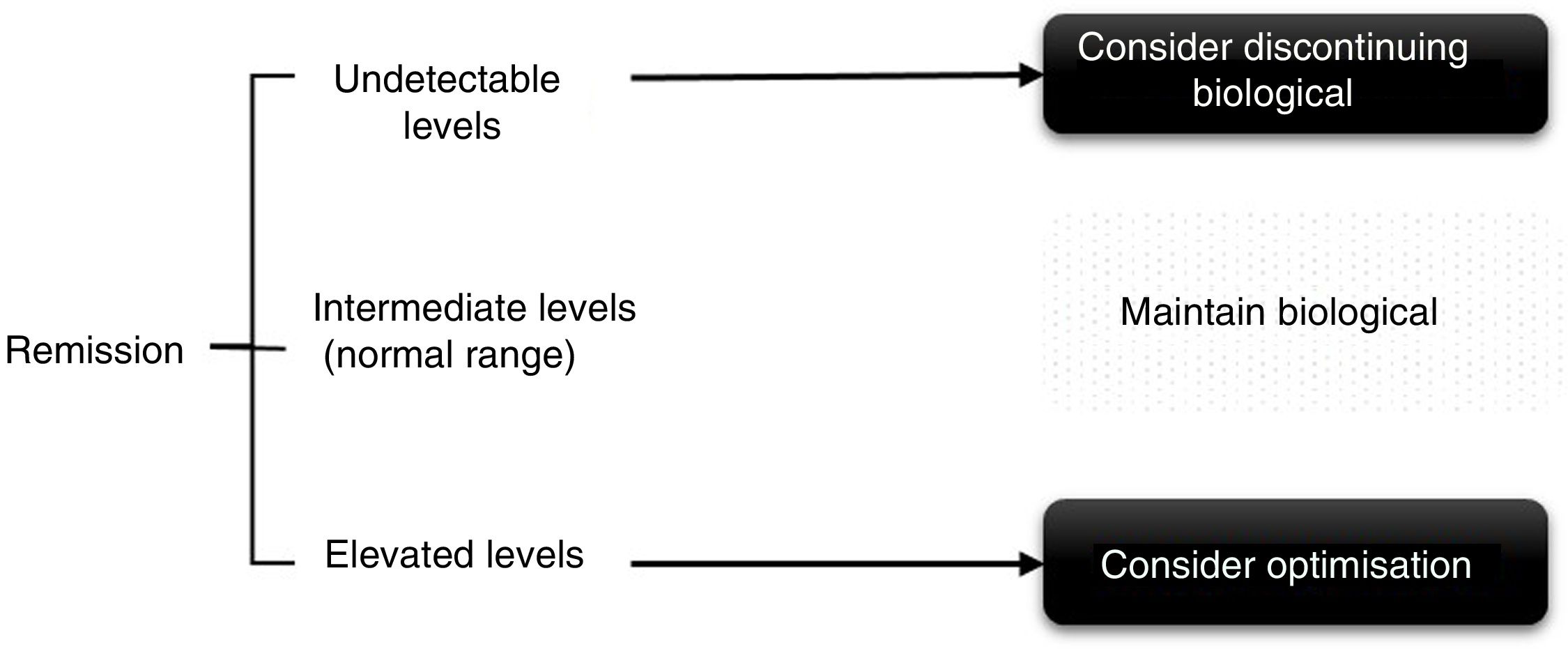
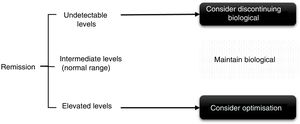
In the case of patients in persistent remission (defined as remission for at least 6 months to one year) or low disease activity, throughput levels can be used for decision-making, both when spacing and decreasing the dose (Fig. 2), although this was supported until recently in a study on adalimumab that demonstrated that persistent response was predicted.11 More recently in a clinical trial, it was observed that patients with adalimumab levels>8μg/ml can maintain response after reduction without a flare-up of activity.15
There are no data to support routine monitoring of all patients in follow-up, treated with a biological drug, unless there is a loss of clinical efficacy or an infusion reaction.
What is the usefulness of determining throughput levels shortly after starting biological treatment?Testing throughput levels in patients who have been on treatment for a short time (for example, with the second dose of intravenous drugs and at one month16 with subcutaneous drugs, or at 6 months after starting treatment, once it has been assessed that there has not been a primary failure), may have some predictive value in decision-making for patients with poor prognostic factors and who need more rapid intervention. However, there is still insufficient evidence in these types of patients to make general recommendations.
If high throughput levels or supratherapeutic range are reached in the first few months, patients are more likely to respond and achieve remission. By contrast, patients who do not reach a level in the therapeutic range in the first months of treatment are less likely to achieve remission. In these cases, adherence to treatment should be specially monitored.
What factors should be taken into account when interpreting throughput levels?The interpretation of throughput levels will depend on multiple factors. In addition to considering the activity of the underlying disease, other factors must be taken into account to assess the therapeutic range of throughput levels, such as assessment of adherence to the drug (low throughput levels make it necessary to investigate compliance with the prescribed regimen and adherence to it), the use of concomitant drugs, as well as adherence and adequate dose, whether dose adjustment or change of therapeutic target is necessary.
Body mass indexObesity may affect the pharmacokinetics of drugs such as the anti-TNFs, as adipokines in adipose tissue can increase the level of pro-inflammatory cytokines. The inverse relationship between throughput levels and body mass index (BMI) has been described in studies with etanercept,17 adalimumab18 and certolizumab.19 Obese people achieve lower throughput levels which can affect treatment efficacy without an increase in immunogenicity.18 However, in the paper by Sigaux et al.,20 no relationship was found between tocilizumab levels and BMI. It seems necessary, therefore, to individualise according to the drug, the patient's BMI and even the clinical situation at the time.
Synthetic anti-rheumatic disease-modifying drugsThe use of DMARDs minimises the appearance of ADAs,21,22 although the data on the relationship between the use of DMARDs and throughput levels depends more on the biological drug and on the baseline disease. For example, in SpA, infliximab is used at higher doses than in RA, and is not usually combined with MTX. By contrast, in patients with RA treated with infliximab and especially adalimumab, treatment with MTX decreases immunogenicity and increases survival of the drug, and there is even an inverse relationship between MTX dose and ADA levels in patients treated with adalimumab.23 Although there is more controversy in SpA, the published studies indicate greater survival of the drug and lower immunogenicity among patients concomitantly treated with DMARDs. In the case of tocilizumab, throughput levels are not influenced by the concomitant use of MTX.20
What is the recommended technique for testing?Most studies on the subject use the ELISA capture technique to study throughput levels and the “bridge” ELISA for autoantibodies. The “bridge” ELISA is an inexpensive technique, accessible in any hospital and with which it is easy to automate measurement; however, it is less sensitive, it does not detect IgG4 antibodies that bind to the drug. On the other hand, the RIA (radioimmunoassay) is more specific, presents less interference by induced artefacts, as it is able to detect IgG1, IgG2 and particularly IgG4 bound to the drug. However, this is a more complex and expensive technique and a nuclear medicine service is required as management of isotopes is involved. Concordance between both techniques is greater when ADA levels are high.24
The standardisation of current techniques for determining biological throughput levels is an issue that has not been fully resolved. In our country, kits generally use the ELISA method (Spanish Grifols kit). In this field, several interlaboratory (using the same sample) and comparison studies with other kits/techniques have been carried out with excellent correlation.25–28
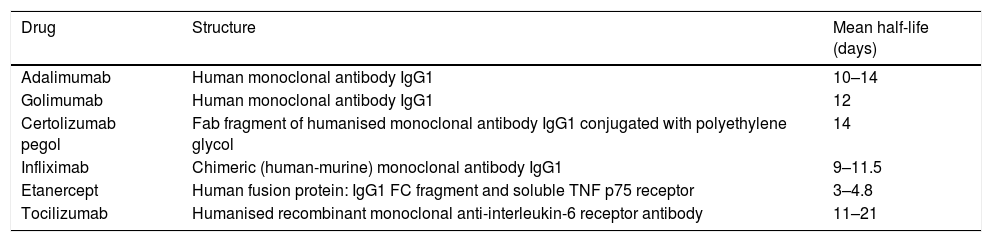
When is the best time to take the sample?Most papers measure trough levels, which could correspond to minimum concentrations, i.e., those collected on the day of administration of the treatment dose, prior to administration, since these levels usually correlate with the clinical response.4 Levels of autoantibodies are also measured at trough time, when plasma levels of the drug are minimal (thus avoiding possible interferences), thus taking into account the half-life of the drugs under study (Table 3).
Half-life of drugs for which throughput and antibody levels are measured.
| Drug | Structure | Mean half-life (days) |
|---|---|---|
| Adalimumab | Human monoclonal antibody IgG1 | 10–14 |
| Golimumab | Human monoclonal antibody IgG1 | 12 |
| Certolizumab pegol | Fab fragment of humanised monoclonal antibody IgG1 conjugated with polyethylene glycol | 14 |
| Infliximab | Chimeric (human-murine) monoclonal antibody IgG1 | 9–11.5 |
| Etanercept | Human fusion protein: IgG1 FC fragment and soluble TNF p75 receptor | 3–4.8 |
| Tocilizumab | Humanised recombinant monoclonal anti-interleukin-6 receptor antibody | 11–21 |
Fab: antigen-binding fragment; CF: crystallisable fragment; IgG1: immunoglobulin G1; TNF: tumour necrosis factor.
This is a relevant aspect, on which there is much confusion in the published literature, especially due to the population used in the studies, the type of therapeutic response studied, the measurement techniques and the different units of measurement used. In fact, it is not certain that the ranges in active disease are the same as in remission.
Studies on adalimumab, where the therapeutic level was determined, and those that performed ROC curve analysis,23 refer to levels of 5μg/ml (equivalent to 5mg/l)13 or even lower.25,29 Kneepkens et al.17 did not find an optimal dose in 162 patients with SpA treated with etanercept. A recently published study indicated in patients with axial SpA that serum levels of golimumab of .7–1.4mg/l were adequate to control disease activity, and that increases in circulating drug dose did not result in greater benefit.30 In the case of infliximab, the analysis of 66 patients with RA who started treatment with infliximab they propose a dose of 4.4μg/ml in week 6.16 Finally, Jani et al. described in 115 patients with RA treated with certolizumab a trend for patients with higher levels of certolizumab (>23–24μg/ml) to improved DAS28 compared to baseline.19 In a model of pharmacokinetics and population pharmacodynamics with tocilizumab therapeutic levels of 3.7μg/ml were established, although with great individual variability. However, only the presence of detectable levels (>1μg/ml) is sufficient to suppress acute-phase response (CRP).31
When should anti-drug antibodies be measured?Measurement of ADAs is only considered clinically relevant in the following cases:
- a)
When throughput levels are low or undetectable. Undetectable throughput levels may be related to the appearance of neutralising ADAs that block the action of the drug. If there is a detectable throughput level, ADAs will not be detected with the ELISA technique, because they will be bound to the drug. In patients with levels of adalimumab<2mg/l a study shows that acid dissociation can separate this binding making ADAs detectable in around 50% of cases,14 although in practice it is not generally used with detectable levels. In patients with ADAs, they can be detected even months after the drug has been discontinued.
- b)
Therapeutic failure. Evaluation of ADAs helps to select the most potentially effective therapeutic option (change of anti-TNF or other therapeutic target or to assess adherence) (Fig. 1). Only a third of secondary failures in patients treated with monoclonal anti-TNF agents are due to the formation of ADAs.4,32
- c)
Infusion reactions. In the event of an infusion reaction, the same anti-TNF should be avoided, but in those patients who have had mild reactions or have halted treatment, detection of ADAs may be useful to assess the possibility of reintroducing the same drug. Most infusion reactions are not due to ADAs, but to the speed of infusion.4,33
The arrival of the biological drugs, in addition to constituting a radical change in the management of immune-mediated diseases, has also led to a major increase in pharmaceutical expenditure. Bearing in mind the great impact that these treatments have had on the pharmacy budget, improving cost-effectiveness must be a priority objective.34
There are several publications, both in the field of rheumatology and gastroenterology, on chronic inflammatory diseases treated with biological therapy, which support the association between clinical response and biological drug serum levels.25,35,36 However, systematic measurement of levels does not seem appropriate, therapeutic decisions must be based fundamentally on clinical data and, in any case, testing throughput and ADA levels could be useful in some of the clinical situations mentioned: secondary failure, especially; patients in persistent remission, candidates for optimisation or suspension of therapy; assessment of adherence and in the event of infusion reactions, although the therapeutic decision will always be made in combination with the clinical data.
After reviewing the literature, this paper aimed to develop preliminary algorithms of possible guidelines for requesting and assessing possible scenarios where throughput level testing could be useful, and thus facilitate their being implemented as a tool in clinical practice. It is suggested that trough drug levels should be extracted, and these are usually classified as low, medium or high according to a therapeutic range defined in each study for the biological agent used and according to the patient's clinical situation (remission or activity after therapeutic failure). The main disadvantage we found is that there are no universally validated therapeutic ranges of throughput levels for each of the biological agents and for each clinical situation, and there is still no standardisation as to which throughput measuring techniques should be used. ELISA is the most recommended technique in Spain, and also acid dissociation in patients with low or subtherapeutic throughput levels and absence of ADAs.14,37
We suggest, therefore, considering optimising the biological drug in patients with controlled disease activity and high throughput levels,15,38–41 keeping the treatment the same if levels are intermediate and assess discontinuing therapy if throughput levels are low or undetectable. In patients with therapeutic failure, if the throughput levels are high, it will be necessary to switch to another drug with a different mechanism of action and/or assess other causes responsible for the symptoms other than disease activity. And conversely, if the levels are low the presence of ADAs and/or adherence to the biological and concomitant DMARDs, if any, must be checked. In cases where the patient could present therapeutic failure with undetectable throughput and ADA levels, it is advisable to check adherence, since adjustment of the dose of the biological drug according to the patient's weight (if the drug allows it) has not been endorsed in the rheumatology literature. Thus, in the PREMIER study42 better results were not achieved by reducing the adalimumab interval in patients with RA. In the Loadet study,43 for example, no clinical benefit was found with more doses of etanercept in patients with axial SpA, and therefore this is not recommended in routine clinical practice, also due to the increased costs and side effects that can be entailed.44
Some factors to take into consideration have also been described that can influence throughput levels, the inverse relationship with BMI (except in a study with tocilizumab20 and the association with the use of concomitant DMARDs (MTX being the most used) with higher throughput levels and less immunogenicity, although there is more controversy with SpA,45,46 and in the case of tocilizumab there are no differences between combining or not combining concomitant DMARDs.20 Most of the published algorithms indicate dose optimisation in patients with elevated serum levels of the drug, since reducing the dose is very likely to reach a level between normal and intermediate. In intermediate levels or normal range the patient should be maintained in order to obtain the maximum benefit, and in this situation dose reduction does not seem reasonable, at least at the same intensity that is usually used for patients in remission, since if we consider the pharmacokinetics of these drugs it would be very likely that the patient would go into subtherapeutic levels and clinical efficacy could be lost. These regimens might also be of interest for patients who have been started on an optimisation regimen to explore whether the dose adjustment can be continued; this is always dependent on the patient's clinical situation.
In recent years, biosimilar anti-TNF drugs have appeared. To be approved these drugs must undergo clinical trials where the immunogenicity data must be similar to the original products. Thus, the immunogenicity of each of the approved biosimilars, of both infliximab47,48 and adalimumab,49,50 was similar to that of the “original” drug. With etanercept, as it is less immunogenic than the monoclonal anti-TNF antibodies, a lower prevalence has also been obtained and much lower prevalence of ADAs detected, as occurred with non-biosimilar etanercept.51–53 Thus the comments made are useful for biologicals, both the original drugs and the biosimilars.
Therefore, monitoring throughput and ADA levels remains a subject of debate; for it to be generally undertaken in clinical practice more studies and more evidence are necessary to universally define the therapeutic ranges of throughput levels. Therapeutic decisions could be based on throughput levels tailored to each patient in those clinical situations, which would enable greater cost savings and a reduction in the side effects associated with biological therapy.
FinancingThis study was financed by Grifols through the Research Association in Rheumatology of Marina Baixa (AIRE-MB). The content is the sole responsibility of the authors and does not necessarily represent the official views of Grifols.
Conflict of interestsMML, TO, JC and LC have no conflict of interest to declare.
JR has participated in consultancies, conferences and training/research projects financed by Abbvie, BMS, Grifols, Lilly, MSD, Novartis, Pfizer, Roche and UCB.
AB has participated in consultancies, conferences and training/research projects financed by Pfizer, Abbvie, UCB, Roche, Novartis, BMS, Sandoz, Celltrion and Nordic.
RS has participated in consultancies, conferences and training/research projects financed by BMS, MSD, Pfizer, Abbvie, UCB and Roche.
JT has received funding for training and/or research projects from Gebro, Jansen, Pfizer, Roche and Sanofi.
Please cite this article as: Rosas J, Martín-López M, Otón T, Balsa A, Calvo-Alén J, Sanmartí R, et al. Aspectos prácticos de la medición de los niveles de fármacos biológicos y de anticuerpos antifármaco en artritis reumatoide y espondiloartritis. Reumatol Clin. 2020;16:378–385.