This article outlines key recommendations for the appropriate prescription of non steroidal anti-inflammatory drugs to patients with different musculoskeletal problems. These recommendations are based on current scientific evidence, and takes into consideration gastrointestinal and cardiovascular safety issues. The recommendations have been agreed on by experts from three scientific societies (Spanish Society of Rheumatology [SER], Spanish Association of Gastroenterology [AEG] and Spanish Society of Cardiology [SEC]), following a two-round Delphi methodology. Areas that have been taken into account encompass: efficiency, cardiovascular risk, gastrointestinal risk, liver risk, renal risk, inflammatory bowel disease, anemia, post-operative pain, and prevention strategies. We propose a patient management algorithm that summarizes the main aspects of the recommendations.
Este artículo señala las recomendaciones claves para una adecuada prescripción de antiinflamatorios no esteroideos a pacientes que presentan indicación de tratamiento con esta medicación, en base a la evidencia científica actual y teniendo en consideración aspectos de seguridad gastrointestinal y cardiovascular. Las recomendaciones se han consensuado por expertos designados por 3 sociedades científicas (Sociedad Española de Reumatología, Asociación Española de Gastroenterología y Sociedad Española de Cardiología), siguiendo una metodología Delphi a 2 rondas. Las áreas que se han tenido en cuenta engloban: eficacia, riesgo cardiovascular, riesgo gastrointestinal, riesgo hepático, riesgo renal, enfermedad inflamatoria intestinal, anemia, dolor postoperatorio y estrategias de prevención. Se propone un algoritmo de manejo de pacientes que recoge los aspectos fundamentales de las recomendaciones.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are drugs with a heterogeneous chemical structure that share antipyretic, anti-inflammatory and analgesic activity through their ability to inhibit cyclooxygenase (COX), involved in the synthesis of prostaglandins, thromboxanes and leukotrienes. They are the cornerstone in the treatment of pain and inflammation in patients with musculoskeletal diseases.
Prostaglandins are produced from an arachidonic acid oxidative pathway and COX enzymes. There are three forms of COX (COX-1, COX-2, and COX-3), with the first two being the most significant. COX-3 is an isoform of COX-1, which only differs in the structure of an amino acid, and its function, but is believed to be antipyretic, although this remains uncertain. In a simple way, it can be said that COX-1 acts at the onset of platelet aggregation and at the defense mechanisms level of the gastrointestinal (GI) mucosa, while COX-2 appears to be involved in pain mediation, fever and inflammation. Inhibition of COX enzymes is related to beneficial and unwanted effects on major organ systems where COX activity plays a key role.
The introduction of drugs that function as selective inhibitors of COX-2 induced high expectations when it was proven that they presented an efficacy equal to that of nonselective NSAIDs but with a safer GI toxicity profile. Subsequently, the observation of an increased frequency of cardiovascular (CV) events cooled these expectations but opened a very important way to understand not only the benefits but also all the side effects associated with the use of NSAIDs. In October 2006, the Committee for Medicinal Products for Human Use of the EMEA issued a report1 regarding the existence of new data on the risks of atherothrombotic CV type events related to traditional NSAIDs. So it is currently considered that all NSAIDs, in varying degrees, are associated with increased GI and CV risk. The increased CV risk due to the use of selective COX-2 is explained by an imbalance between inhibition of thromboxane and prostacyclin, but given that the increased CV risk is also seen with nonselective classic NSAIDs, the mechanism appears to be more complex. Moreover, both traditional and COX-2 NSAIDs are associated with an increase in blood pressure and edema, to varying degrees.
In 2003, the Spanish Gastroenterological Association (ALA) and the Spanish Society of Rheumatology (SER) contributed to the publication of a “clinical strategy for the prevention of adverse effects on the digestive tract by NSAIDs”,2,3 and then, a consensus document of the SER and the Mexican College of Rheumatology for the “Appropriate use of NSAIDs in rheumatology”4 was published in the year 2009. The current review is a collaboration of ALA, the SER and the Spanish Society of Cardiology (SEC). This review aims to promote a rational use of NSAIDs according to new studies published in recent years and help in an optimal decision-making process for the routine use of these drugs.
Operational Definitions of the DocumentThe term NSAID in these recommendations refers to traditional NSAIDs as a group, to selective COX-2 (coxibs) inhibitors and acetylsalicylic acid (ASA) at anti-inflammatory doses. If a statement refers only to traditional NSAIDs, coxibs or ASA, this will be well specified in the recommendation. We have not included other NSAID analgesics, such as lysine clonixinate or metamizole.
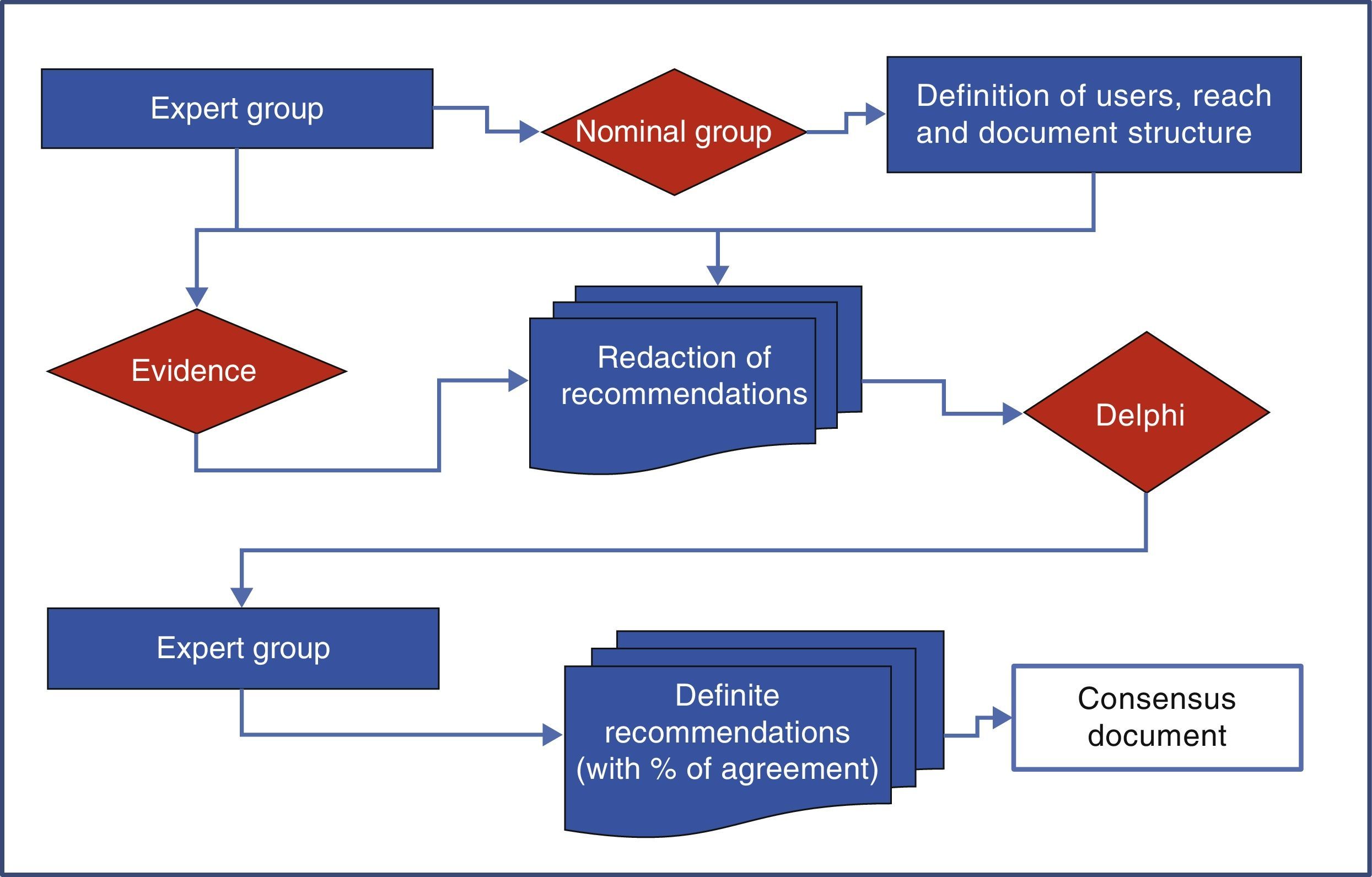
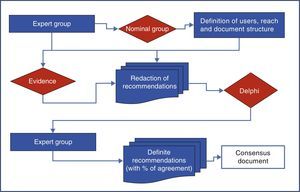
MethodologyThe development of consensus was performed using a nominal group and the Delphi technique as shown in Fig. 1.
Nominal GroupNine panelists (three rheumatologists, 3 gastroenterologists and 3 cardiologists) from their respective scientific societies (SER, ALA and SEC) and appointed by the boards of the same based on their experience and/or publications on the topic in journals included in Medline, formed the nominal group. This group was coordinated by a methodologist of the Research Unit of the SER that established the scope, users, and paragraphs of consensus, and the definitions used in the document.
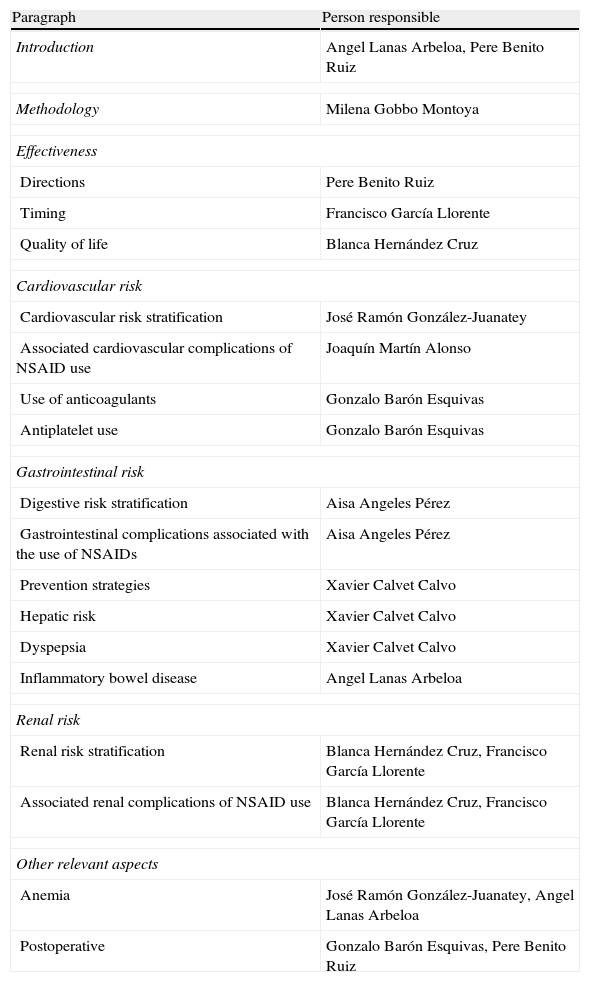
To carry out the group, the panelists were provided with documents published previously on the subject, and asked everyone to, with no interaction among them, develop their personal proposal following a scheme provided by the methodologist of the Research Unit of the SER, who subsequently developed a joint document that included all proposals. On 15 June 2012 the actual meeting where the background of the problem and the relevant methodological aspects were revised consensus took place. Then, the group worked on the entire document and tested each of the aspects to agree on (user, scope and topics to be included). Each expert participant had the opportunity to vote on the appropriateness or otherwise of the inclusion of each of the proposed recommendations, arguing the reasons for it. Thus, the options, the final structure of the document and the heads of the sections hereof, were successively rearranged. Finally it was decided that the consensus would address primary care physicians, rheumatologists, cardiologists and gastroenterologists (as users), and would focus on issues of efficacy, CV security and kidney safety (range). Those responsible for each of the paragraphs were distributed as shown in Table 1.
Sections of the Consensus and Person Responsible.
| Paragraph | Person responsible |
| Introduction | Angel Lanas Arbeloa, Pere Benito Ruiz |
| Methodology | Milena Gobbo Montoya |
| Effectiveness | |
| Directions | Pere Benito Ruiz |
| Timing | Francisco García Llorente |
| Quality of life | Blanca Hernández Cruz |
| Cardiovascular risk | |
| Cardiovascular risk stratification | José Ramón González-Juanatey |
| Associated cardiovascular complications of NSAID use | Joaquín Martín Alonso |
| Use of anticoagulants | Gonzalo Barón Esquivas |
| Antiplatelet use | Gonzalo Barón Esquivas |
| Gastrointestinal risk | |
| Digestive risk stratification | Aisa Angeles Pérez |
| Gastrointestinal complications associated with the use of NSAIDs | Aisa Angeles Pérez |
| Prevention strategies | Xavier Calvet Calvo |
| Hepatic risk | Xavier Calvet Calvo |
| Dyspepsia | Xavier Calvet Calvo |
| Inflammatory bowel disease | Angel Lanas Arbeloa |
| Renal risk | |
| Renal risk stratification | Blanca Hernández Cruz, Francisco García Llorente |
| Associated renal complications of NSAID use | Blanca Hernández Cruz, Francisco García Llorente |
| Other relevant aspects | |
| Anemia | José Ramón González-Juanatey, Angel Lanas Arbeloa |
| Postoperative | Gonzalo Barón Esquivas, Pere Benito Ruiz |
The recommendations were drafted by experts, either individually or in working groups. A common approach was established for its development, so that each responsible manager should include a short, synthetic statement of recommendation, followed by its argumentative development, supporting the recommendation with the best available evidence-based literature searches made for such a purpose by each panelist. The level of evidence and strength of recommendation were established according to the SIGN5 scale.
Delphi SurveyTo establish the degree of consensus, a 2 round Delphi survey was conducted. The recommendations submitted by the panelists were reformulated in the form of items to be answered using a Likert scale that measured the degree of agreement with the statement, scoring it as 1 (completely disagree) to 5 (completely agree). Questionnaires were sent electronically via email to the panelists insuring that there was no interaction between them.
The first round was sent on January 28, 2013 (66 items grouped in 17 sections). In the first round, panelists were able to add new claims, comments, quotes or arguments for each item when, from their point of view, they were important aspects that should be included in the consensus document.
It was felt that there was agreement with the item when the average score was equal to or greater than 4, and disagreement when the score was less than or equal to 2. In both cases (agreement and disagreement) there is consensus. In the rest of the possibilities, it was considered that the item was problematic and no consensus could be established. Based on the comments of the panelists the wording of some items was modified, others were broken up and a list of possible new recommendations to be included in the questionnaire for the second round was added.
The second round was sent on February 11, 2013 (73 items grouped in 17 sections). Analysis of the second round was performed using the same methodology and the same criteria were used as in the first (agreement or disagreement and conflicting items). It was felt that there was a consensus when more than 75% of the panelists agreed with the statement (agreement score ≥4). The remaining cases (conflicting items) are considered recommendations without consensus, and are not included herein.
RecommendationsEfficacyDirectionsThe approved indications for NSAID on the insert are varied, according to the type of pain ranging from those originated in the musculoskeletal or neurological system to dysmenorrhea. This makes the target population very broad and heterogeneous. NSAID responses vary among individuals, which obliges the individualization of the indication and evaluation of such a response to these drugs.4
One cannot recommend any NSAID over another based on their clinical response. Several publications provide sufficient evidence to assert that efficacy is similar.6,7
Time of UseTo control pain, NSAIDs share indications with other pharmacological and non-pharmacological interventions. NSAIDs should be used for the shortest duration and the lowest possible dose, adapting their use to different approved indications. This recommendation is collected by regulatory agencies,1,8 based on data showing dose-dependent adverse effects and that the risk is maintained over time. Therefore, given the potential for adverse effects, its use is recommended when the alternatives are not possible, both for intolerance as for ineffectiveness.9–11
In osteoarthritis, NSAIDs continuous treatment is most effective in controlling the symptoms than on-demand use. Nevertheless, the latter is often recommended because of possible undesirable side effects, as stated in a study lasting 22 weeks for the prevention of outbreaks of osteoarthritis of the knee and hip.12 In another prospective multicenter randomized trial of 24 weeks in patients with hip or knee osteoarthritis, the frequency of adverse effects was similar in those taking celecoxib continuously compared to those taking it intermittently depending on the symptoms of13 the patients. In psoriatic arthritis, due to the intestinal and CV specific risks of these patients, NSAIDs are recommended at the lowest dose and for the shortest time possible due to their potential toxicity.14
In ankylosing spondylitis NSAIDs are recommended as first-line therapy if patients present with inflammatory back pain and stiffness, being continued as treatment of choice if the disease is active and persistently symptomatic.15–18 NSAIDs improve function and decrease disease activity in ankylosing spondylitis, while disease-modifying drugs have no effect on these parameters.19 There has also been a reduction in the degree of radiographic progression in patients who take NSAIDs continuously associated with anti-TNF drugs.20 Similarly, continued treatment with celecoxib is associated with a lower radiographic progression compared with on demand use.21 The German Early Spondyloarthritis Inception Cohort notes that patients with ankylosing spondylitis associated with a high rate of NSAID use for more than 2 years have a slowing of new spinal bone formation compared to the those that have a low rate. This apparent protective effect is exclusive of those patients with high C-reactive protein in time and the presence of syndesmophytes at onset.22 Patients with elevated acute phase reactants benefit more from continuous treatment with NSAIDs.23–25
Quality of LifeThe efficacy of NSAIDs in reducing pain and symptoms associated with inflammation in patients with acute or chronic rheumatic disease is unquestionable.26,27 These benefits produced significant improvement in the physical function subdomain and in the quality of life of patients.28–30 This improvement occurred more frequently in patients with a worse baseline physical function, fewer comorbidities and shorter duration of symptoms.
Retention rates (days of treatment without abandonment) of NSAIDs, especially COXIB remain high, particularly in chronically ill and patients over 65 years. The average duration of treatment in rheumatic patients for celecoxib, rofecoxib, naproxen and ibuprofen was 15, 13, 10 and 10months, respectively.7 Differences in safety, especially GI, could have significant implications for the quality of life of patients.31,32
Cardiovascular RiskStratification of Vascular RiskGiven the CV risk associated with NSAID therapy, it is important to evaluate this parameter when prescribing it and choosing it as an appropriate treatment. The Third Joint Task Force on Cardiovascular Disease Prevention in Clinical Practice recommended a Systematic Coronary Risk Evaluation (SCORE) model for CV risk estimation in Europe,33 which is based on studies on European population. Given the geographic variability of CV risk in Europe, 2 SCORE models have been developed, for countries with high or low risk, setting Spain in the low incidence zone.34,35
The CV risk is stratified into very high, high, moderate or low CV risk36,37 (Table 2). This new classification of CV risk levels is directly related to the achievement of therapeutic goals over increasingly stringent classic CV risk factors. Thus, specifically defined lipid goals are 70mg/dL in plasma LDL-cholesterol levels for patients at very high CV risk 100mg/dL for high-risk patients and 115hp mg/dL in patients with moderate CV risk.
Assessment of Cardiovascular Risk.
| Very high |
| (a) Cardiovascular disease documented by invasive or non-invasive techniques (coronary angiography, isotope techniques, stress echocardiography and evidence of atherosclerotic plaques by carotid ultrasound), previous myocardial infarction, previous acute coronary syndrome, coronary revascularization, other revascularization procedures, stroke and peripheral arterial disease |
| (b) Diabetes mellitus type 2 or type 1 diabetes mellitus associated with target organ damage (microalbuminuria: 30–300mg/24h) |
| (c) chronic moderate to severe renal disease (estimated glomerular filtration rate <60ml/min/l.73m2) |
| (d) SCORE≥10% |
| High |
| (a) Any markedly elevated individual risk factor, familial hypercholesterolemia or severe hypertension |
| (b) ≥SCORE 5% and <10% |
| Moderate |
| SCORE≥1% and <5% |
| Low |
| SCORE<1% absence of other risk factors |
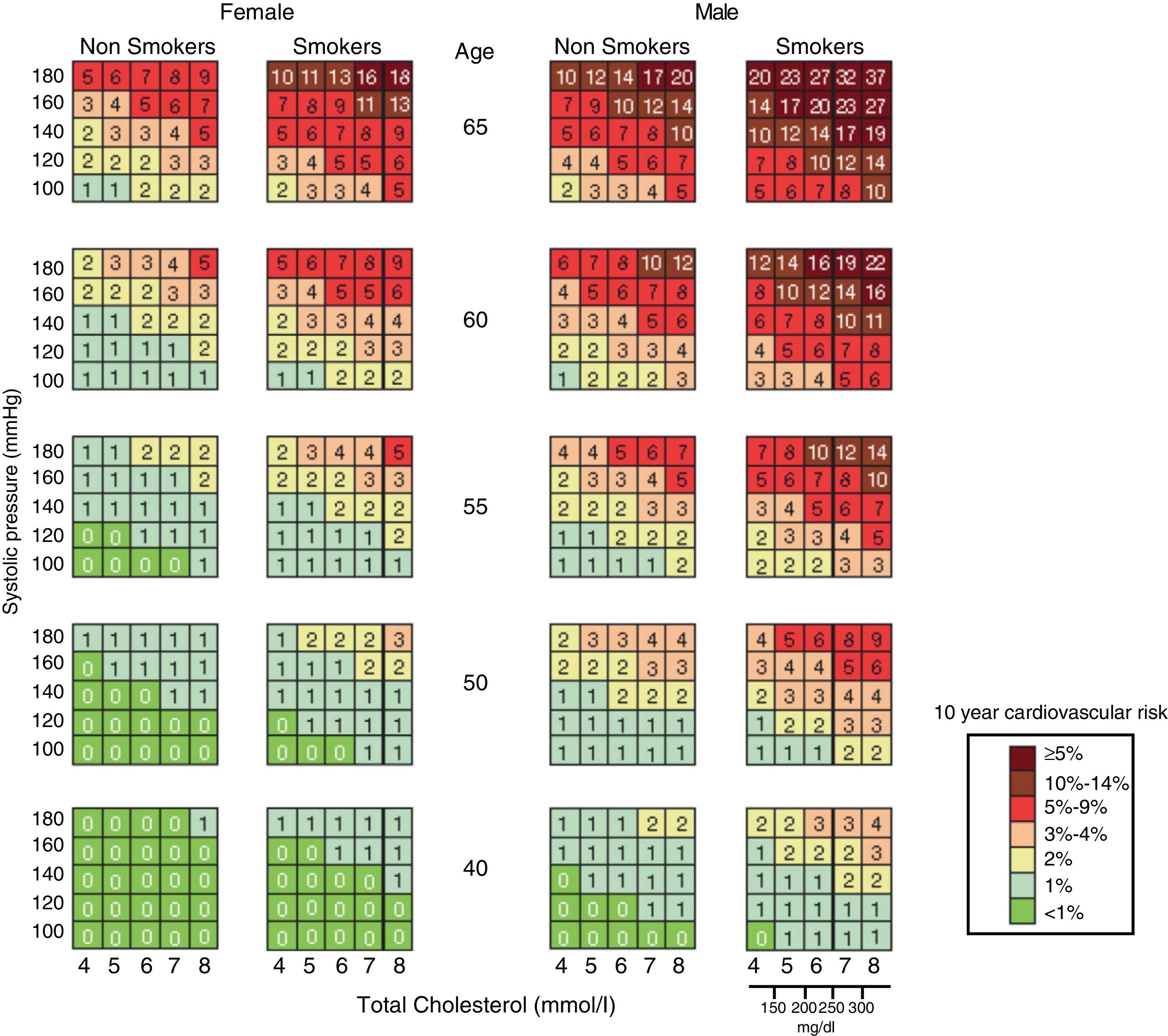
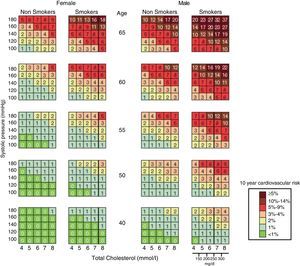
The first “EULAR Standing Committee for International Clinical Studies Including Therapeutics” consensus for CV risk management in patients with rheumatoid arthritis and other inflammatory arthritis (spondyloarthropathy) was published in 2010. These diseases, in particular rheumatoid arthritis, have been linked directly to an increased cardiovascular risk and long-term cardiovascular mortality. This consensus recommends evaluating CV risk through the modified SCORE chart (Fig. 2), and proposes a Decalogue of recommendations based on scientific evidence.38 In the general aspects of estimation and CV risk management, these recommendations could also be extended to patients with osteoarthritis.
Cardiovascular Complications Associated With the Use of Nonsteroidal Anti-inflammatory DrugsIn the absence of treatment, administration of NSAIDs increases the risk of ACS or other CV atherothrombotic problems. The clinical trials that determined the association between NSAIDs and CV events were not specifically designed for this purpose.39,40 Observational studies and meta-analyses of observational and clinical trials indicate that all NSAIDs increase the CV risk although to varying and, in general, low degrees. A recently conducted review by the Spanish Agency for Medicines and Health Products (AEMPS) and the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA)41 has concluded that recent evidence confirms previous findings published in 2006, which showed an increased CV risk of atherothrombotic type for NSAIDs, although the risk/benefit balance remains positive.
The increase in CV risk varies greatly depending on the type of NSAID used.
LE: 1++, DR: A, LA: 100%
Naproxen is one of the safest NSAID in terms of CV risk.
LE: 1++, DR: A, LA: 100%
Rofecoxib, diclofenac, indomethacin, etodolac etoricoxib and NSAIDs are the drugs most associated to CV risk.
LE: 1+, DR B, LA: 100%
Different meta-analyses have indicated to a greater or lesser extent that all NSAIDs studied increased CV risk, although the absolute magnitude is limited.42,43 A meta-analysis of observational studies published by McGettigan and Henry44 in populations with high CV risk studied the most currently used NSAIDS (naproxen, ibuprofen, celecoxib, diclofenac, indomethacin, piroxicam, meloxicam, etodolac, etoricoxib and valdecoxib). The overall conclusions of the review suggested that naproxen and low doses of ibuprofen are associated with smaller increases in CV risk. Diclofenac clearly increased CV risk. The data for etoricoxib are sparse, but comparisons with naproxen and ibuprofen showed a higher CV risk. Indomethacin was also associated with a significant increase in CV risk. In the MEDAL study, etoricoxib was associated to a CV risk equal to that of diclofenac.45
The most recent large meta-analysis of clinical trials with individual patient data indicates that NSAIDs and coxibs have increased CV risk compared to placebo, with no significant differences between them globally. Among the nonselective NSAIDs, CV risk was higher with diclofenac, which presented a similar risk to that of coxibs. Naproxen meanwhile, at doses of 500mg/12h, was not associated with increased CV risk, unlike ibuprofen and diclofenac, the only nonselective NSAIDs most studied.46 There is also a clear association between the risk of developing heart failure and the administration of these drugs46 in addition to the well-known relationship of NSAIDs and the development of episodes or exacerbations of heart failure.
Patients with ischemic heart disease have a higher risk of CV events when given NSAIDs. A Spanish47 case–control study, which included 6000 participants concluded that NSAID use is associated with an overall increase of 16% in coronary risk (OR: 1.16, 95% CI: 0.95–1.42). The use of high doses increased it by 64% (OR: 1.64, 95% CI: 1.06–2.53) compared to those taking low doses. In patients with ischemic heart disease, the increased risk was 84% (OR: 1.84, 95% CI: 1.13–3.00). The meta-analysis of44 McGettigan and Henry also supports the fact that high doses are associated with increased risk.
Anticoagulant UseEvidence through observational studies showing an increased risk of upper gastrointestinal bleeding in patients using NSAIDs and anticoagulants is extensive. In general, NSAIDs should be avoided in patients using these drugs.48–51 The safety of novel anticoagulants such as dabigatran, rivaroxaban and apixaban associated with NSAIDs does not seem different from the classical anticoagulants.52
Interactions with anticoagulants are different for different NSAIDs. Aspirin, phenylbutazone and mefenamic acid very significantly enhance the anticoagulant effect of warfarin, displacing albumin and decreasing its metabolism in the liver.53 Tenoxicam, meloxicam, lornoxicam, nimesulide, etodolac, nabumetone and celecoxib did not significantly increase the international normalized ratio (INR) in anticoagulated individuals. Conversely, diclofenac, ibuprofen and naproxen increased bleeding time.54–56
A study that evaluated 98,821 elderly patients on continuous treatment with warfarin indicated that the risk of upper gastrointestinal bleeding associated with NSAIDs or coxibs was similar.57 However, more recent studies have indicated that celecoxib combined with warfarin was associated with lower risk of GI bleeding hospitalizations than the association of warfarin with ibuprofen, indomethacin, etodolac, nabumetone and naproxen.58,59 All this means that, even with reservations, COXIBs should be considered as a first choice in situations where an NSAID is needed and the problem cannot be handled using other therapies.
Use of Antiplatelet AgentsAvoid the use of NSAIDs, even in the short term, in patients with acute myocardial infarction taking ASA as it is associated with an increased CV risk.
LE: 2+, DR: B, LA: 78%
In patients taking low-dose aspirin, the association with ibuprofen and naproxen interferes with the antiplatelet effect of aspirin, so one should avoid using them together.
LE: 1+, DR: C, LA: 100%
Ibuprofen, naproxen and indomethacin, but not paracetamol, diclofenac or coxibs, interfere with aspirins ability to irreversibly acetylate the platelet COX-1 enzyme, so it is likely that these drugs reduce the protective effect of aspirin on the risk of atherosclerotic events.60–62
The evidence is not yet sufficient to make firm recommendations.63 However, ibuprofen should not be taken until at least 1h after ingestion of aspirin. For patients taking enteric coated-ASA (mostly in Spain) the problem is greater because the peak maximum release is late (beyond 4h), which makes interaction with ibuprofen is very likely. Thus, a study showed that the antiplatelet effect of low dose enteric coated aspirin is attenuated when ibuprofen is administered 2, 7 and 12h after ASA.64,65 In the case of naproxen, interaction was lower when administered at least 2h after ASA.66 There is controversy about the extent to which this interaction may have clinical impact in terms of CV mortality in patients taking aspirin and ibuprofen, as the 2 studies are contradictory.67,68
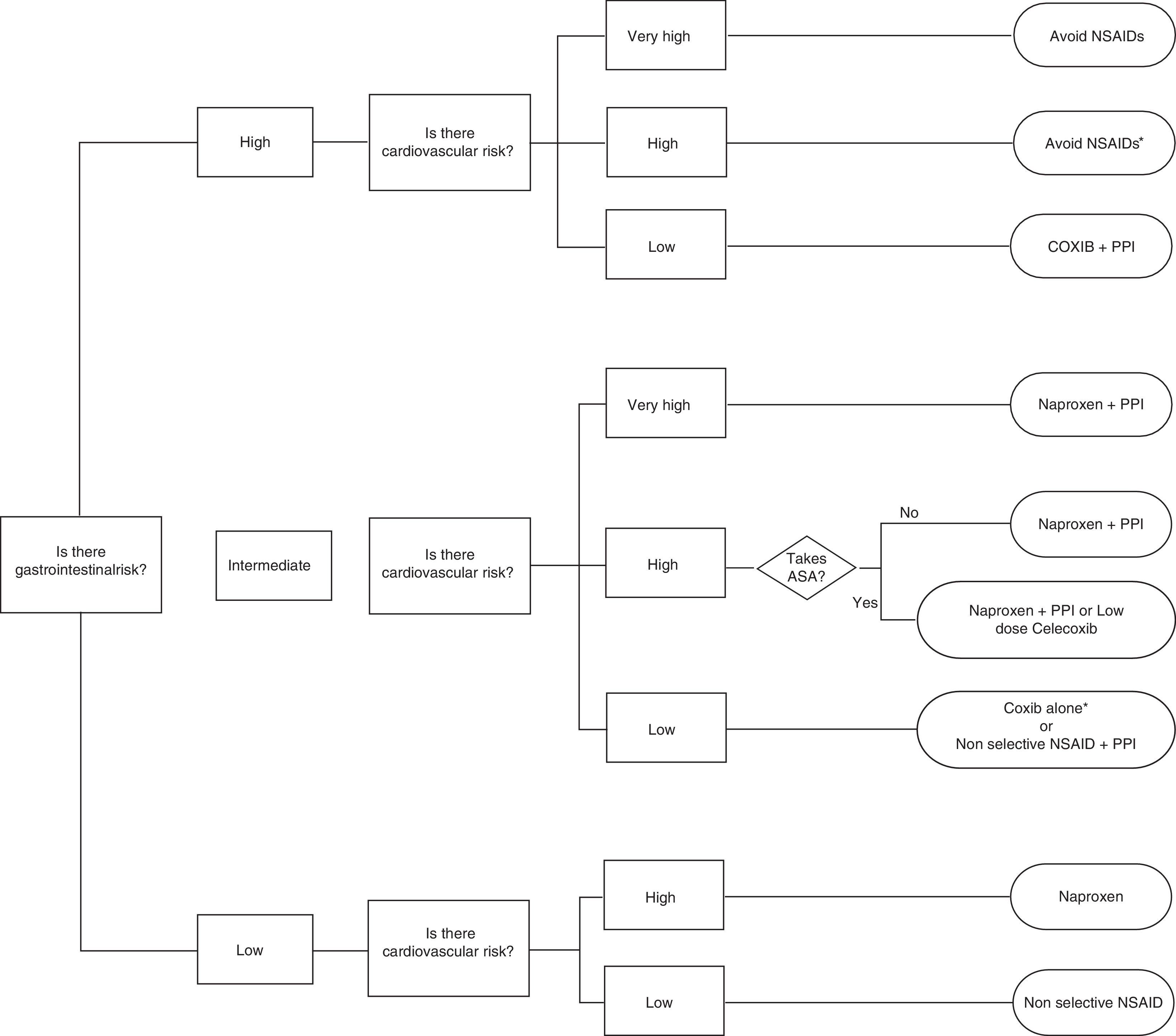
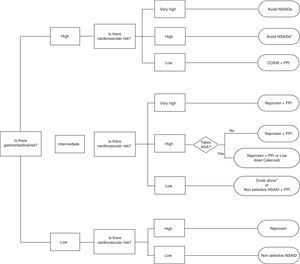
Celecoxib, rofecoxib, and lumiracoxib do not interfere with ASA in its ability to irreversibly acetylate platelet COX-1.60,69 Of these, only celecoxib is available in the market, which reduces the options, as there are no data for etoricoxib. The trouble with using ASA and COXIB is determined by the CV risk associated with them and the fact that the EMA, but not the FDA, established a contraindication to the use of coxibs in patients with previous history of CV events. This limits considerably the treatment options in this population, given also that naproxen, although neutral from the point of view CV risk at doses of 500mg/12h, is associated with increased GI risk and may interfere with ASA. So, in patients with ASA who have not presented a CV event, celecoxib at low doses and for the shortest possible time is a reasonable therapeutic option based on epidemiological studies indicating that the drug has no greater risk than other traditional NSAIDs.44 Prescribing recommendations according to CV and GI risk are developed in the algorithm of the attached as Fig. 3.
Algorithm recommendations for the use of NSAIDs according to the cardiovascular and gastrointestinal risk (upper and lower digestive tract).
* If necessary naproxen+PPI or celecoxib at low doses (200mg/24h)±PPI as soon as possible in both cases.
** More robust information for celecoxib than for etoricoxib
# Naproxen should be taken 2h before ASA. Ifenteric coated aspirin are used, interaction possibilities are greater.
## If there is a prior cardiovascular event, EMA and AEM currently contraindicate this option.
It is assumed that patients with very high cardiovascular risk should be treated with ASA. Therefore, in the group of low gastrointestinal risk, very high cardiovascular risk is not contemplated, because use of ASA, and gastrointestinal risk is intermediate at best.
The keys for this custom evaluation are those outlined in this section. The risk profile is established for each patient according to Table 3 and will be the one to guide prevention strategy.
Evaluation of Gastrointestinal Risk.
| High |
| History of complicated peptic ulcers |
| Use of anticoagulants or |
| Combination>2 other risk factors |
| Medium |
| Nonanticoagulated patients or those with a history of complicated ulcer who have some other isolated risk factor |
| Low |
| Patients without risk factors. Does not take acetylsalicylic acid |
Accepted risk factors: Age>60, history of complicated and uncomplicated peptic ulcer. Concomitant use of NSAIDs or aspirin plus COXIB with clopidogrel, anticoagulants, corticosteroids or inhibitors of serotonin reuptake, high dose NSAIDs, or 2 NSAID, serious comorbidity.
As age increases, the risk of developing GI complications in patients taking NSAIDs also increases progressively.70,71 Patients over 60 years of age alone is a risk factor for the occurrence of GI complications, and this risk is increased in patients receiving NSAIDs.72,73 It is estimated that approximately 6 upper GI complications occur per 1000 persons/year in patients taking NSAIDs and with an age between 60 and 69years. This estimate increases to 16 per 1000persons/year in patients aged between 70 and 79 years.
The existence of a previous history of uncomplicated gastric or duodenal ulcer or a history of GI bleeding or perforation is an independent risk factor for the development of peptic ulcer complications associated with taking NSAIDs. This finding has been confirmed in most observational studies and clinical trials.51,71,74–79
The combination of 2 or more NSAIDs increases the risk of bleeding over the individual risk for each individual NSAIDs.80 Similarly, and possibly linked to this same phenomenon, it has been noted that the change of an NSAID to another during an episode also increases the risk of upper GI bleeding.81
This increased risk with the combination of 2 NSAIDs is also observed with the combination of a classic NSAIDs or coxibs with aspirin at low doses used for preventing CV events, currently the most commonly used combination. In the CLASS study82 patients who received a classic NSAID plus low-dose ASA had an annualized complication rate of 2.1%, higher than that seen in patients receiving NSAIDs alone (1.2%). A number of observational studies have shown that the combination of NSAIDs or coxibs with aspirin increases the risk of upper gastrointestinal bleeding over the risk estimates for each of the drugs individually.81,83,84 Post hoc analysis comparisons of nonrandomized and observational studies have suggested that the risk associated with traditional NSAID combination+ASA is possibly greater than that associated with COXIB+ASA.85,86
Finally, the concomitant use of steroids has been included in some previous reviews among arisk factors for GI complications. However, the latest evidence shows that the incidence of complications is very low, so it cannot be established that this combination of drugs constitutes an essential GI risk factor of.87,88
High GI risk complication in patients treated with NSAIDs is dose dependent. While it has been suggested that the risk of developing high GI complications is higher during the first month of treatment and then stays stable during the remaining period of treatment, long-term clinical trials indicate that the risk of developing complications is stable throughout time.89
A study in patients with prior acute myocardial infarction noted that short-term treatment with most NSAIDs is associated with an increased CV risk. Diclofenac was associated with an increased CV risk from the onset of treatment to its end. Ibuprofen also showed an increased risk when used for more than a week. Celecoxib was associated with increased risk of death after a treatment duration of 14–30days. Naproxen was the only NSAID evaluated that was not associated with an increased risk of heart attack or death.90
There is no strong evidence that the use of acetaminophen is associated with increased cardiovascular risk.91
Gastrointestinal Complications Associated With the Use of NSAIDsNSAID treatment is associated with serious adverse events which affect the upper and lower gastrointestinal tract. They can occur as uncomplicated symptomatic ulcers or upper gastrointestinal bleeding, lower GI bleeding and GI perforation. These side effects occur in the 1%–4% of patients treated with NSAIDs.82 The relative risk for developing serious upper GI complications in patients treated with NSAIDs has been estimated between 3 and 5 times compared to those untreated.92
The incidence of serious complications of the lower digestive tract is not well defined, but it could account for 20% of the morbidity associated with NSAIDs.93 Studies show that 70% of patients taking NSAIDs chronically develop lesions of the lower GI tract, although many of them have little clinical relevance. Among the lesions described, increased intestinal permeability, inflammation, erosions, ulcers, strictures, anemia, protein losing enteropathy, diverticulitis, bleeding and perforation are included. Recent data on the rate of upper and lower GI perforation in patients with rheumatoid arthritis show that 83% occur in the lower tract.94 Additionally, some patients with certain rheumatic of inflammatory diseases also have associated inflammatory bowel disease, often subclinical, that possibly, but not exclusively be due to chronic NSAID use.
Prevention StrategiesH2 antagonists have been shown to be inferior to proton pump inhibitors (PPI), in both healing and prevention of ulcers associated with NSAID or ASA use.95–99
PPIs have been shown to be superior to H2 antagonists for the prevention of NSAID-induced endoscopic ulcers and similar to misoprostol,100,101 but better tolerated. Observational studies and clinical trials have suggested that PPIs reduce the risk of GI complications in patients taking NSAIDs.26,53,71,76
Coxibs have been proven superior to the combination of NSAID plus placebo and as effective as the use of nonselective NSAID associated to PPIs for the prevention of GI lesions and69,82,102–105 complications. Data from randomized trials indicate that coxibs are associated with a lower frequency of low GI lesions produced by the association of a nonselective NSAID plus PPI.103,106,107
These drugs have been developed with the intent of reducing GI toxicity associated with NSAID use. Several meta-analyses have indicated that the risk of upper GI complications with COXIB was 50% less than the risk seen with conventional NSAIDs.85,108
The CONDOR and GI REASON studies, meanwhile, have shown that celecoxib was associated with a lower frequency and a reduction in all clinically significant episodes of GI tract toxicity when compared to PPI and associated diclofenac (CONDOR) or traditional NSAIDs associated or not to PPI (GI REASON).107,109 One study showed a lower (not significant) rate of complications with etoricoxib versus diclofenac.110 The MEDAL study indicates in turn that etoricoxib was not superior to diclofenac regarding the incidence of upper and lower GI complications.110
Antiplatelet doses of aspirin doubles the risk of GI bleeding and hospitalization compared to people who do not use it.92 This causes different clinical situations in patients with CV and GI risk, and doubts may arise between maintaining or interrupting treatment with ASA. Such disruption can precipitate serious CV effects, including death.111,112
ASA therapy is associated with the development of peptic ulcers, including esophageal, gastroesophageal reflux disease (GERD) or dyspepsia, besides upper GI complications. Therefore, the benefits of treatment with PPIs in patients taking ASA also extend to a better control of symptoms and lesions associated with GERD.113
Recent data indicate that the combined use of PPI, low dose aspirin and clopidogrel in patients with CV risk is associated with a low frequency of GI bleeding (1.8 cases per 100 patient-years). However, a shift in the location thereof to the lower GI tract is observed since, for all bleeding episodes, 73% were found in the small intestine and in the colon (many of them as a result of vascular injury, possibly preexisting). These hemorrhages occurred predominantly at an early stage during the first year of follow up.114
Liver RiskSeveral systematic reviews assessing patients in clinical trials show a very low incidence of serious hepatic effects associated with NSAID use. A systematic review including 132 studies shows that the need to discontinue NSAID due to adverse effects was not significantly higher than the placebo group, except for diclofenac.115 Another analysis that brings together 41 studies showed the incidence of hepatic adverse effects of celecoxib to be 1.1%, 4.2% for diclofenac, 1.5% for ibuprofen and 0.9% for placebo116; the rate of serious hepatic adverse events was 5/100000 for celecoxib and 21/100000 for diclofenac. The need for hospitalization due to toxicity has been estimated at between 3 and 23 per 100000 patients.117,118 Transient elevations of transaminases are common, and in most cases do not progress.115 Although cases of toxic hepatitis, and even cases of severe hepatitis and death have been described due to NSAID,115,119–122 these are exceptional, and there is no monitoring system to determine which patients will have a serious adverse liver reaction, so monitoring measures are not recommended. The risk of liver toxicity is significantly increased with diclofenac than with other NSAIDs.115,116
The use of NSAIDs in patients with cirrhosis has been linked to the onset of variceal bleeding,123 and it is well known for his ability to impair renal function in patients with both compensated and decompensated liver cirrhosis.124,125 For these reasons NSAIDs are considered contraindicated in patients with chronic liver disease and cirrhosis.
A study in 28 patients with cirrhosis and ascites assessed for 3 days the effect of celecoxib 200mg every 12h, naproxen 500mg every 12h placebo on renal function and platelet aggregation. Neither celecoxib nor placebo induced changes in renal or platelet function. In contrast, naproxen induced a marked reduction in glomerular filtration, renal plasma flow and urine output, along with a significant antiplatelet effect.126 On the other hand, Agrawal et al. noted that etoricoxib127 was well tolerated in patients with moderate or severe hepatic impairment. However, given the limited experience, it is also recommended to avoid the use of coxibs in patients with liver disease, and especially if presenting decompensated liver cirrhosis.
DyspepsiaIt is estimated that between 10% and 30% of patients receiving NSAIDs have dyspepsia, and dyspepsia leads to discontinuation of treatment in 5%–15% of patients.128 The addition of a PPI versus placebo was associated with a lower frequency of dyspepsia in101 patients treated with NSAIDs. A meta-analysis by Spiegel et al.,129 evaluated 26 studies comparing a COXIB vs traditional NSAIDs or traditional NSAID plus PPI vs NSAIDs alone. Comparing COXIB vs traditional NSAIDs showed a relative risk reduction of symptoms of 12% and an absolute reduction of 3.7%. Comparison of traditional NSAIDs {3+} PPI vs traditional NSAIDs showed a relative risk reduction of 66% and an absolute risk reduction of 9%. The number needed to treat to prevent dyspepsia was 27 for coxibs and 11 for traditional NSAIDs plus PPI. These results should be evaluated carefully, since a direct comparison between coxibs and traditional NSAIDs+PPI was not made. In an ad hoc systematic search performed, 6 studies directly comparing with a traditional NSAID COXIB+PPI were found.102,103,106,130–132 The meta-analysis of 6 studies with data on dyspepsia suggests, however, that this effect is true. Dyspepsia incidence was 11.8% (112/947) in those receiving one COXIB vs 7.4% (72/977) in patients treated with a traditional or nonselective NSAIDs+PPI (OR: 2.2, 95% CI: 1.3–3.6). Given the high efficacy of PPIs in the treatment of dyspepsia associated with NSAIDs, it seems reasonable to recommend their use in the case of patients having dyspepsia associated with the use of coxibs.
Inflammatory Bowel DiseaseIn patients suffering from inflammatory bowel disease (IBD), the use of NSAIDs should be avoided.
LE: 1−, DR: B, LA: 89%
In patients suffering from IBD and if it should be necessary to use NSAIDs in quiescent phases of the disease, the use of COXIBs is recommended at low doses for a short time.
LE: 1−, DR: B, LA: 100%
It is estimated that up to one-third of patients with IBD develop arthritis which may or may not be linked133 pathogenically with the disease. This implies that many patients with IBD require some type of NSAID at any given point in their evolution. Existing epidemiological studies are generally of poor quality and results clearly contradictory.134–136 Moreover, there are no clinical trials to show that taking NSAIDs produce worsening or recurrence of IBD, although there are case series with varying numbers of patients that do suggest it.134 In this sense, a relatively recent study indicated that 17%–28% of patients in remission with Crohn's disease or ulcerative colitis who received nonselective NSAIDs such as naproxen, diclofenac, indomethacin or nabumetone for 4 weeks, had recurrence in the first 9days after taking the drug. This did not occur in those taking nimesulide (selective inhibitor of COX-2), low-dose aspirin or paracetamol.137 Few clinical trials have tested whether the use of selective COX-2 inhibitors is associated with quiescent IBD relapses. A double-blind randomized trial compared etoricoxib (60–120mg/day) versus placebo over a period of 3months in patients with Crohn's disease or ulcerative colitis. The recurrence rate was 10% in both situations.138 In another similar trial, but with a shorter observation period of 14 days celecoxib 200mg/12h was compared to placebo in patients with ulcerative colitis, with the same recurrence rates below 3%.139
Renal RiskRenal Risk StratificationNSAIDs interfere with renal homeostasis. This has implications for renal function, known in classic NSAIDs, which as a result of the most recent investigations have been better defined in COXIBs. Systematic reviews of the risk of developing renal comorbidity in rheumatic patients have difficulty concluding on the magnitude of the problem, because clinical trials do not measure systematically renal outcomes or as part of the primary events of interest.140–142
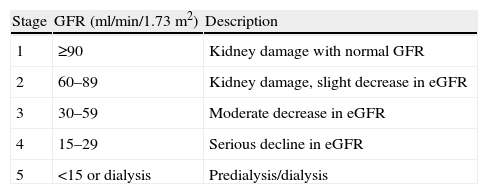
The Spanish Society of Nephrology recommended an annual estimate of renal function by glomerular filtration. Accordingly, the presence of chronic renal disease is categorized according to Table 4.143 A moderate risk of developing renal adverse event is present if patients have chronic kidney disease stage 3 and/or intravascular volume depletion, hypertension or congestive heart failure and peripheral edema is.
Estimation of Renal Damage.
| Stage | GFR (ml/min/1.73m2) | Description |
| 1 | ≥90 | Kidney damage with normal GFR |
| 2 | 60–89 | Kidney damage, slight decrease in eGFR |
| 3 | 30–59 | Moderate decrease in eGFR |
| 4 | 15–29 | Serious decline in eGFR |
| 5 | <15 or dialysis | Predialysis/dialysis |
GFR, glomerular filtration rate; eGFR, estimated glomerular filtration rate.
Stages 4 and 5 are considered at high risk of developing renal adverse event.
Renal Complications Associated With the Use of Nonsteroidal Anti-inflammatory DrugsIn patients with chronic kidney disease stage 3 or renal comorbidity and/or associated CV, use of NSAIDs, except in special situations and with close clinical monitoring, is not recommended.
LE: 2+, DR: C, LA: 100%
In patients with chronic kidney disease stage 3 or renal comorbidity and/or associated CV, the use of higher doses than recommended of NSAIDs should be avoided, especially COXIB.
LE: 2+, DR: C, LA: 89%
In patients with chronic kidney disease stage 4 and 5 NSAID use is contraindicated.
LE: 2+, DR: C, LA: 78%
NSAIDs lead to a decreased renal function and sodium and water retention. Clinically, this may present as elevated serum creatinine, edema, hypertension, water and electrolyte imbalance, renal failure, renal papillary necrosis or nephrotic syndrome.140 The relative risk of renal adverse event is different for each type of NSAID, and apparently there is no class effect. As with other toxic effects, these are dose dependent and accumulate over time. Moreover, in rheumatic patients renal and CV comorbidities are often associated, which increases the risk.140,141,144
Decreased renal function (elevated serum creatinine>1.3 times the upper normal limit) occurs with a frequency of 1%. This elevation was observed equally in patients receiving therapeutic doses of NSAIDs, and is higher with NSAIDs than placebo.
Another clinically relevant side effect associated with NSAIDs is the development of peripheral edema, with an estimated risk of 3%. This risk is even greater when COXIBs are used than with placebo (5%–38% higher in clinical trials in which edema is a major variable in the research). However, celecoxib at doses of 200mg/day was not associated with increased risk of edema.145 This suggests that the presence of edema does not follow a specific effect of the coxib class. The development of heart failure due to the retention of sodium and water in susceptible patients is another common side effect of all NSAIDs.70
All NSAIDs are associated with increased blood pressure in hypertensive subjects, and this has little effect in individuals with normal blood pressure. In general, the frequency of hypertension associated with NSAIDs is ≈2% and occurs more frequently in subjects with renal comorbidity and/or pre-existing CV disease. The minimum mean change in blood pressure after initiation of NSAID is an elevation of 5mmHg but the clinical significance of this increase is uncertain. The risk is higher for coxibs (rofecoxib>etoricoxib and lower for celecoxib). It is important to monitor blood pressure after initiation of NSAID, especially in the elderly, in patients with pre-hypertension and in patients with chronic renal disease. The use of NSAIDs is contraindicated in patients with uncontrolled hypertension.146–148
The presence of severe renal damage associated with NSAIDs is a rare event, with little information about its occurrence and risk factors.
Other Relevant AspectsAnemiaNSAID use is associated with GI bleeding made clinically apparent by hematemesis, melena or rectal bleeding, but also hidden GI bleeding and anemia. The origin of this occult blood loss may be due to mucosal lesions that affect the entire GI tract, from the stomach to the small intestine and the colon.149 Different studies in healthy volunteers have demonstrated the appearance of petechiae, intraluminal blood and mucous substance losses compatible with erosions and ulcers in the small intestine. In these studies it has been observed that ibuprofen or naproxen with a PPI were more associated with these injuries than celecoxib at doses of 400mg/day or placebo.106,107 In patients on chronic NSAIDs or coxibs it has also been seen, by capsule endoscopy, that 40%150,151 of them had lesions in the small intestine, although the frequency was numerically lower in patients treated with coxibs. The CONDOR103 study showed that patients with osteoarthritis or rheumatoid arthritis treated with celecoxib 200mg/12h had 4 times the risk of developing lower hematocrit or hemoglogin decreases (>2g/dL) of GI origin or, presumably, from the small bowel versus diclofenac 75mg/12h plus omeprazole 20mg/day after 6 months of treatment. This study has been confirmed by another randomized multicenter study, the PROBE trial, designed to compare celecoxib versus traditional NSAIDs associated or not to PPI.152 In the MEDAL program, however, no significant difference between diclofenac etoricoxib and the number of GI tract complications was observed.110
PostoperativeWe recommend the use of paracetamol–NSAID combination in postoperative pain in the short term, provided there is no contraindication to the administration of the latter.
LE: 1+, DR: A, LA: 78%
One NSAID cannot be prioritized over another regarding their postoperative use.
LE: 2++, DR: B, LA: 89%
The combination in terms of dose and type of drug use in the postoperative period must be balanced empirically.
LE: 3, DR: D, LA: 78%
The non-opioid analgesics, paracetamol and NSAIDs are often given with opioids as part of multimodal analgesia after major surgery. The choice of method of postoperative pain relief should be well balanced and combining different drugs and different routes of administration may be employed with the aim of using smaller doses to minimize potential side effects.
A recent systematic review153 demonstrated a statistically significant reduction in morphine consumption at 24h after surgery, when using combination therapy with acetaminophen or NSAIDs versus placebo; there was also a clear difference between paracetamol and NSAIDs, in favor of the latter. A statistically significant difference was also shown in the level of sedation and the occurrence of adverse effects (post operative nausea and vomiting) with any of the combinations, with no superiority of one over another. In another review154 renal function was evaluated after administration of NSAIDs for postoperative pain control. In 1459 patients from 23 randomized trials, creatinine clearance (48h), serum creatinine, urine volume, Na/K urinary and need for dialysis were determined. The results show a decrease in creatinine clearance 16ml/min but no differences in other parameters. Nor difference was seen between NSAIDs or cases of renal failure requiring dialysis were observed.
A single oral dose of celecoxib or etoricoxib155,156 (120mg) is effective for postoperative pain relief and adverse events did not differ from placebo.
Dexketoprofen administered parenterally has a similar analgesic efficacy as postoperative ketoprofen and diclofenac.157,158 The recommended dose is 50mg every 8or 12h limited to the acute symptomatic period (no more than 2days). The total daily dose should be limited to 50mg on the following assumptions: (a) in the elderly who have a mild physiological decline in renal function, (b) in patients with mild to moderate hepatic impairment (Child–Pugh score 5–9), which also should be carefully monitored, and (c) for mild renal impairment (creatinine clearance 50–80ml/min).
Summary and Final RecommendationsA summary of the recommendations of this consensus, and the graphic expression of the secondary effects of commonly used NSAIDs are detailed in Tables 5 and 6. Finally, in Fig. 3 an algorithm guiding the correct prescription of NSAIDs, based on the presence of different levels of GI and CV risk has been constructed, according to the evidence available in 2013.
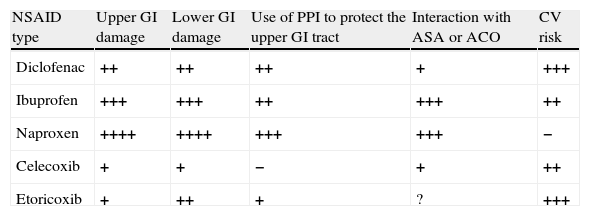
Summary of the Effects of Different NSAIDs at Anti-inflammatory Doses.
| NSAID type | Upper GI damage | Lower GI damage | Use of PPI to protect the upper GI tract | Interaction with ASA or ACO | CV risk |
| Diclofenac | ++ | ++ | ++ | + | +++ |
| Ibuprofen | +++ | +++ | ++ | +++ | ++ |
| Naproxen | ++++ | ++++ | +++ | +++ | − |
| Celecoxib | + | + | − | + | ++ |
| Etoricoxib | + | ++ | + | ? | +++ |
The number of crosses indicates a semi-quantitative assessment of the level of risk vs non-use. The levels of evidence are not identical and sometimes limited (see specific recommendations).
ASA, acetylsalicylic acid; ACO, anticoagulants; CV, cardiovascular; GI, gastrointestinal; PPI, proton pump inhibitors.
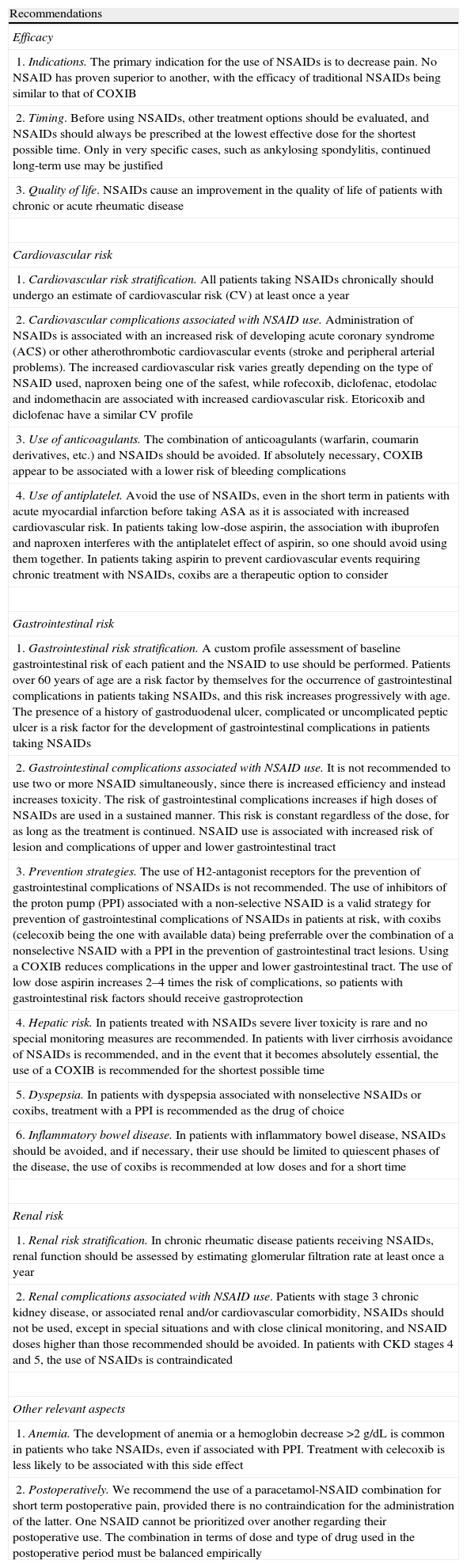
Summary of Consensus Recommendations.
| Recommendations |
| Efficacy |
| 1. Indications. The primary indication for the use of NSAIDs is to decrease pain. No NSAID has proven superior to another, with the efficacy of traditional NSAIDs being similar to that of COXIB |
| 2. Timing. Before using NSAIDs, other treatment options should be evaluated, and NSAIDs should always be prescribed at the lowest effective dose for the shortest possible time. Only in very specific cases, such as ankylosing spondylitis, continued long-term use may be justified |
| 3. Quality of life. NSAIDs cause an improvement in the quality of life of patients with chronic or acute rheumatic disease |
| Cardiovascular risk |
| 1. Cardiovascular risk stratification. All patients taking NSAIDs chronically should undergo an estimate of cardiovascular risk (CV) at least once a year |
| 2. Cardiovascular complications associated with NSAID use. Administration of NSAIDs is associated with an increased risk of developing acute coronary syndrome (ACS) or other atherothrombotic cardiovascular events (stroke and peripheral arterial problems). The increased cardiovascular risk varies greatly depending on the type of NSAID used, naproxen being one of the safest, while rofecoxib, diclofenac, etodolac and indomethacin are associated with increased cardiovascular risk. Etoricoxib and diclofenac have a similar CV profile |
| 3. Use of anticoagulants. The combination of anticoagulants (warfarin, coumarin derivatives, etc.) and NSAIDs should be avoided. If absolutely necessary, COXIB appear to be associated with a lower risk of bleeding complications |
| 4. Use of antiplatelet. Avoid the use of NSAIDs, even in the short term in patients with acute myocardial infarction before taking ASA as it is associated with increased cardiovascular risk. In patients taking low-dose aspirin, the association with ibuprofen and naproxen interferes with the antiplatelet effect of aspirin, so one should avoid using them together. In patients taking aspirin to prevent cardiovascular events requiring chronic treatment with NSAIDs, coxibs are a therapeutic option to consider |
| Gastrointestinal risk |
| 1. Gastrointestinal risk stratification. A custom profile assessment of baseline gastrointestinal risk of each patient and the NSAID to use should be performed. Patients over 60 years of age are a risk factor by themselves for the occurrence of gastrointestinal complications in patients taking NSAIDs, and this risk increases progressively with age. The presence of a history of gastroduodenal ulcer, complicated or uncomplicated peptic ulcer is a risk factor for the development of gastrointestinal complications in patients taking NSAIDs |
| 2. Gastrointestinal complications associated with NSAID use. It is not recommended to use two or more NSAID simultaneously, since there is increased efficiency and instead increases toxicity. The risk of gastrointestinal complications increases if high doses of NSAIDs are used in a sustained manner. This risk is constant regardless of the dose, for as long as the treatment is continued. NSAID use is associated with increased risk of lesion and complications of upper and lower gastrointestinal tract |
| 3. Prevention strategies. The use of H2-antagonist receptors for the prevention of gastrointestinal complications of NSAIDs is not recommended. The use of inhibitors of the proton pump (PPI) associated with a non-selective NSAID is a valid strategy for prevention of gastrointestinal complications of NSAIDs in patients at risk, with coxibs (celecoxib being the one with available data) being preferrable over the combination of a nonselective NSAID with a PPI in the prevention of gastrointestinal tract lesions. Using a COXIB reduces complications in the upper and lower gastrointestinal tract. The use of low dose aspirin increases 2–4 times the risk of complications, so patients with gastrointestinal risk factors should receive gastroprotection |
| 4. Hepatic risk. In patients treated with NSAIDs severe liver toxicity is rare and no special monitoring measures are recommended. In patients with liver cirrhosis avoidance of NSAIDs is recommended, and in the event that it becomes absolutely essential, the use of a COXIB is recommended for the shortest possible time |
| 5. Dyspepsia. In patients with dyspepsia associated with nonselective NSAIDs or coxibs, treatment with a PPI is recommended as the drug of choice |
| 6. Inflammatory bowel disease. In patients with inflammatory bowel disease, NSAIDs should be avoided, and if necessary, their use should be limited to quiescent phases of the disease, the use of coxibs is recommended at low doses and for a short time |
| Renal risk |
| 1. Renal risk stratification. In chronic rheumatic disease patients receiving NSAIDs, renal function should be assessed by estimating glomerular filtration rate at least once a year |
| 2. Renal complications associated with NSAID use. Patients with stage 3 chronic kidney disease, or associated renal and/or cardiovascular comorbidity, NSAIDs should not be used, except in special situations and with close clinical monitoring, and NSAID doses higher than those recommended should be avoided. In patients with CKD stages 4 and 5, the use of NSAIDs is contraindicated |
| Other relevant aspects |
| 1. Anemia. The development of anemia or a hemoglobin decrease >2g/dL is common in patients who take NSAIDs, even if associated with PPI. Treatment with celecoxib is less likely to be associated with this side effect |
| 2. Postoperatively. We recommend the use of a paracetamol-NSAID combination for short term postoperative pain, provided there is no contraindication for the administration of the latter. One NSAID cannot be prioritized over another regarding their postoperative use. The combination in terms of dose and type of drug used in the postoperative period must be balanced empirically |
The authors declare that no experiments have been performed on humans or animals.
Data confidentialityThe authors state that no patient data appear in this article.
Right to privacy and informed consentThe authors state that no patient data appear in this article.
Conflicts of InterestDr. Lanas claims to have received research grants from AstraZeneca, Pfizer and Bayer over the past two years. He has also participated in Advisory Board meetings organized by Pfizer and Bayer.
Dr. Calvet states he has participated, for the past two years, in Boards organized by Pfizer.
Dr. P. Benito claims to have received, over the past two years, research grants from Pfizer, Abbott, Roche, UCB and Esteve.
Dr. García Llorente states that he has participated, for the past two years, at scientific meetings sponsored by Abbott, Pfeizer, UCB, Roche, BMS, as well as a UCB advisory board.
Dr. Blanca Hernández Cruz states that in the past two years she has received research grants, consulting fees paid and fee-for-papers with various companies, the Spanish Society of Rheumatology, Abbott, Roche, Pfizer, MSD and BMS.
The rest of the participants declared no conflicts of interest.
Please cite this article as: Lanas A, Benito P, Alonso J, Hernández-Cruz B, Barón-Esquivias G, Perez-Aísa Á, et al. Recomendaciones para una prescripción segura de antiinflamatorios no esteroideos: documento de consenso elaborado por expertos nominados por 3 sociedades científicas (SER-SEC-AEG). Reumatol Clin. 2014;10:68–84.
By agreement with the authors and the editors, this article is published in complete form simultaneouslyin the Journal Gastroenterología y Hepatología doi:10.1016/j.gastrohep.2013.11.014.