We report a case of acute left ventricular dysfunction due to myocarditis, in the setting of a scleroderma renal crisis. The case is particularly intriguing for the favorable outcome of both symptoms and heart function following immunosuppressive therapy. We also highlight the changes observed over time with image techniques as well as in electrocardiograms.
Presentamos un caso de disfunción ventricular izquierda secundaria a miocarditis en el contexto de una crisis renal esclerodérmica. Su principal atracción reside en la gran mejoría experimentada por el paciente, no solo en lo que a los síntomas se refiere sino también en su función cardiaca, tras el inicio del tratamiento inmunosupresor. Es muy llamativa la evolución radiológica y electrocardiográfica documentada.
Systemic sclerosis (SSc) is an autoimmune connective tissue disease targeting small size vessels, and leading to fibrosis of affected organs.1 Along with the skin, these principally include lungs, kidney, and gastrointestinal tract. A scleroderma renal crisis (SRC) has been reported to occur in 10% of the patients.2
Also considered to be common, myocardial involvement is often unsuspected, as it can be silent or draw nonspecific symptoms.3 However, symptomatic heart disease is associated to a poor survival. The most frequent presentation is progressive deterioration of both left and right systolic and diastolic functions resulting from an abnormal microvascular resistance.4 More unusual is acute heart failure due to myopericarditis, but its occurrence during an SRC should be watched.2
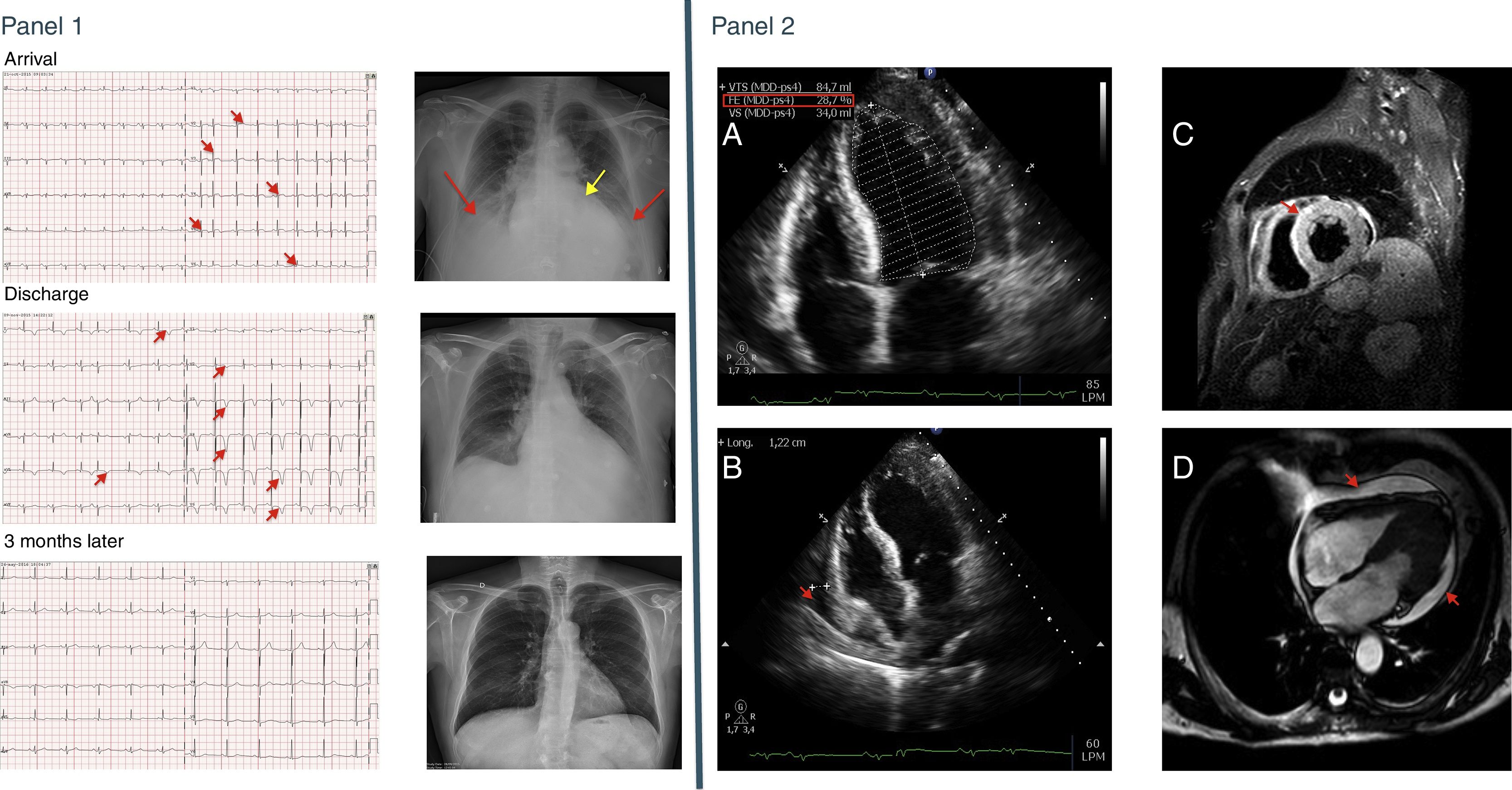
CaseA 56 year-old man without cardiovascular risk factors had been recently diagnosed with anti-RNA polymerase III antibodies (ARA) positive SSc. Because of rapid progression of skin thickening, he received methylprednisolone pulses followed by 1mg/kg/day prednisone and methotrexate. Few days later, the patient attended the Emergency Room complaining of weakness and shortness of breath. Vitals showed low blood pressure, bilateral rales, jugular vein engorgement and increased respiratory effort. Indurated edema at upper limbs and tight fingers were evident. An electrocardiogram (ECG) showed sinus tachycardia and low voltage and mild ST segment elevation in almost all precordial leads (Fig. 1 top, Panel 1). Blood tests yielded an increased creatinine and a moderate thrombocytopenia, along with raised markers of myocardial damage (Table 1). Signs of pulmonary congestion were observed in the chest X-ray (CXR) (Fig. 1 top, Panel 1). An echocardiogram showed severe global hypokinesia, a left ventricular ejection fraction (LVEF) of 28% and mild pericardial effusion (Panel 2 A and B), findings which could be later on confirmed with MRI (Panel 2 C and D).
Panel 1: Top, ECG (left panel) and CXR (right panel) at admission. At the ECG sinus tachycardia and ST-segment elevation from V2-V6 are visible (red arrows); the CXR shows cardiomegaly (yellow arrow) and bilateral pulmonary congestion (red arrows). Middle, the same tests at discharge. ECG with sinus rhythm and deep negative T waves from V2-V6 and in I and AvL (red arrows); CXR with clear lung fields. Botton, ECG and CXR 3 months after the episode. ECG shows normalization of the T wave, and CXR is normal. Panel 2: A: Echocardiogram at admission showing severe LV dysfunction (28.7% calculated by Simpson method). B: four chambers views from the echocardiogram at admission showing pericardial effusion (red arrow). C: short-axis view from a Cardiac MR (STIR sequence) showing more white density in the anterior and septum zone suggesting myocardial edema (red arrow). D: four chambers view from a Cardiac MR (CINE sequence) showing generalized pericardial effusion (red arrows).
Blood Test Results During the First Year.
| Admission | Day 2 | Day 5 | 1 Week | 2 weeks | 1 month | 3 months | 6 months | 1 year | |
|---|---|---|---|---|---|---|---|---|---|
| Hb (g/dl) (13–17) | 14.8 | 14.5 | 11.9 | 10.8 | 10.0 | 11.7 | 11.9 | 12.0 | 11.9 |
| Plat (pl/dl) (150,000–450,000) | 52,000 | 55,000 | 41,000 | 53,000 | 79,000 | 128,000 | 191,000 | 169,000 | 217,000 |
| Prothrombin time (s) (10–14) | 12.5 | 11.1 | 10.9 | 12.9 | 10.9 | 10.5 | 9.5 | – | 10.5 |
| aPTT (s) (26–36) | 16.6 | 29.0 | 26 | 34.6 | 25.6 | 30.2 | 30.7 | – | 28.2 |
| INR (0.85–1.15) | 1.14 | 1.06 | 1.00 | 1.18 | 1.00 | 0.96 | 0.88 | – | 0.97 |
| Cr (mg/dl) (0.7–1.3) | 1.8 | 2.0 | 2.2 | 2.4 | 3.0 | 3.8 | 3.6 | 2.6 | 1.7 |
| GFR (ml/kg/min) | 42 | 37 | 33 | 30 | 23 | 18 | 21 | 28 | 44 |
| LDH (mg/dl) (230–460) | 2082 | 1309 | 1125 | 1000 | 744 | 503 | 494 | 376 | 415 |
| CK (UI/l) (1–175) | 127 | 107 | 91 | – | – | – | – | – | – |
| CK-MB (ng/ml) (<3.6) | 9.41 | 9.90 | 2.3 | – | – | – | – | – | – |
| Troponin I (ng/dl) (<0.12) | 0.85 | 1.36 | 0.19 | – | – | – | – | – | – |
| C3 (mg/dl) (90–170) | – | 68 | 68 | 53 | 66 | 79 | 78 | 85 | 88 |
| C4 (mg/dl) (12–36) | – | 5 | 5 | 8 | 14 | 20 | 21 | 20 | 14 |
| Haptoglobin (mg/dl) (40–240) | – | <1 | <1 | <1 | <1 | 3 | 84 | 110 | 150 |
The patient was admitted into the Coronary Care Unit (CCU), where standard treatment for acute heart failure was started. In the next days, his hemoglobin levels fell and schistocytes were found in blood smears, while his plasma creatinine continued to increase (Table 1). Additional diagnostic workup confirmed the existence of a thrombotic microangiopathy (TMA), with a normal activity of von Willebrand-cleaving protease ADAMTS13, negative microbiologic studies and low complement levels. Together, these features led to a diagnosis of an SRC presenting with an atypical hemolytic uremic syndrome type of TMA and myopericarditis. Cyclophosphamide, immunoglobulins (IVIG), plasma exchange sessions (PLEX), and the endothelin 1 (ET1) receptor antagonist bosentan were started.
As initial difficulties in raising his blood pressure to normal range were overcome, the patient's condition improved and his heart function was restored to normal. ECG showed progressive recovering of voltage and appearance of negative T waves (Fig. 1 middle, Panel 1). Platelet counts and Hb also reached stable levels. However, his renal function continued to worsen, and complement and haptoglobin remained low, indicating persistent TMA (Table 1). At this point, eculizumab, a C5 blocking monoclonal antibody, was started.
The patient showed an immediate response, and he could be discharged to continue treatment with cyclophosphamide, IVIG and eculizumab. Six months later, his LVEF was normal, creatinine levels went down and skin returned to normal, but for sclerodactylia. Background therapy was changed to mycophenolate mofetil, with the disease remaining stable thereafter (Table 1; Fig. 1 bottom, Panel 1).
DiscussionThe subgroup of ARA+ SSc patients are at particular risk of developing an SRC during early disease. The intake of high doses of corticosteroids is considered a precipitating factor. An association to microangiopathic hemolytic anemia and thrombocytopenia, such as in our patient, has been found in up to 20%–25% of patients with SRC,2 an entity which in fact could be considered as a special subset of TMA.5
As regards mechanisms involved in SSc-associated cardiac injury, it is generally considered that local immune complex deposition and complement activation drive connective tissue disorders’ acute myocarditis. Some autoantibodies could further exert a direct toxic effect to cardiomyocytes.6 Accordingly, IVIG and PLEX along with immunosuppresors are usually employed in an attempt to remove autoantibodies and inactivate complement.
In addition, a particular feature of SSc is the tendency of myocardial inflammation to progress to established fibrosis. This fact confers a high severity to cardiac disease in the patients.1,4 In this fibrotic tendency, the microvascular alterations which define systemic sclerosis are supported by the local up-regulation of ET1 and the renin-angiotensin-aldosterone system (RAAS).7,8 The increased microvascular resistance leads to ischemia and to an abnormal remodeling of smooth muscle cells in blood vessel walls.7 This provides a rationale for the use of vessel-protective measures targeting thses systems in patients presenting with SSc-related myopericarditis.4,8 TMA represents an extreme situation of this pathogenic process, in which endovascular damage is further enhanced by a vicious circle of complement activation.5,9 In this regard, eculizumab could provide an additional benefit in life-threatening SRC, as we could observe in our patient and had been already reported.10
In summary, this case illustrates how SSc can present as a life-threatening acute heart failure with ischemic-like electric changes, as well as the benefit of an early immune-targeted therapy for achieving a favorable outcome. This fact underlines the need of a multidisciplinary approach in the management of this complex syndrome.
Conflict of interestsThe authors declare no conflict of interests.