To develop multidisciplinary recommendations to improve the management of rheumatoid arthritis-related interstitial lung disease (RA-ILD).
MethodsClinical research questions relevant to the objective of the document were identified by a panel of rheumatologists and pneumologists selected based on their experience in the field. Systematic reviews of the available evidence were conducted, and evidence was graded according to the Scottish Intercollegiate Guidelines Network (SIGN) criteria. Specific recommendations were made.
ResultsSix PICO questions were selected, three of which analysed the safety and effectiveness of glucocorticoids, classical synthetic disease-modifying anti-rheumatic drugs (DMARDs) and other immunosuppressants, biological agents, targeted synthetic DMARDs, and antifibrotic therapies in the treatment of this complication. A total of 12 recommendations were formulated based on the evidence found and/or expert consensus.
ConclusionsWe present the first official SER-SEPAR document with specific recommendations for RA-ILD management developed to resolve some common clinical questions, reduce clinical healthcare variability, and facilitate decision-making for patients.
Elaborar unas recomendaciones multidisciplinares para mejorar el manejo de la enfermedad pulmonar intersticial difusa asociada a la artritis reumatoide (EPID-AR).
MétodosUn panel de reumatólogos y neumólogos expertos identificó preguntas clínicas de investigación relevantes para el objetivo del documento. Se realizaron revisiones sistemáticas de la evidencia, que se graduó de acuerdo con los criterios del SIGN. Tras ello, se formularon las recomendaciones.
ResultadosSe seleccionaron seis preguntas PICO, tres de las cuales específicamente evaluaron la seguridad y eficacia de los glucocorticoides, fármacos de acción lenta moduladores de la enfermedad (FAME) sintéticos clásicos e inmunosupresores, FAME biológicos, FAME sintéticos dirigidos y antifibróticos en el tratamiento de los pacientes con EPID-AR. Se formularon un total de 12 recomendaciones con base en la evidencia encontrada y/o consenso de expertos.
ConclusionesSe presenta el primer documento oficial SER-SEPAR de recomendaciones específicas para el abordaje terapéutico de la EPID-AR, con el fin de ayudar en la toma de decisiones a los clínicos directamente implicados en su manejo.
Interstitial lung disease (ILD) is one of the most common and severe extra-articular manifestations of rheumatoid arthritis (RA) and is currently the second leading cause of premature death from the disease.1–5
The treatment of patients with RA-ILD is a particularly difficult and complex clinical scenario. These patients are usually excluded from randomised clinical trials (RCTs), and there have been no controlled studies that have specifically evaluated the efficacy of drugs to treat this complication. There is little published evidence on the subject, most is of low methodological quality, and shows contradictory results in some aspects. To date there are no consensus recommendations from scientific societies.
There is also a growing body of literature suggesting that some of the drugs that we commonly use in the management of RA may trigger ILD or worsen pre-existing ILD.
On this basis, and to respond to this healthcare need, we present the first official document prepared by the Spanish Societies of Rheumatology (SER) and Pneumology and Thoracic Surgery (SEPAR) with specific recommendations on the therapeutic approach to RA-ILD, resolving some common clinical questions and facilitating decision-making.
MethodsThese recommendations were drafted based on a qualitative synthesis of the scientific evidence and consensus techniques, and reflect the agreement of experts based on the available evidence and their clinical experience.6
The procedure for the project was as follows:
- 1
Creation of the working group. An interdisciplinary working group was formed comprising 5 rheumatologists from SER (JN, GB, IC, NMV, and AMO) and 5 pneumologists from SEPAR (MA, ECJ, MAN, CV, and JARP). The participants were endorsed by their society to participate in this document. The clinical and methodological aspects were coordinated, respectively, by one of the rheumatologists (JN) and a pneumologist (JARP), as principal investigators (PI), and two methodology specialists from the SER Research Unit.
- 2
Identification of key areas. The contents and key aspects of the document were defined, and the clinical research questions with the greatest impact on clinical practice were formulated. The questions were reformulated in patient, intervention, comparison, and outcome (PICO) format.
- 3
Literature search. A search for published scientific evidence was conducted and successively expanded until October 2020. The databases PubMed (MEDLINE), EMBASE, and Cochrane Library (Wiley Online) were used for this purpose. The process was completed with a manual search of the references of the studies identified, as well as posters and conference abstracts that the reviewers and experts considered to be of interest.
- 4
Analysis and synthesis of the scientific evidence. Rheumatologists from SER who are experts in evidence review conducted the systematic reviews and synthesis of the scientific evidence. The level of scientific evidence was assessed using Scottish Intercollegiate Guidelines Network (SIGN)7 criteria (Annex).
- 5
Formulation of recommendations. Once the critical reading had been completed, the PIs, together with the members of the expert group reviewing the evidence for each of the PICO questions, formulated the recommendations, based on formal evaluation or "reasoned judgement" of the evidence for each of the questions. The quality, quantity, and consistency of the scientific evidence, the generalisability, applicability, and clinical impact of the results were considered. The strength of the recommendations was graded using the SIGN7 system. For questions where the evidence was insufficient, recommendations were formulated based on consensus of the expert group.
The recommendations have been divided into five main areas: incidence and prevalence of ILD in RA, risk factors for the onset of ILD in RA, prognostic factors for mortality and pulmonary progression, as well as the safety of drug treatment in patients with RA-ILD, and its efficacy in managing this complication.
- 6
External review. The draft document was subjected to external review to ensure the validity and accuracy of the recommendations and, subsequently, to public exposure so that other SER and SEPAR members, as well as different groups and potentially interested entities, could evaluate the document, and offer criticism or suggestions.
A total of 18 recommendations have been formulated, 12 of which relate to the management of patients with RA-ILD (Table 1).
SER-SEPAR recommendations for the treatment of interstitial lung disease associated with rheumatoid arthritis.
| Recommendations | Grade of recommendation |
|---|---|
| For the treatment of patients with rheumatoid arthritis-associated interstitial lung disease, multidisciplinary therapeutic management is recommended | √ |
| If interstitial lung disease is present at RA debut, an individualised assessment for the use of MTX is recommended, as there is a risk of drug-induced acute pneumonitis, albeit low | A |
| In these cases, the drafting group considers that the best strategy to minimise risks is to use another conventional synthetic DMARD whenever possible | √ |
| In patients with RA, when ILD is diagnosed or worsens during the first year of MTX treatment, MTX should be temporarily discontinued until it is clear whether or not there is a causal relationship | √ |
| In patients with RA on methotrexate for more than one year who are diagnosed with interstitial lung disease, the drug can be maintained as there is no evidence to justify discontinuation | D |
| In patients with RA-ILD who are not of Asian descent, leflunomide can be considered a safe drug | √ |
| In patients with rheumatoid arthritis and interstitial lung disease requiring biologic therapy, abatacept or rituximab should be used interchangeably as safer options | D |
| In patients with rheumatoid arthritis and interstitial lung disease, in case of contraindication or inadequate response to abatacept and rituximab, the use of an IL-6 inhibitor or a targeted synthetic DMARD can be considered | D |
| In patients with rheumatoid arthritis being treated with anti-TNF and stable interstitial lung disease, there is inconclusive evidence to recommend discontinuation if the drug has achieved good control of joint symptoms | √ |
| In patients with rheumatoid arthritis-associated interstitial lung disease with an inflammatory radiological pattern (NSIP, OP, LIP, etc.) in whom GLC treatment is considered necessary, their use is always recommended at the lowest dose and for the shortest possible time | √ |
| The drafting group considers that the available evidence is insufficient to issue a conclusive recommendation on the use of immunosuppressants in the treatment of rheumatoid arthritis-associated interstitial lung disease | D |
| If it is decided to use them, the drafting group suggests the use of mycophenolate because of its better safety profile | √ |
| Although evidence of efficacy of biologic DMARDs in the treatment of rheumatoid arthritis-associated interstitial lung disease is scarce, real-life data suggest that both abatacept and rituximab could be useful in stabilising or improving lung function, particularly in patients with a non-fibrotic radiological pattern | D |
| In the subgroup of patients with rheumatoid arthritis-associated interstitial lung disease with a progressive fibrosing phenotype, the use of nintedanib is recommended, while maintaining background rheumatoid arthritis treatment | B |
DMARDs: slow-acting disease-modulating anti-rheumatic drugs; IL: interleukin; LIP: lymphocytic interstitial pneumonia; NSIP: non-specific interstitial pneumonia; OP: organising pneumonia.
For further information on any of the sections summarised in this article, the full content of the document can be accessed on the SER website (www.ser.es).
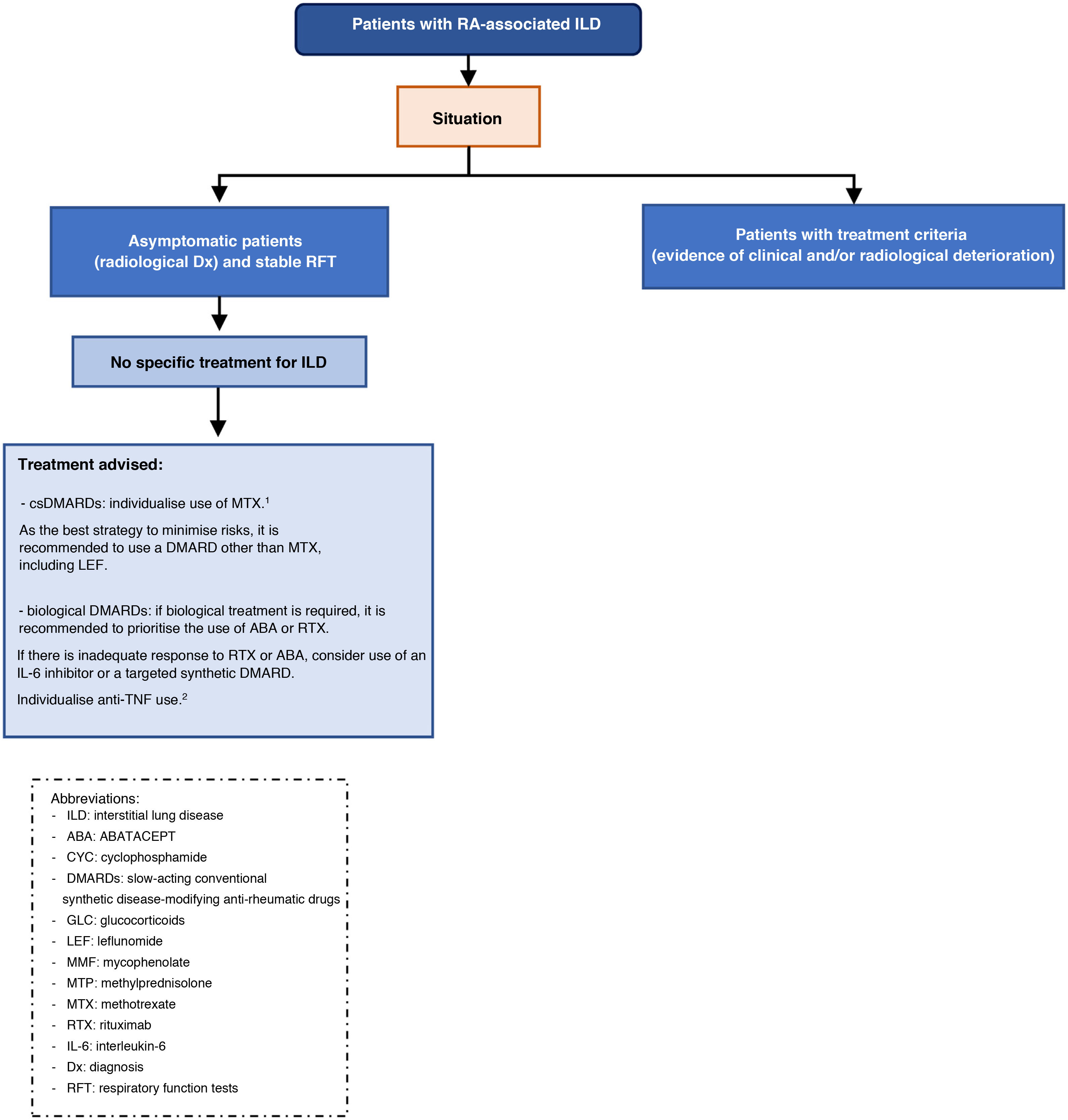
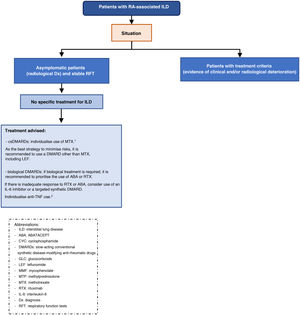
Multidisciplinary approach in the diagnosis and treatment of RA-ILD- □
Recommendation. Multidisciplinary therapeutic management is recommended in the treatment of patients with RA-ILD (recommendation grade √).
The multidisciplinary approach brings multiple advantages in the different stages of care for these patients: it reduces the time to accurately identify ILD, ensures adequate staging of the severity of both ILD and extrapulmonary manifestations, reduces the time between diagnosis and initiation of treatment, improves the choice of pharmacological treatments, ensures pro-activity in the management of lung disease with a holistic approach, reduces the time for decision-making in cases that require intensification or a change in therapeutic strategy, and guarantees a protocolised follow-up reducing variability.8 In addition, diagnostic and therapeutic resources are optimised, reducing costs.8
The therapeutic approach to patients with RA-ILD should be individualised and comprehensive to control underlying disease activity and prevent, where necessary, the progression of fibrotic pulmonary changes. Therefore, it is important that therapeutic decisions are made by consensus between rheumatologists and pneumonologists.
Safety of slow-acting conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs), and targeted synthetic DMARDs (tsDMARDs) in patients with RA-ILD— Is treatment with methotrexate (MTX) or leflunomide (LEF) safe in patients with RA-ILD?
- □
Recommendation. If ILD is present at RA debut, an individualised assessment for the use of MTX is recommended, as there is a risk of drug-induced acute pneumonitis, albeit low (grade A recommendation). In these cases, the drafting group considers that the best strategy to minimise risks is to use another conventional synthetic DMARD whenever possible (recommendation grade √).
- □
Recommendation. In patients with RA, when ILD is diagnosed or worsens during the first year of MTX treatment, MTX should be temporarily discontinued until it is clear whether or not there is a causal relationship (recommendation grade√).
- □
Recommendation. In patients with RA on MTX for more than one year who are diagnosed with ILD, the drug can be maintained as there is no evidence to justify discontinuation (recommendation grade D).
- □
Recommendation. In patients with RA-ILD who are not of Asian descent, LEF can be considered a safe drug (recommendation grade √).
The pulmonary safety of the two most commonly used csDMARDs in the treatment of RA9 has been questioned by cohort studies, a case-control study, case series, and a systematic review of the literature (SRL), suggesting their possible involvement as triggers of ILD, or as a cause of worsening in patients with pre-existing ILD.10–16
As to whether or not there is a risk of induced or exacerbated ILD in RA patients on MTX treatment, according to the conclusions of a meta-analysis of controlled trials (22 studies with 8584 patients; I2: 3%), MTX use is associated with an increased risk of respiratory complications (relative risk [RR]:1.10; 95% confidence interval [CI]: 1.02−1.19), primarily due to an increased risk of infections (RR: 1.11; 95% CI: 1.02–1.21) and acute pneumonitis (RR: 7.81; 95% CI: 1.76–34.72).17 In this meta-analysis, MTX treatment was not shown to increase the risk of death from lung disease (RR: 1.53; 95% CI: .46–5.01) (level of evidence 1+).
Acute MTX pneumonitis usually occurs during the first year of treatment (median 36 weeks)10,18 and is caused by a mechanism of hypersensitivity to the drug, and does not depend on the total cumulative dose.10–14,17,18 It has some characteristics to differentiate it from RA-ILD (Table 2). Criteria have been proposed for its diagnosis that require the systematic exclusion of other causes, especially infections (Table 3).19 Risk factors for its onset include advanced age (>60 years), previous use of other csDMARDs, the presence of insulin-dependent diabetes mellitus (DM) and a history of pre-existing ILD secondary to RA (odds ratio [OR]: 7.1).12,18 The latter is the main argument against its use in patients with RA-ILD.
Main features of methotrexate pneumonitis and rheumatoid-arthritis interstitial lung disease.
| MTX pneumonitis | RA-associated ILD | |
|---|---|---|
| Frequency | .3% | Symptomatic ILD: 10%–29% |
| Clinical course | Acute or sub-acute onset during the first year of treatment | Generally in the first 5–10 years since onset of the disease |
| Often identified late (ILD is often asymptomatic or paucisymptomatic until advanced stages | ||
| Clinical symptoms | Febrile or fever, non-productive cough and dyspnoea often progressing to respiratory failure | Repetitive dry cough and dyspnoea on exertion, which may progress more or less rapidly to respiratory failure |
| Peripheral blood eosinopilia (50%) | ||
| Findings on high resolution chest CT | NSIP or acute alveolar damage (AIP) | UIP > NSIP |
| Less frequent: OP, RB-ILD, CPFE, DIP | ||
| Rarely: LIP, AFOP | ||
| Broncoalveolar lavage | CD4-lymphocyte predominance with increased CD4/CD8 ratio, eosinophilia rarely present | Usually neutrophilic predominance |
AFOP: acute fibrinous and organising pneumonia; AIP: Acute Interstitial Pneumonia; CPFE: combined pulmonary fibrosis + emphysema pattern; CT: computerised tomography; DIP: desquamative interstitial pneumonia; LIP: lymphoid interstitial pneumonia; NSIP: non-specific interstitial pneumonia; OP: organising pneumonia; RB-ILD: respiratory bronchiolitis-associated interstitial lung disease; UIP: usual interstitial pneumonia.
Proposed criteria for the diagnosis of methotrexate pneumonitis.19
| Major criteria | Minor criteria |
|---|---|
| 1. Histopathologic: hypersensitivity pneumonitis | Dyspnoea <8 weeks duration |
| 2. Radiological: interstitial pattern and /or nodular or patchy alveolar infiltrates | Non-productive cough |
| O2 saturation <90% | |
| 3. Microbiological: blood cultures, sputum cultures, broncoalveolar lavage (BAL), and negative serological tests | DLCO <70% |
| Leucocytes <15.000 |
Defined: 1 or 2 and 3 major + 3 of the five minor criteria.
Probable: 2 and 3 major + 2 of the five minor criteria.
Although acute MTX pneumonitis is a clinical reality, the SLR clearly shows that it is overdiagnosed. Thus, a meta-analysis analysing the same issue in other diseases treated with MTX but not involving pulmonary manifestations (7 studies with 1630 patients with psoriasis, psoriatic arthropathy, or inflammatory bowel disease; I2: 0%), no increased risk was observed of respiratory (RR: 1.03; 95% CI: .90–1.17), infectious (RR: 1.02; 95% CI: .88–1.19), or non-infectious (RR: 1.07; 95% CI: .58–1.96) complications.20
Along the same lines, the results of a recently published international multicentre case-control study (410 patients with RA-ILD and 673 controls with RA) do not demonstrate that treatment with MTX is a risk factor for developing this complication (OR: 0.43; 95% CI: .26–.69; p = .0006)21 (level of evidence 2++). Neither was MTX observed to increase the risk of ILD in RA patients in two cohort studies: a Danish population-based study with data from 30,512 patients at 5 years after MTX initiation (hazard ratio [HR]: 1.0; 95% CI:.78−1.27)22 (level of evidence 3), and a multicentre study conducted in the UK in 2692 patients with recent onset RA (1578 exposed to MTX and 1114 unexposed: OR: .85; 95% CI: .49–1.49; p = .578)23 (level of evidence 2+).
Finally, data from the Cardiovascular Inflammation Reduction Trial (CIRT), an RCT that evaluated the possible efficacy of treatment with MTX (≤20 mg/week orally) to prevent the development of cardiovascular events in patients with previous ischaemic heart disease, DM, or metabolic syndrome, confirm that acute pneumonitis is a potential adverse effect of MTX, but its actual prevalence in patients without RA is shown to be very low, at .3%.24–26
Not only has MYX not been confirmed to increase the risk of developing ILD in RA patients, but it even seems to delay its onset and improve its prognosis21,23,27–31 (level of evidence 2++, 2+, 2–, 3). It is now known that moderate or high maintained RA activity according to DAS28 score is one of the risk factors for developing ILD (level of evidence 2++) and one of the prognostic factors associated with increased mortality in RA-ILD (LE: 2+). Therefore, keeping RA activity under control (remission or low activity) prevents the onset of ILD and improves it prognosis.32,33
The possible involvement of LEF in both the development of ILD and the worsening of pre-existing ILD in RA patients is mainly supported by two post-marketing pharmacovigilance studies conducted in Japan34 and Korea,35 and a review of cases published in the literature.15 The prevalence of induced or exacerbated ILD attributed to LEF in these studies was 1 %–1.2%. Most often the clinical presentation occurred within the first 20 weeks of treatment, and mortality ranged from 18% to 41%. The main risk factors for its development were identified as administration of the loading dose of LEF (100 mg/day for 3 days), smoking, low weight, and a history of pre-existing ILD.15,34,35
In a Canadian case-control study nested in a large RA cohort that assessed the risk of ILD with LEF versus MTX and biologic agents, an increased risk of this complication was found only in the group of patients with pre-existing ILD or who had received MTX (RR: 2.6; 95% CI: 1.2–5.6).36 Finally, in a subsequent meta-analysis that analysed this question (8 studies, including 4 RCTs, with a total of 4579 patients; I2: 0%), no increased risk of respiratory (RR: 0.99; 95% CI: .56–1.78), infectious (RR: 1.02; 95% CI: .58–1.82), or non-infectious (RR: .64; 95% CI: .41–.97)37 complications was observed (level of evidence 1+). Seven of the eight included studies were conducted in non-Asian populations.
The overall analysis of these five studies means that LEF should be used with caution in patients of Asian descent, as a risk not yet well defined cannot be ruled out. However, this risk has not been demonstrated in the Caucasian population. In a SLR, the frequency of this complication in Western countries was less than .1%.16 This disparity could be due to pharmacogenetic differences between different racial groups, such as those described in MTX metabolisation pathways. The safety of LEF in combination with abatacept (ABA), rituximab (RTX), or tocilizumab (TCZ) has been confirmed in several open-label studies in patients with progressive RA-ILD in real clinical practice.38–44 All these studies were conducted in European countries (four of them in Spain).38–41
— In patients with RA-ILD, which is the safest biologic DMARD or tsDMARD?
- □
Recommendation. In patients with RA-ILD requiring biologic therapy, ABA or RTX should be used interchangeably as safer options (recommendation grade D).
- □
Recommendation. In patients with RA-PID, in case of contraindication or inadequate response to ABA and RTX, the use of an IL-6 inhibitor or a tsDMARD can be considered (recommendation D).
- □
Recommendation. Recommendation. In patients with RA being treated with an anti-TNF and stable ILD, there is inconclusive evidence to recommend discontinuation if the drug has achieved good control of joint symptoms (recommendation grade √).
Anti-tumour necrosis factor-alpha (anti-TNF) agents, and to a lesser extent TCZ, have been implicated in both the development of ILD and the worsening of pre-existing ILD in patients with RA.
The possible involvement of anti-TNF is mainly supported by systematic reviews of case series and case reports,16,45–47 a retrospective case-control study (with a high risk of bias due to its design, small sample size, and short follow-up),48 and some observational studies.49,50 According to these studies, the prevalence of induced or exacerbated ILD attributed to anti-TNF therapy ranges from .5% to 3%. This complication has been reported with all anti-TNF drugs. It occurs in the first 6 months after biologic initiation (in most cases during the first 20–26 weeks) and is usually severe with a poor outcome (29%–35% mortality). In addition to the classic patterns of interstitial pneumopathy, sarcoid-like lesions have also been described, with the development of non-caseating pulmonary granulomas, especially in patients treated with etanercept (ETC).51,52
The main risk factors involved in the development of this complication are advanced age, pre-existing ILD, and concomitant treatment with MTX or LEF,16,45–47 which makes it difficult to clarify whether there is a causal relationship. This possible causal relationship is further questioned by the recent publication of two retrospective observational studies (both based on data from persons insured in North American mutual health insurance companies) that found no statistically significant differences in the frequency of ILD, either when comparing patients treated with anti-TNF versus patients treated with synthetic DMARDs (adjusted HR anti-TNF versus DMARDs: 1.03; 95% CI: .51–2.07),53 or when comparing the different anti-TNF agents with other biologics (RTX, ABA, or TCZ).28 The latter study also found no significant differences in the risk of hospitalisation for ILD among the different biologic therapies analysed.
These results are consistent with a previous study using a database of 17,598 RA patients, which also found no apparent causal relationship between the development of ILD and different treatments, including ETC and infliximab (IFX).54
In this scenario, even national registries of biologic therapies do not provide consistent results. Thus, according to data from the British Society for Rheumatology Biologics Register (BSRBR), patients treated with an anti-TNF agent have an increased prevalence of ILD (anti-TNF: 2.9% versus synthetic DMARDs: 1.8%; p = .02) and numerically higher mortality attributed to this complication (anti-TNF: 21% versus DMARDs: 7%; p = NS).49 Furthermore, in this register, 5-year mortality in patients with RA-ILD who received a first-line anti-TNF was also higher than those who received RTX: the all-cause mortality rate was 94.8 (95% CI: 74.4–118.7) with anti-TNF and 53 (95% CI: 22.9–104.6) with RTX per 1000 patient-years. The adjusted mortality risk was halved in the first line RTX-treated cohort, although the difference was not statistically significant (HR: .53; 95% CI: .26–1.10).55 In contrast, in a study from the Spanish Registry of Adverse Events of Biological Therapies in Rheumatic Diseases (BIOBADASER), neither the incidence of ILD nor mortality from this complication was higher in patients treated with anti-TNF compared to another cohort of RA patients without biologic therapy (EMECAR).56
Published experience with interleukin-6 (IL-6) inhibitors in patients with RA-ILD is limited to TCZ and also shows conflicting results.28,42,47,57–62 However, treatment with TCZ has been associated with both the development of ILD and the worsening of pre-existing ILD in RA patients. A 2010 SLR analysing this question included data from 3 RCTs with a total of 589 patients47 (level of evidence 2++). Of these, 6 (1%) developed non-infectious pulmonary adverse events, including 3 exacerbations of pre-existing ILD (with one death) and 2 cases of onset ILD. Furthermore, its possible involvement is supported by some clinical cases57,58 (level of evidence 3), and by a post-marketing surveillance study that analysed cumulative safety data from 7901 Japanese patients.59 In this study, the incidence of ILD was 10 cases/1000 patient-years, which is clearly higher than the estimated incidence of the disease (between 1.05 and 4.1 cases/1000 patient-years) (level of evidence 2+). Therefore, the drug’s label includes a specific warning about this risk under special precautions for use.
However, as mentioned above, a recently published retrospective observational study found no statistically significant differences in the frequency of ILD and the risk of hospitalisation for ILD when comparing the different biologic agents (level of evidence 2).28 Another recent case-control study retrospectively reviewed 395 patients with RA treated with TCZ, dividing the sample into two groups: patients with and without ILD.60 None of the patients without ILD at the start of treatment developed this complication de novo. In the subgroup of patients with previous ILD, only 8% worsened. The only risk factor associated with worsening was poor control of inflammatory activity (clinical disease activity index [CDAI] >10 at 24 weeks) (level of evidence 2). Based on these results, the authors suggest that the progression of ILD in these patients seems to be more related to RA activity than to pulmonary toxicity of the drug. Along the same lines, and questioning this causal relationship, a growing number of clinical cases61,62 and a retrospective cohort study42 (level of evidence 3) have been published, showing improved or stabilised lung function in most patients with RA-ILD treated with TCZ, although not all cases had progressive lung disease.
Both the new edition of the SER clinical practice guidelines for the management of patients with rheumatoid arthritis (GUIPCAR)63 and the British Society of Rheumatology guidelines64 agree in their systematic reviews that ABA and RTX are the safest biologic agents in patients with RA-ILD. With ABA, only two cases of apparently drug-related induced or exacerbated ILD have been published to date.65,66 Furthermore, in a post-marketing surveillance study that collected integrated safety data from 3173 patients included in its pivotal studies and followed for up to 8 years, the incidence of ILD in the ABA-treated group was 1.1/1000 patient-years (95% CI: .06–.20), similar to that estimated for RA (level of evidence 2+).67 With respect to RTX, an SLR up to June 2010 identified only three cases of ILD apparently related to the drug in patients with RA47 (level of evidence 2++). Of these 3 patients, one also had lymphoma and one had concomitant Castleman’s disease, and therefore in both cases RTX was combined with other drugs, including chemotherapy. Two of the patients had also been treated with MTX (level of evidence 3). Thereafter no additional cases have been published to date. Furthermore, there is a growing number of open studies (level of evidence 2 or 3) confirming its safety in this scenario.38–41,43,44,68–72 In clinical practice, it is well known that prolonged use of RTX in patients with RA-ILD is associated with a slightly increased risk of respiratory or urinary tract infections, mostly non-serious and related to the development of hypogammaglobulinaemia as an adverse effect of the drug.73
The safety of tsDMARDs at the pulmonary level has not been evaluated in any RCT and the available information is also scarce. In tofacitinib (TOFA) clinical trials and in the post-marketing phase, cases of ILD (some of them fatal) have been reported, mainly in Asian patients. For this reason, a specific mention of this risk is included on the drug's data sheet under special warnings and precautions for use. However, a post hoc analysis has recently been published that specifically investigated this issue in 7061 RA patients included in 21 TOFA RCTs, estimating an incidence of ILD of 1.8 cases/1000 patient-years74 (level of evidence 2+). This incidence is similar to that estimated in RA and remained stable over time. The data from this study are consistent with those of a previous meta-analysis that analysed the pulmonary safety of tsDMARDs75 (level of evidence 1+). According to this work, which included 47 RCTs, 25 observational studies, and 7 post-marketing surveillance studies (with a total of 159,652 patients), treatment with JAK inhibitors increases the risk of different types of respiratory infection, but not of non-infectious pulmonary complications, including ILD. Some clinical cases of RA-ILD treated with TOFA have also been published without evidence of pulmonary worsening.76 With baricitinib there are no alerts to date on its possible involvement in the development of ILD or in the worsening of pre-existing ILD, following analysis and long-term follow-up of patients included in their RCTs.77
In conclusion, both ABA and RTX are safe drugs in patients with RA-ILD, therefore the drafting group recommends prioritising their use as first-choice biologic agents without distinction. Based on the available evidence, IL-6 inhibitors or tsDMARDs would not be formally contraindicated either, although their use should be individualised.
The causality evidence supporting the possible involvement of anti-TNFs is for the most part of low quality and difficult to interpret due to confounding biases. Moreover, several studies of the same methodological quality have obtained completely opposite results, and therefore the drafting group considers it impossible to make a conclusive recommendation in one direction or the other. However, the fact that this complication has been described in patients treated with anti-TNF monotherapy, or that cases of ILD have been described in patients treated for ulcerative colitis or spondyloarthritis,47 obliges caution, as it cannot be ruled out that there is a risk as yet not well defined and probably overestimated. Therefore, a strategy of risk minimisation is advised, avoiding its use in patients with RA-ILD in whom biologic therapy is to be initiated. In patients on anti-TNF therapy who are diagnosed with ILD, there is no conclusive evidence to recommend discontinuation if the drug has achieved good control of joint symptoms, as long as it is verified that the ILD remains stable.
Efficacy of glucocorticoids (GLC), csDMARDs and other immunosuppressants, biologic DMARDs, tsDMARDs and antifibrotic agents in the treatment of RA-associated ILD- □
Recommendation. In patients with RA-ILD with an inflammatory radiological pattern in whom GLC treatment is considered necessary, their use is always recommended at the lowest dose and for the shortest possible time (recommendation grade √).
- □
Recommendation. The drafting group considers that the available evidence is insufficient to issue a conclusive recommendation on the use of immunosuppressants in the treatment of RA-ILD (recommendation grade D). If it is decided to use them, the drafting group suggests the use of mycophenolate because of its better safety profile (recommendation grade √).
- □
Recommendation. Although evidence of efficacy of biologic DMARDs in the treatment of RA-ILD is scarce, real-life data suggest that both ABA and RTX could be useful in stabilising or improving lung function, particularly in patients with a non-fibrotic radiological pattern (recommendation grade D).
- □
Recommendation. In the subgroup of patients with RA-ILD with a progressive fibrosing phenotype, the use of nintedanib is recommended, while maintaining background RA treatment (recommendation grade B).
In clinical practice, GLCs are commonly used in the treatment of RA-ILD, in combination or not with a csDMARD or immunosuppressant. There are no RCTs that have evaluated the efficacy of GLCs in this complication, and therefore the evidence supporting their use is based primarily on clinical experience and real-life data. The drafting group endorses their use in ILD patterns with a relevant inflammatory component: non-specific interstitial pneumonia (NSIP), organising pneumonia, lymphoid interstitial pneumonia, as well as in respiratory bronchiolitis associated with ILD, and in desquamative interstitial pneumonia if no improvement after smoking cessation or when it occurs in non-smoking patients. Its use in fibrotic patterns (usual interstitial pneumonia [UIP] and fibrosing NSIP) is questionable, except in acute exacerbations.
Because of their adverse effect profile, the drafting group recommends the use of GLCs at the lowest dose and for the shortest duration possible. Prolonged treatment with prednisone (PDN) doses >7.5 mg/day increases the risk of serious infections and worsens cardiovascular risk and mortality in patients with RA.78–82
A strategy to reduce iatrogenesis would be to apply new knowledge on the mechanisms of action of GLCs in daily clinical practice. It is currently known that they exert their anti-inflammatory action via two pathways: a classic genomic and a non-genomic pathway.83,84 The genomic pathway has a slow onset of action, a persistent effect (which is responsible for the adverse effects of GLCs), and is 100% active at PDN doses of 30 mg/day. Therefore, if we give doses higher than 30 mg/day (the classic 1 mg/kg/day) we only manage to increase toxicity without substantially increasing its anti-inflammatory effect. In contrast, the non-genomic pathway exerts a much more intense and rapid anti-inflammatory action. This pathway begins to activate appreciably at 100 mg/day of methylprednisolone, with a maximum effect above 250 mg/day. Intravenous pulse therapy above 100−250 mg/day for 3 days has greater efficacy and lower toxicity than prolonged treatment with high-dose PDN.
Based on this knowledge, it is now recommended not to exceed 30 mg/day of PDN, regardless of the patient's clinical picture. If necessary due to initial severity or acute exacerbation, methylprednisolone pulses (125 mg or 250 mg per day for 3 days, or 500 mg/day in the most severe cases) will be considered, which are more effective and faster (generally in less than 24 h) than prolonged treatment with doses of 1 mg/kg/day.
Cyclophosphamide (CF), azathioprine (AZA), mycophenolate (MMF) and cyclosporine A (CsA) have also been used in the treatment of RA-ILD. The studies that have evaluated the efficacy of these immunosuppressants are of very low methodological quality (clinical cases, case series, and some observational studies) (level of evidence 3),85–97 and therefore the available evidence is insufficient to make a conclusive recommendation on their use. If it is decided to use an immunosuppressant, MMF appears to have the best safety profile. In a multicentre study that analysed mortality over the last 25 years in a cohort of 290 patients with RA-ILD versus 290 age- and sex-matched controls with RA without this complication, mortality, both overall and respiratory, was higher in patients treated with CF or AZA than in those treated with MMF.98
No RCTs evaluating the efficacy and safety of biologic DMARDs or tsDMARDs in the treatment of RA-ILD have been conducted to date. Published experience with biologic DMARDs is generally limited to observational studies with RTX39,40,44,70–72 and ABA38,43,68,69,99,100 (level of evidence 2 or 3). In addition to having no control group, the limitations of these studies include the fact that not all patients included had active ILD, as evidenced by the lack of a protocolised assessment with RFT in some of the cases. Despite these limitations, the real-life observational studies are consistent and suggest that both ABA and RTX, in addition to being safe, also appear to be potentially useful in the treatment of RA-ILD, stabilising and even improving respiratory function parameters and HRCT findings in at least two-thirds of patients, including cases whose ILD had worsened despite previous treatment with GLC and csDMARDs or immunosuppressants and patients with chronic fibrosing ILD with a progressive phenotype.39
Clinical cases61,62 and a retrospective observational study42 have been published with TCZ. In this study, which included 28 patients treated with TCZ (23 in monotherapy), improvement or stabilisation in RFT was observed in 76% of cases (20% improvement) and in radiological changes on HRCT in 92.8% at the end of 30 months’ follow-up (median) (level of evidence 3).
Indirectly supporting the possible beneficial effect of non-anti-TNF biologic agents (RTX, ABA, and TCZ) in the treatment of RA-ILD, another study in Spain demonstrates that there is less lung progression with these drugs than that observed with anti-TNF.41
Published experience with tsDMARDs is limited to a few clinical cases of RA-ILD treated with TOFA without evidence of pulmonary worsening.76
Of the two antifibrotic drugs marketed for the treatment of idiopathic pulmonary fibrosis (IPF) (nintedanib and pirfenidone), only nintedanib has so far been approved by the Spanish Agency of Medicines (AEMPS) for the treatment of RA-ILD with a progressive fibrosing phenotype.101 The criteria defining this phenotype are shown in Table 4. Approval for this indication is based on data from the phase III INBUILD RCT,102 which evaluated the efficacy of the drug in different types of progressive fibrosing IPD other than IPF, including a group of patients with SAD-ILD, mostly with RA or scleroderma (level of evidence 1++). Of the patients, 69.5% received SLN at doses <20 mg/day and 78% received concomitant treatment with csDMARDs (MTX, LEF, or antimalarials) and/or biologic DMARDs (ABA, TCZ, ETC, IFX, or adalimumab). In addition, at 6 months into the trial, salvage therapy with AZA, MMF, cyclosporine A, tacrolimus, RTX, CF, or PDN >20 mg/day was allowed in case of pulmonary or baseline disease worsening.102–104 At the end of 52 weeks of treatment, nintedanib was able to slow the decline in forced vital capacity (FVC) in this group of patients by 58% compared to placebo, although there were no significant differences between groups in quality of life as measured by the King’s Brief Interstitial Disease (K-BILD) questionnaire, or in the frequency of first acute exacerbation or mortality.102 The safety profile of the drug was similar to that already known, and no new safety alerts emerged when administered in combination with GLCs, csDMARDs, immunosuppressants, and/or biologic DMARDs.
Definition of progressive fibrosing ILD according to the INBUILD randomised controlled trial.102
| Fibrotic changes affecting more than 10% of the lung parenchyma on high-resolution chest computed tomography (HRCT) and any of the following: |
| • A decrease in FVC > 10% in the 24 previous 24 months despite treatment |
| • A decrease in FVC between 5%–10% with evidence of progression of fibrotic changes on HRCT in the previous 24 months despite treatment |
| • A decrease in FVC between 5%–10% with worsening of respiratory symptoms (dyspnoea and dry cough) in the previous 24 months despite treatment |
| • Worsening of dyspnoea with fibrosing progression on HRCT in the previous 24 months despite treatment |
DLCO: diffusing capacity of the lung for carbon monoxide; FVC: forced vital capacity.
Some experts also include as a definition of progression a decrease in FVC between 5%–10% with worsening of DLCO greater than 15% in the previous 24 months despite treatment (George PM, et al. Lancet Respir Med 2020;8:925−34).
This is the first official document produced by the SER and SEPAR with specific recommendations for the therapeutic approach to RA-ILD, with the aim of assisting clinicians directly involved in the management of the condition in their decision-making.
The document clarifies the safety of MTX and LEF, biologic DMARDs and tsMARDs. One of the most important conclusions is that the actual risk of MTX-induced acute pneumonitis is confirmed to be low (.3%),24–26 and the drug has not been shown to significantly increase the risk of developing ILD in RA patients.21,23,27–33 Typically, MTX pneumonitis has been erroneously overdiagnosed, thereby condemning the use of a drug that has not only shown great efficacy in the control of joint symptoms, but also seems to improve the prognosis of the lung disease. It should also be noted that in patients with RA-ILD who are not of Asian descent, LEF can be considered a safe drug,16,36,37 and that both ABA and RTX are confirmed as the biologic drugs of first choice in these patients due to their safety.38–41,43,44,47,63–72
Furthermore, there is a growing number of open studies showing that ABA and RTX also appear to be useful in the treatment of this complication, stabilising and even improving respiratory function parameters and HRCT findings in two thirds of patients, particularly in those with a non-fibrotic radiological pattern.38–40,43,44,69–72,99,100
Finally, nintedanib has been added to the therapeutic arsenal for the treatment of fibrosing forms of RA-ILD (UIP and fibrotic NSIP), preferably in combination with background treatment of the disease. According to the indication approved by the AEMPS, it can only be used in cases with RA-ILD with a progressive fibrosing phenotype despite treatment; therefore, as second- or third-line therapy.101
Finally, some studies have shown that the UIP pattern is less responsive to treatment and has a worse prognosis compared to non-UIP patterns.39,43,44 However, although less responsive, there is sufficient evidence confirming the efficacy of immunosuppressive/biologic treatments in patients with UIP, both in real-life data,38–40,43,44,69–72,99,100 and in RCTs on scleroderma.105,106 This evidence justifies their use in patients with RA-ILD with this pattern, whether or not combined with antifibrotic treatment.
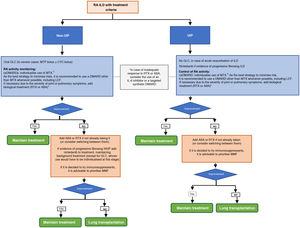
There is a clear need for further clinical research to help clarify the safety and efficacy of different drugs in the management of patients with this complication. Pending further studies, a treatment algorithm for patients with RA-ILD and UIP or non-UIP pattern is proposed in Fig. 1. In no case, should either the algorithm or the recommendations be considered restrictive rules of use but as an aid to decision making. We recognise that the guidelines are intended to provide guidance only and that many of the recommendations in the document are derived from the consensus opinion of the expert panel and from studies with a low level of evidence.
Treatment algorithm for rheumatoid arthritis-associated interstitial lung disease (UIP or non-UIP pattern).1In patients with RA under treatment with MTX for more than 1 year, diagnosed with ILD, the drug can be maintained as there is no evidence to justify its discontinuation.,
2In patients on anti-TNF therapy and with stable ILD, there is no conclusive evidence to recommend discontinuation if the drug has achieved good control of joint symptoms.
Protection of humans and animals. The authors declare that no experiments on humans or animals have been conducted for this research.
Data confidentiality. The authors declare that no patient data appear in this article.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
FundingThis project was funded by the Spanish Society of Rheumatology (SER) and the Spanish Society of Pneumology and Thoracic Surgery (SEPAR).
AuthorshipAll the authors made substantial contributions to a) the conception and design of the study and the data analysis, b) drafting of the article or critical review of the intellectual content, and c) final approval of the version presented.
Conflict of interestsJavier Narváez has received funding from Bristol, Kern, Lilly, Pfizer, Roche, and Sanofi for attendance at courses/congresses; honoraria from Abbvie, Bristol, Boehringer, Gebro Pharma, GSK, Kern, Lilly, Pfizer, Sanofi, and Sobi for presentations and consultancies for pharmaceutical companies or other technologies, and has participated in clinical trials funded by Boehringer, GSK, Janssen, Roche, and Vorso.
Petra Díaz del Campo Fontecha has no conflict of interests to declare.
Noé Brito García has no conflict of interests to declare.
Gema Bonilla has received funding from Abbvie and Janssen for attendance at courses/congresses and honoraria from Abbvie, BMS, Boehringer, Novartis, Roche, and UCB for presentations.
Myriam Aburto has received funding from Boehringer and Roche for attendance at courses/congresses; honoraria from BMS, Boehringer, and Roche for presentations; and financial support from Boehringer for consultancy for pharmaceutical companies and other technologies. She has also participated in a Boehringer clinical trial.
Iván Castellví has received funding from Actelion, BMS, Boehringer, Kern, Lilly, Novartis, Pfizer, and Sanofi for attendance at courses/congresses; honoraria from Actelion, BMS Boehringer, Nordic, Pfizer, and Roche for presentations; funding from Actelion for educational programmes or courses, and has received financial support from Actelion, Boehringer, Gebro, and Kern for consultancy for pharmaceutical companies and other technologies.
Esteban Alberto Cano Jiménez has received funding from Boehringer and Roche for attendance at courses/congresses; honoraria from Boehringer, Chiesi, Roche, and Rovi for presentations; and has received financial support from Galapagos for consultancy for pharmaceutical companies and other technologies.
Natalia Mena Vázquez has received funding from Abbvie, Novartis, Pfizer, and Roche for attendance at courses/congresses; honoraria from Abbvie, MSD, Pfizer, and Roche for presentations, and has received funding from Abbvie and MSD for educational programmes and courses.
María Asunción Nieto has received funding from Boehringer, MSD, Roche, and TEVA for attendance at courses/congresses; honoraria from Boehringer, BMS, and Roche for presentations and for educational programmes or courses; funding from Boehringer and Roche for participation in research and has received financial support from Roche, Boehringer, and Astra for consultancy for pharmaceutical companies and other technologies.
Ana María Ortiz has received funding from Lilly, Novartis, Pfizer, Roche, Sandoz, Sanofi, and UCB for attending courses/congresses; honoraria from Abbvie, Lilly, MSD, and Roche for presentations; funding from Bristol, Gilead, MSD, and Roche for participating in research and has received financial support from Abbvie, Gilead, Janssen, Lilly, and Pfizer for consultancy for pharmaceutical companies and other technologies. She has also participated in clinical trials for MSD/Amgen and Bristol.
Claudia Valenzuela has received funding from Boehringer and Roche for attendance at courses/congresses; honoraria from Roche for presentations; and has received financial support from Boehringer, Galapagos, and Roche for consultancy for pharmaceutical companies and other technologies.
Miguel Ángel Abad Hernández has no conflict of interests to declare.
Isabel Castrejón has no conflict of interests to declare.
María Correyero Plaza has no conflict of interests to declare.
Félix Manuel Francisco Hernández has no conflict of interests to declare.
María Vanesa Hernández has no conflict of interests to declare.
José Antonio Rodríguez Portal has received funding from Roche and Sanofi for attendance at courses/congresses; honoraria from Roche, Boehringer, and Janssen for presentations; financial support from Bristol, Janssen, Roche, and Boehringer for consultancy for pharmaceutical companies and other technologies; and has participated in clinical trials funded by Gilead, Roche, and Boehringer.
The group of experts in this study would like to thank Mercedes Guerra Rodríguez, documentalist at the SER, for her collaboration in the evidence search strategies.
They would also like to expressly thank Dr. Raimon Sanmartí Sala and Dr. Gustavo Enrique Zabert, as expert reviewers of the document, for their critical review and contributions to it.
They would also like to thank Dr. Federico Díaz González, Director of the SER Research Unit, for his participation in the review of the final manuscript and for helping to preserve the independence of this document.
Please cite this article as: Narváez J, Díaz del Campo Fontecha P, Brito García N, Bonilla G, Aburto M, Castellví I, et al. Recomendaciones SER-SEPAR para el manejo de la enfermedad pulmonar intersticial difusa asociada a la artritis reumatoide. Parte 2: tratamiento. Reumatol Clin. 2022;18:501–512.