In this study, we aimed to evaluate LIF levels and its possible relationship with disease activity in patients with Takayasu's (TAK) and Giant cell arteritis (GCA) patients.
Materials and methods23 Takayasu's arteritis, 9 Giant cell arteritis patients and 25 healthy volunteers were included in the study. Serum LIF levels were measured ELISA.
ResultsThe mean age of Giant cell arteritis patients was statistically significantly higher than the other groups (p<0.001). The rate of women was found to be higher in Takayasu's arteritis (p=0.021). When healthy control, patients with GCA and Takayasu arteritis were compared, there was a difference in LIF values (p=0.018). In subgroup analyzes, LIF values were found to be higher in GCA patients compared to healthy controls (p<0.05). There was no statistically significant correlation between LIF and CRP (Rho=−0.038, p=0.778), ESR (Rho=0.114, p=0.399) and ITAS (Rho=−0.357, p=0.094). While CRP was statistically significantly higher in patients with disease activity (p=0.003), there was no statistically significant difference between patients in terms of ESR and LIF values. While there was a statistically significant relationship between CRP (OR=1.19 [1.03–1.37], p=0.018) and disease activity in univariate analyses, no statistically significant variable was found in multivariable analyses.
ConclusionsLIF values were significantly higher in patients with Giant cell arteritis compared to healthy controls.
En este estudio nos propusimos evaluar los niveles de factor inhibidor de la leucemia (LIF) y su posible relación con la actividad de la enfermedad en pacientes con Takayasu (TAK) y arteritis de células gigantes (ACG).
Materiales y métodosEn el estudio se incluyeron 23 pacientes con arteritis de TAK, nueve con ACG y 25 voluntarios sanos. Los niveles séricos de LIF se midieron mediante ELISA.
ResultadosLa edad media de los pacientes con ACG fue significativamente superior a la de los demás grupos (p<0,001). Se observó que la tasa de mujeres era superior en la arteritis de TAK (p=0,021). Cuando se compararon controles sanos, pacientes con ACG y arteritis de TAK, se observaron diferencias en los valores de LIF (p=0,018). En los análisis de subgrupos, se observó que los valores de LIF eran más elevados en los pacientes con ACG que en los controles sanos (p<0,05). No hubo correlación estadísticamente significativa entre el LIF y la reacción en cadena de la polimerasa (PCR) (Rho=-0,038, p=0,778), la velocidad de sedimentación globular (VSG) (Rho=0,114, p=0,399) y el ITAS (Rho=-0,357, p=0,094). Aunque la PCR fue estadísticamente significativa en los pacientes con actividad de la enfermedad (p=0,003), no hubo diferencias estadísticamente significativas entre estos en cuanto a los valores de VSG y LIF. Aunque hubo una relación estadísticamente significativa entre la PCR (odds ratio [OR]=1,19 [1,03-1,37], p=0,018) y la actividad de la enfermedad en los análisis univariantes, no se encontró ninguna variable estadísticamente significativa en los multivariantes.
ConclusionesLos valores de LIF fueron significativamente más elevados en los pacientes con arteritis de células gigantes en comparación con los controles sanos.
Takayasu's arteritis (TAK) is a chronic idiopathic inflammatory and obliterative disease characterized by large vessel vasculitis affecting the aorta and its main branches. It is known that the expression of interleukin (IL)-1 and IL-6 is increased in aortic tissues in patients with TAK and serum levels of IL-6 are correlated with disease activity.1,2 Temporal or Giant cell arteritis is an inflammatory systemic vasculitis involving medium and large arteries. The systemic manifestations of Giant cell arteritis are characteristic and vascular involvement may be common, but the most common involvement is in the extracranial branches of the carotid artery and often in the temporal artery. The feared complication of Giant cell arteritis is vision loss.3 The effects of infections on disease development and the importance of T cell-derived cytokines are known in Takayasu's and Giant cell arteritis. Macrophages are found in various layers of the vessel wall and secrete IL-6, IL-1 and reactive oxygen radicals. Oxidative stress leads to an increase in endothelial and smooth muscle damage, and by increasing metalloproteinases, leading to the breakdown of the elastic membranes of the involved vessel. IL-6 is a proinflammatory cytokine. After being synthesized by macrophages at the initial stage of inflammation, IL-6 enters to the liver via the bloodstream, followed by C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), which are usually high in the early phase of the disease.4–7 Many studies have shown the relationship of IL-6 with disease activity and pathogenesis in Takayasu's and Giant cell arteritis. Tocilizumab, a monoclonal antibody that inhibits IL-6 receptor, is used as a treatment alternative in resistant patients.4,8–10
Leukemia inhibitory factor (LIF) is a cytokine that is a member of the interleukin-6 cytokine family and has the capacity to stimulate acute phase reactants synthesis.11,12 LIF is induced under inflammatory stress and LIF expression is also regulated by many cytokines (IL-1α, IL-1β, TGF-β, TNF-α).12 LIF is known to be a multifunctional protein with wide biological functions in the neuronal, hepatic, endocrine, inflammatory, and immune systems.12,13 It has been detected especially in the serum of patients with Giant cell arteritis. It has also been reported that LIF levels are significantly increased in patients with elevated IL-6 and CRP.13
In this study, we aimed to investigate whether LIF can be used as a biomarker in the evaluation of disease activity in Takayasu's and Giant cell arteritis.
Materials and methodsBetween April 2019 and May 2021, 23 Takayasu's arteritis, 9 Giant cell arteritis patients and 25 healthy volunteers followed in Ankara University Faculty of Medicine, İbni-Sina Hospital, Department of Rheumatology were included in the study. Patients with a diagnosis of Takayasu's arteritis and Giant cell arteritis were selected according to the American Rheumatology Society (ACR) classification criteria.14,15 Patients’ acute phase reactants, including ESR, and CRP, were measured. Smoking, comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, etc.), diagnosis dates, and medications used in treatment were recorded. The activities of Takayasu patients were evaluated with the Indian Takayasu clinical activity score (ITAS) (Active disease was defined as an ITAS score of 2 or higher for Takayasu patients)16 and the activities of Giant cell arteritis patients with the Birmingham vasculitis activity scores (BVAS) version 3.17 Active disease was defined as the presence of clinical findings that required glucocorticoid initiation or dose increase, or the presence of weakness, fatigue, or musculoskeletal system findings together with acute phase elevation in patients with Giant cell arteritis. LIF levels were measured in pg/L by the USCN human LIF (Cloud-Clone Corp., Wuhan, China) using the quantitative double sandwich enzyme immunoassay technique according to the manufacturer's instructions.
Statistical analysisData were analyzed with SPSS version 25 software (SPSS, Chicago, USA). Categorical data were expressed by frequencies and percentages. The variables’ fitness to normal distribution was examined using visual (histogram and probability graphics) and analytical methods. When comparing quantitative data, One-Way ANOVA test was used for normally distributed data, and Kruskal–Wallis test was used for data that did not show normal distribution. Chi-squared or Fisher's exact test was used when comparing rates in categorical data. Spearman's correlation test was used for analysis of relationship between LIF, CRP, ESR and ITAS score. Multivariable analysis was performed by logistic regression analysis, which modeled independent predictors to predict active disease, by using possible factors identified in univariate analyses. (Variables with a p-value<0.25 on univariate analysis were subsequently entered into the final multivariable model.) A multiple linear regression model was used to identify independent predictors of LIF value. The model fit was assessed using model used appropriate residual and goodness-of-fit statistics. For effect size=1.41 and alpha error=0.05, the power of current study with the G*Power 3.1.9.4 program was calculated as 97%. p<0.05 was considered statistically significant.
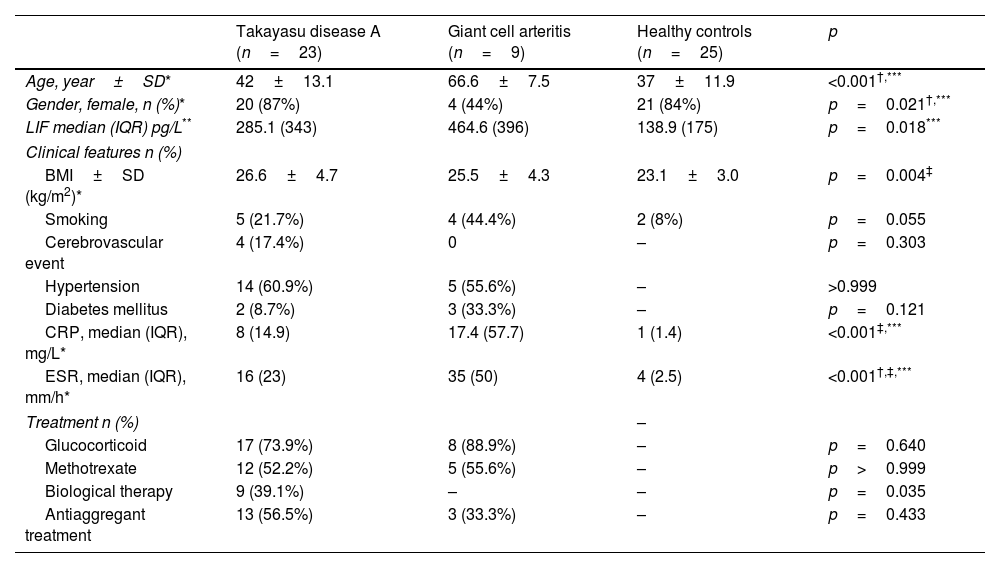
ResultsThe mean age of Giant cell arteritis patients was statistically significantly higher than the other groups (p<0.001) (Table 1). In the patient group (Takayasu's+Giant cell arteritis), the mean age was significantly higher and statistically significant when compared to healthy controls (48.9±16.2 vs 37.1±11.9, p=0.003). Female patients in Giant cell arteritis were less (p=0.021). There was no difference in body mass index (BMI) between patients with Takayasu's and Giant cell arteritis, but BMI was lower in the control group compared to patients with Takayasu's arteritis (p=0.013). When the patient group (Takayasu's+Giant cell arteritis) (mean: 26.3, SD: 4.6) was compared with the control group (mean: 23.1, SD: 3), BMI was lower in the control group (p=0.004). CRP and ESR were statistically significantly lower in the healthy group (p=0.001) (Table 1).
Clinical, laboratory and demographic characteristics of Takayasu and Giant cell arteritis patients and healthy controls.
| Takayasu disease A (n=23) | Giant cell arteritis (n=9) | Healthy controls (n=25) | p | |
|---|---|---|---|---|
| Age, year±SD* | 42±13.1 | 66.6±7.5 | 37±11.9 | <0.001†,*** |
| Gender, female, n (%)* | 20 (87%) | 4 (44%) | 21 (84%) | p=0.021†,*** |
| LIF median (IQR) pg/L** | 285.1 (343) | 464.6 (396) | 138.9 (175) | p=0.018*** |
| Clinical features n (%) | ||||
| BMI±SD (kg/m2)* | 26.6±4.7 | 25.5±4.3 | 23.1±3.0 | p=0.004‡ |
| Smoking | 5 (21.7%) | 4 (44.4%) | 2 (8%) | p=0.055 |
| Cerebrovascular event | 4 (17.4%) | 0 | – | p=0.303 |
| Hypertension | 14 (60.9%) | 5 (55.6%) | – | >0.999 |
| Diabetes mellitus | 2 (8.7%) | 3 (33.3%) | – | p=0.121 |
| CRP, median (IQR), mg/L* | 8 (14.9) | 17.4 (57.7) | 1 (1.4) | <0.001‡,*** |
| ESR, median (IQR), mm/h* | 16 (23) | 35 (50) | 4 (2.5) | <0.001†,‡,*** |
| Treatment n (%) | – | |||
| Glucocorticoid | 17 (73.9%) | 8 (88.9%) | – | p=0.640 |
| Methotrexate | 12 (52.2%) | 5 (55.6%) | – | p>0.999 |
| Biological therapy | 9 (39.1%) | – | – | p=0.035 |
| Antiaggregant treatment | 13 (56.5%) | 3 (33.3%) | – | p=0.433 |
LIF: leukemia inhibitory factor, BMI: body mass index, ITAS: Indian Takayasu's Clinical Activity Score, BVAS. Birmingham Vasculitis Activity Score, CRP: C-reactive protein, ESR: erythrocyte sedimentation ratio.
LIF value was found to be significantly higher in Giant cell arteritis than healthy control (p=0.018) (Table 1). LIF value (Takayasu's+Giant cell arteritis median: 286.1, IQR: 392.8 and healthy group median: 138.9, IQR: 175.2) were significantly higher in the patient group when compared healthy group (p=0.014).
While CRP was statistically significantly higher in patients with disease activity (p=0.003), there was no statistically significant difference between patients in terms of ESR and LIF values (Table 2).
Comparison of acute phase reactants and LIF values of active and inactive patients.a
| Active disease (n=19) | Inactive disease (n=13) | p | |
|---|---|---|---|
| CRP, mg/L | 17.4 (18.7) | 5.5 (6.9) | 0.003 |
| ESR, mm/h | 28 (35) | 18 (13.5) | 0.195 |
| LIF, pg/L | 283.8 (423.7) | 438.9 (301.7) | 0.223 |
There was no statistically significant correlation between LIF and CRP (Rho=−0.038, p=0.778), ESR (Rho=0.114, p=0.399) and ITAS (Rho=−0.357, p=0.094).
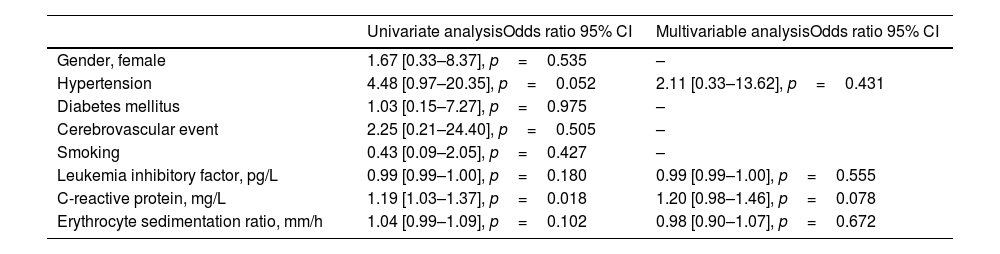
Univariate and multivariable analyzes of factors associated with active disease are presented in Table 3. While there was a statistically significant relationship between CRP (OR=1.19 [1.03–1.37], p=0.018) and disease activity in univariate analyses, no statistically significant variable was found in multivariable analyses.
Factors associated with active disease.
| Univariate analysisOdds ratio 95% CI | Multivariable analysisOdds ratio 95% CI | |
|---|---|---|
| Gender, female | 1.67 [0.33–8.37], p=0.535 | – |
| Hypertension | 4.48 [0.97–20.35], p=0.052 | 2.11 [0.33–13.62], p=0.431 |
| Diabetes mellitus | 1.03 [0.15–7.27], p=0.975 | – |
| Cerebrovascular event | 2.25 [0.21–24.40], p=0.505 | – |
| Smoking | 0.43 [0.09–2.05], p=0.427 | – |
| Leukemia inhibitory factor, pg/L | 0.99 [0.99–1.00], p=0.180 | 0.99 [0.99–1.00], p=0.555 |
| C-reactive protein, mg/L | 1.19 [1.03–1.37], p=0.018 | 1.20 [0.98–1.46], p=0.078 |
| Erythrocyte sedimentation ratio, mm/h | 1.04 [0.99–1.09], p=0.102 | 0.98 [0.90–1.07], p=0.672 |
CI; confidence internal.
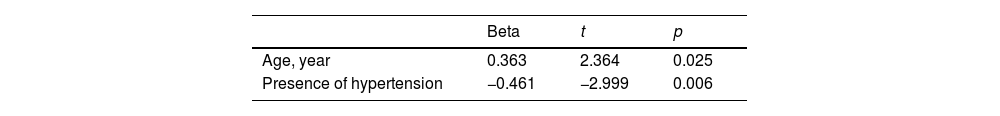
Possible clinical and demographic characteristics that may affect the LIF value were analyzed by multiple linear regression analysis. There was a positive relationship with age with LIF value, and a negative relationship with the presence of HT (Table 4).
DiscussionIn our study, LIF values were found to be significantly higher in Giant cell arteritis patients compared to the healthy control group. In a previous study, LIF was detected in the serum of 11 of 20 patients with Giant cell arteritis. Also, some patients who exhibited elevated IL-6 and CRP levels had significantly elevated LIF levels; however, it has been reported that no correlation was observed between circulating LIF levels and IL-6 or CRP levels.13 The role of LIF in Takayasu's arteritis has not been extensively studied. In a study which four patients with Takayasu's arteritis and one patient with Giant cell arteritis were included, LIF could not be detected.17
The relationship of IL-6 with disease activity and pathogenesis in Takayasu's arteritis and Giant cell arteritis has been shown in many studies, and anti-IL-6 (tocilizumab) is effectively used in the treatment of the disease.8,10 The fact that LIF cytokine, a member of the IL-6 cytokine family, was found to be high in our study when compared to controls, it suggests that it is one of the cytokines associated with the diseases. When we investigated whether LIF could be used as a biomarker in the evaluation of disease activity in Takayasu, no correlation was found between ITAS activity scores and LIF values. Tocilizumab was used as a biological therapy in 9 of patients with Takayasu's arteritis. Since tocilizumab blocks IL-6, these patients may also have low LIF values. Therefore, no relationship could be found between LIF value and ITAS scores in patients with Takayasu's arteritis in the current study. It may also explain why LIF values are not statistically significant in patients with Takayasu compared to healthy patients. Keser et al. suggested that systemic inflammation pathways and vessel wall inflammation pathways do not match each other, and that the two inflammation pathways may progress through different cytokines.4 The fact that LIF values were not correlated with active patients indicates that this cytokine is associated with different inflammation pathways constituting disease activity. New cytokines should be investigated to determine disease activity in this patient group in which inflammation is dominant at the vascular level, not at the systemic level. Many cytokines to determine the serum profiles of inflammatory cytokines and correlations with disease activity in patients with Takayasu's arteritis to allow early detection of the presence and activity of Takayasu's arteritis and to initiate medical treatment during this period when it is more susceptible to treatment; Serum TNF-α, IFNγ, IL-6, IL-12 and IL-18 levels have been investigated.2 In addition, different molecule that may reflect disease activity such as pentraxin-3, ghrelin and leptin are still being investigated.18
It has also been reported that LIF levels are significantly increased in patients with high IL-6 and CRP.13 In our study, we found no correlation between serum LIF level and CRP and ESR. Since intensive immunosuppressive therapy is required in the active periods of patients in Takayasu's arteritis, close follow-up of the patients is of great importance. However, there is currently no gold standard method for determining active disease. Acute phase reactants such as ESR and CRP, which are markers of inflammation, may increase in active disease but do not show a direct correlation with disease activity.4,8,9
The important limitations of our study are that it had a cross-sectional design and that it was performed with a relatively small number of patients. The strength of our study is that it is one of the few studies investigating LIF levels in large vessel vasculitis.
In conclusion, the inadequacy of clinical signs/symptoms and acute phase reactants such as ESR and CRP values to show vascular inflammation necessitates investigation of different cytokine parameters in order to evaluate disease activity more clearly. In our study, we found higher LIF values in patients with Takayasu's and Giant cell arteritis compared to healthy controls. We also showed that there was no difference in serum LIF levels between active patients and inactive patients.
Informed consentThis study was approved by the Ankara University Faculty of Medicine Ethics Committee (05-404-19, March 2019).
FundingThis research was supported by the Scientific Research Project Coordinator. Unit of Ankara University. Project code: 20B0230002.
Conflict of interestThere is no conflicts of interest with respect to the authorship and/or publication of this article.