A 15 years old caucasian girl was diagnosed with systemic juvenile idiopathic arthritis (sJIA) by the age of four and was initially treated with nonsteroidal anti-inflammatory drugs and oral corticosteroids (prednisolone 1mg/kg/day). Although there was some clinical improvement, the disease progressed with 1–2 articular and systemic exacerbations a year and, in the beginning of the year 2007, when she was 7 years old, she started weekly methotrexate (10mg/m2).
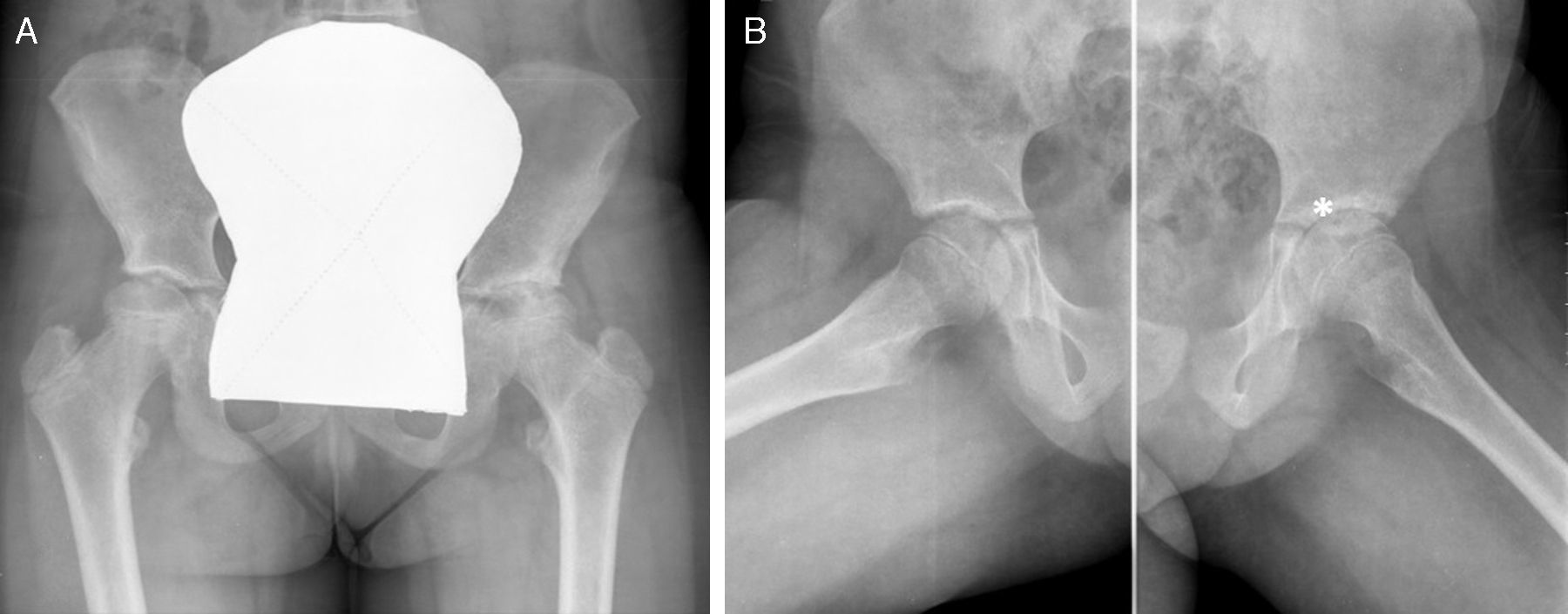
Despite methotrexate treatment escalation (up to 25mg/m2/week subcutaneously) the patient had persistent disease activity and developed severe left coxitis (Fig. 1) in the beginning of 2009, which did not respond to ultrasound guided joint injection with triamcinolone hexacetonide. Due to the persistence of arthritis and elevated inflammatory markers she was started on Anakinra (1mg/kg/day) in July 2009.
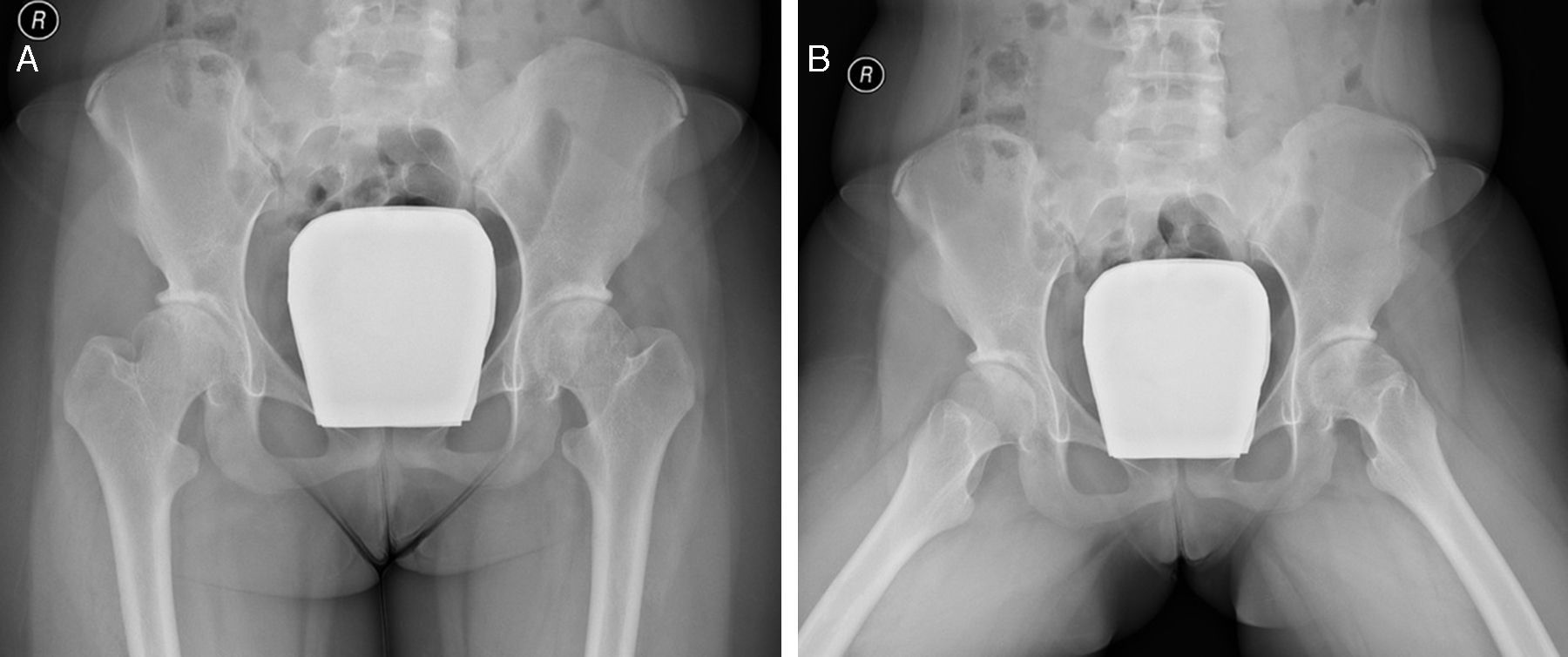
Since then there has been sustained improvement with resolution of clinical symptoms, including complete imagiological regression of coxitis (Fig. 2), which allowed discontinuation of methotrexate and corticosteroids. The patient is in clinical remission on medication with Anakinra 100mg/day (2–2.5mg/kg/day) since 2011.
DiscussionsJIA is an autoinflammatory rheumathologic disease that accounts for 5–10% of all patients classified as JIA.1 Hip involvement in sJIA is relatively common and is a cause of significant functional impairment and a marker of poor prognosis.2,3 A significant number of patients with sJIA has persistent disease despite the treatments used.
Anakinra is a recombinant form of human IL-1 receptor antagonist (IL-1Ra) that is recommended as first line disease-modifying therapy in sJIA selected patients and in refractory disease.4 In this case, the authors report the therapeutic success with Anakinra in a patient with refractory systemic and articular disease, emphasizing the regression of structural damage of the hip joint, which is rarely reported in these cases.
Conflict of interestThe authors declare that there are no conflicts of interest.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.