Since the administration of subcutaneous abatacept (ABA)1,2 has been approved many patients with rheumatoid arthritis (RA) have had their administration route changed.3 The switch from intravenous to subcutaneous administration route may imply a risk of disease outbreak, which in our centre we decided to control clinically at the time of change, after 3 months and after 12 months. Musculoskeletal ultrasound (MSU) provides more precise information than clinical examination in the assessment of joints and tendons, and we therefore also undertook both baseline ultrasound scans and scans after 3 months.
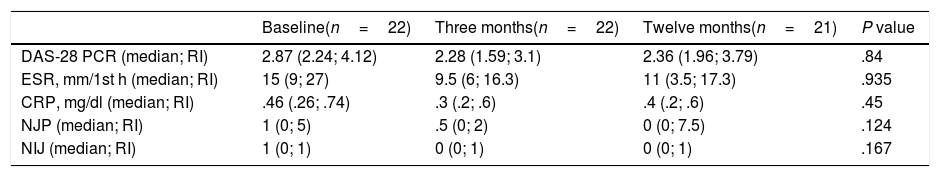
The clinical activity data in Table 1 showed no differences between the baseline moment and after follow-up at 3 and 12 months. Of the 23 patients with RA, 21 (91.3%) continued in treatment with subcutaneous ABA a year after the switch.
Evolution of the activity data and the acute phase reactants after changing administration route.
| Baseline(n=22) | Three months(n=22) | Twelve months(n=21) | P value | |
|---|---|---|---|---|
| DAS-28 PCR (median; RI) | 2.87 (2.24; 4.12) | 2.28 (1.59; 3.1) | 2.36 (1.96; 3.79) | .84 |
| ESR, mm/1st h (median; RI) | 15 (9; 27) | 9.5 (6; 16.3) | 11 (3.5; 17.3) | .935 |
| CRP, mg/dl (median; RI) | .46 (.26; .74) | .3 (.2; .6) | .4 (.2; .6) | .45 |
| NJP (median; RI) | 1 (0; 5) | .5 (0; 2) | 0 (0; 7.5) | .124 |
| NIJ (median; RI) | 1 (0; 1) | 0 (0; 1) | 0 (0; 1) | .167 |
DAS-28 PCR: Disease Activity Score of 28 joints; NPJ: number of painful joints; NIJ: number of inflamed joints; CRP: C-reactive protein; IR: interquartile range; ESR: erythrocyte sedimentation rate.
The characteristics of our patents are representative of those attending any rheumatology outpatients department, with a mean age of 63.5 years and mean disease duration of 15.5 years. 65.2% had had other previous treatment with biologics, with a mean of 2.3 biologics. 39.1% had received an additional DMAID and 34.8% a corticoid (mean dose of 3.6mg/day of prednisone).
The administration route of a drug is obviously relevant when choosing treatment, particularly in young patients since they are unable to periodically attend hospital appointments due to their employment. Despite the fact that clinical trials support the safety of ABA administration route changes, clinical practice has reported different results. In fact, results published by other researchers, with 27% of patients who reverted to the intravenous route 3 months after changing, suggest that the risk of clinical worsening of the patient is high. However, only one of our patients changed treatment after 3 months, which tends to confirm that change is not associated with an assumable risk of outbreak.4
The change in administration route affects patients treated with ABA and also those treated with other biologics such as tocilizumab. Current trends in the use of biologics are for the use of more subcutaneous than intravenous treatments and there are many patients who have been undergoing intravenous treatment with these drugs for years and who are requesting a more convenient administration route. Our results support the change of administration route as safe, but also highlight the need for strict control when this change is carried out.
Although there were no significant differences in the baseline and 3-month control scans, the use of MSU in our patients enabled us to detect subclinical synovitis in one patient and to change treatment after 3 months, which according to the usual activity markers (DAS-28), may not have happened. We believe that this provides another piece of evidence in favour of using routine MSU in the follow-up of RA patients, and supports the fact that the change of administration route is not associated with an exacerbation of inflammatory activity.
To conclude, the change of administration route of intravenous to subcutaneous ABA maintains efficacy in clinical practice. The application of MSU in monitoring of inflammatory RA activity is a useful tool when taking treatment decisions.
Please cite this article as: Nieto-González JC, Ovalles-Bonilla JG, Estrada E, Monteagudo I. Cambio de abatacept intravenoso a subcutáneo: experiencia de nuestro centro. Reumatol Clin. 2020;16:187–188.