To characterize the orofacial abnormalities in patients with rheumatoid arthritis (RA) and compare them with those in a reference population.
MethodsThe study included 30 RA patients and 30 consecutive patients in an odontology clinic in whom RA was ruled out. Patients underwent a clinical dental examination which included: (1) clinical and radiographic abnormalities of the temporomandibular joint; (2) biomechanical craniocervical analysis; (3) state of dentition and treatment needs; (4) periodontal status; (5) oral hygiene status; and (6) facial pain, which was compared among study groups. In addition, the association between the variables studied was determined through correlation tests.
ResultsPatients with RA showed a higher prevalence of temporomandibular abnormalities, both clinical (100.0% vs 60.0%, P<.001) and radiographic, including erosions (50.0% vs 16.0%, P=.010), compared with individuals in the control group. Likewise, patients with RA had a greater number of missing teeth (6.9±5.7 vs 3.0±2.0, P=.001), more caries (13.4±5.4 vs 4.9±6.5, P=.001), periodontitis (1.3±0.9 vs 0.8±0.8,P=.015), poorer oral hygiene (43.3% vs 13.3%, P=.005) and greater facial pain (66.7% vs 20.0%, P<.001). The cephalometric analysis of Rocabado showed differences in the craniocervical angle and hyoid triangle between RA and controls. Significant correlations were obtained between oral and temporomandibular abnormalities.
ConclusionsPatients with RA showed a greater orofacial deterioration, which reflects the importance of multidisciplinary care, including periodic dental examination.
Caracterizar las afecciones orofaciales en pacientes con artritis reumatoide (AR) y compararlas con las presentes en pacientes sin la enfermedad de la ciudad de Chihuahua, Chihuahua, México.
MétodosEl estudio incluyó a 30 pacientes con diagnóstico de AR y 30 pacientes consecutivos en una consulta de odontología. A través de una revisión clínica odontológica, se compararon entre los grupos variables relacionadas con: 1) trastornos clínicos y radiográficos de la articulación temporomandibular, 2) análisis biomecánico craneocervical, 3) estado de la dentición y necesidades de tratamiento, 4) estado periodontal, 5) estado de higiene oral y 6) dolor facial. Además se determinó la asociación entre las variables estudiadas a través de pruebas de correlación.
ResultadosLos pacientes con AR tuvieron una mayor prevalencia de alteraciones en la articulación temporomandibular, tanto clínicas (100 vs 60%; p<0,001) como radiográficas incluyendo erosiones (50 vs 16; p=0,010), en comparación con la población de referencia. Además los pacientes con AR tuvieron mayor cantidad de pérdidas dentales (6,9±5,7 vs 3±2; p=0,001), caries (13,4±5,4 vs 4,9±6,5; p=0,001), periodontitis (1,3±0,9 vs 0,8±0,8; p=0,015), higiene oral deficiente (43,3 vs 13,3%; p=0,005) y más dolor facial (66,7 vs 20%; p<0,001). El análisis de cefalometría de Rocabado mostró diferencias en el ángulo craneocervical y triángulo hioideo entre AR y controles. Se obtuvieron correlaciones significativas entre las alteraciones orales y las temporomandibulares.
ConclusionesLos pacientes con AR mostraron un mayor deterioro orofacial, lo que refleja la importancia de atención multidisciplinaria incluyendo la evaluación odontológica periódica.
Rheumatoid arthritis (RA) is a worldwide public health problem due to its severe functional consequences in addition to its high economic and social impact. Worldwide prevalence of the disease is approximately 1%, and it is more common in women than in men at a ratio of 3:1.1 In Mexico RA rates reported vary from .7% to 2.8%, and specifically in the population of Chihuahua the rate is 1.9%.2 RA is a progressive, destructive and profoundly limiting disease which also impoverishes quality of life and considerably reduces life expectancy.3 It is characterized by inflammation of the synovial joints which may lead to cartilage damage and bone destruction, with the involvement of many different joints.1 On an orofacial level, RA may produce symptoms which include involvement of the temporomandibular joint (TMJ), secondary Sjögren's syndrome, periodontitis and cranial neuropathy, among others.4
TMJ disorder is frequently found to affect patients with RA and prevalence of involvement ranges between 17% and 88% depending on the population studied.5–8 Although the TMJ is not usually affected in initial stages, its gradual involvement may cause resorption of the mandibular condyles and impairment of the entire stomatognathic system.9
Changes to joints in patients with RA also impede oral hygiene leading to a greater accumulation of bacterial plaque and the potential development of infections and periodontal diseases.10 A dose–response pattern has been demonstrated in the association between severity of periodontitis and disease activity11 and it has been indicated that the presence of periodontitis could trigger an autoimmune response leading to RA.12 These oral complications in patients contribute considerably to their impoverished quality of life which has already been compromised by the actual manifestations of arthritis.13
RA diagnosis focuses on peripheral arthritis and orofacial changes are not considered priority in the treatment of these patients. Participation from odontologists is therefore reserved for symptomatic cases. Previous studies which assess orofacial involvement, including the TMJ, in patients with RA are disperse and their outcomes are dependent upon specific study sample characteristics and also on the tools used for their detection.
Although scientific evidence concludes the existence of orofacial involvement in patients with RA and the need for their treatment, in Mexico, the main medical guidelines for management of the disease, including the clinical practice guide for diagnosis and treatment of RA in adults issued by the Federal Government14 and the Mexican Guide for the pharmacological treatment of RA from the Mexican College of Rheumatology,15 do not include in their contents the diagnosis and clinical or pharmacological management of oral health problems associated with the disease. For their part, the official governmental entities responsible for epidemiological monitoring of mouth diseases have demonstrated that the Mexican population has severe oral health problems. However, their reports do not stratify findings in populations at the greatest risk, such as those patients with RA.16 The above shows that the levels of medical/rheumatologic and odontological care in Mexico do not provide comprehensive care to patients that could have any impact on improving their quality of life associated with oral health.
In light of the above, and to determine the real situation of the Mexican population, we decided to classify orofacial conditions, including those of the TMJ, in patients with RA and compare them with those in people without RA, in the city of Chihuahua, Chihuahua, Mexico.
Materials and methodsThe study was conducted during the period between March and October 2017, and comprised people aged over 18 who had signed an informed consent form to participate in the study. The sample size was estimated to be 30 individuals per group. To determine sample size of proportions for 2 groups a statistical power of .8 was used, a 95% confidence interval and a risk rate of 42.1% for the group with RA and of 13.6% for the group without RA, in accordance with that reported by Bono et al.17 One of the groups comprised 30 patients diagnosed with RA in accordance with the ACR/EULAR 201018 classification criteria, with over one year onset. The control group comprised 30 people without any diagnosis of RA who had presented for a dental check-up at the facial pain clinic of the Faculty of Odontology of the Autonomous University of Chihuahua. Individuals who presented with TMJ problems from trauma were excluded. This study was approved by the Research Ethics Committee of the Faculty of Medicine and Biomedical Science of the Autonomous University of Chihuahua.
The patients diagnosed with RA were seen by rheumatologists, who conducted a detailed medical history review of the disease which included date and form of onset, relevant previous treatments, current treatment, comorbidities and complications.
The patients with RA and the subjects in the control group were given an odontological check-up by dental surgeons which consisted in the assessment of: (1) clinical TMJ disorder, (2) radiographic TMJ disorder, (3) cranio-cervical biomechanical analysis, (4) state of teeth and treatment requirements, (5) periodontal status, (6) oral hygiene status and (7) facial pain. The variables for each assessment are described below.
For diagnosis of clinical TMJ disorder the Helkimo19 index was used. This includes markers of (1) maximum range of movement in opening mouth, (2) range of movement of the right side, (3) range of movement of the left side, (4) change in joint function, (5) presence of pain on making any movement, (6) muscular pain and (7) pain in the TMJ. Each marker was given a score on a scale of 1–5 in increasing order of disorder and a total score was obtained which enabled the temporomandibular (TMD) disorder to be classified: absent (0 points), mild (1–9 points), moderate (10–19 points) and severe (20–25 points).
Diagnosis of radiographic TMJ disorder was undertaken through evaluation of the panoramic radiographs of the study subjects, in which the following were assessed: integrity of the bone cortex (normal or changed), size of mandibular condyles comparing one to another (symmetrical or asymmetrical), reduction of joint space (normal, reduced or collapsed), shape of condyles (normal or flattened) and number of bone erosions.
Cranio-cervical biomechanical analysis was assessed in a side radiography of the brain using the tracing described by Dr. Rocabado20 which included the measurement of: (1) cranio-vertebral angle, consisting of the functional ratio of the occipital bone with the atlas (C1) and the axis (C2) and the normal value of which is 96±5°; (2) distance between C0 and C1, i.e., the space at the base of the occipital to the posterior arch of the atlas, the functional ranges of which measure between 4mm and 9mm; and (3) hyoid triangle (measurement of its height) and the position of the hyoid bone in relation to the third cervical bone, for which the expected value was 4±.6mm.
Assessment of dental status and treatment requirements for each tooth was classified as: (1) repair and sealing status, (2) cavities and (3) missing teeth, using the markers and codes established by the International Cavities Detection and Assessment System (ICDAS).21 For each patient the number of missing teeth and the number of sealed teeth, repaired teeth and untreated cavities were determined.
Periodontal status of the study subjects was assessed by a dental sextant (18–14, 13–23, 24–28, 48–44, 33–43 and 34–38) classifying them as 0: no evidence of inflammation or gingival bleeding (healthy), 1: presence of inflammation and gingival bleeding on probing, 2: presence of subgingival calculus, without any presence of periodontal pocket, 3: pocket of 4–5mm and 4: pocket over 6mm. An average of sextants per individual was obtained.
For assessment of oral hygiene status the level of dentobacterial plaque (detritus) was assessed, together with calculus in teeth 16, 11, 26, 46, 31 and 36. The level of dentobacterial plaque was measured using a disclosing tablet and was classified as: 0: absent, 1: up to a third, 2: up to 2 thirds and 4: up to 3 thirds. The calculus was classified as: 0: absent, 1: up to one third, 2: 2 thirds or mild subgingival and 3: 3 thirds or subgingival band. The averages of scores of the 6 teeth reviewed for detritus and calculus were obtained together with the sum of these 2 averages to obtain the simplified oral hygiene index.
In addition to this a survey was applied with questions relating to oral hygiene habits which included the frequency with which the patients brushed their teeth, flossed their teeth and visited the dentist, as well as the perception they had of the importance of caring for their teeth.
Facial pain was assessed by a self-report with which the presence or non-presence was determined. In positive cases of pain, this was classified depending on the area (anterior, middle or posterior), the intensity (using a visual analogue scale of 0–10) and type (neuropathic and somatic).
In addition to this, in the our population total, through the obtainment of correlation coefficients, the association between the following variables was determined: (1) radiographic TMD: reduction of joint space, shape of the condyles and number of erosions; (2) Clinical TMD: total scoring and interpretation of the Helkimo index and facial pain; (3) state of oral health (cavities, missing teeth, oral hygiene); and (4) cranio-cervical biomechanical analysis.
Statistical analysis was performed in IBM SPSS Statistics, version 22.0 (IBM Corp, Armonk, NY). Absolute and relative frequencies were used for the ordinal, nominal or categorical variables. A descriptive analysis was performed with central and dispersion tendencies for continuous variables. To determine the differences between the groups the χ2 test was used for categorical variables, and the Student's t-test for continuous variables. The correlations between variables of radiographic TMD, clinical TMD and oral health were calculated with the Spearman correlation coefficient. A P value of ≤.050 was considered statistically significant for differences.
ResultsThis study comprised 30 subjects in each group, the socio-demographic characteristics of which are contained in Table 1. There was no difference in age and gender between the study groups. The frequencies of these variables showed that the patients with RA were dedicated mostly to activities in the home whilst the people from the group without RA had a job or profession outside the home. In the self-report on drug addiction and current illnesses, a higher alcohol consumption and greater frequency of diabetes mellitus was present in the patients without RA.
Socio-demographic characteristics of the sample.
| Variable | Without RAn=30 | RAn=30 | P |
|---|---|---|---|
| Age (mean±SD) | 41.7±12.25 | 44.30±13.50 | .432a |
| Sex (women/men) | 24/6 | 28/2 | .129b |
| Civil status | |||
| Married (%) | 50 | 60 | .302b |
| Single (%) | 50 | 40 | |
| Occupation | |||
| Home carer (%) | 16.7 | 63.3 | <.001b |
| Professional (%) | 30 | 3.3 | .006b |
| Student (%) | 6.7 | 0 | .246b |
| Employee (%) | 23.3 | 10 | .149b |
| Trade (%) | 23.3 | 6.7 | .073b |
| Others (%) | 0 | 16.7 | .026b |
| Drug addictions and physical activity | |||
| Tobacco (%) | 10 | 6.7 | .500b |
| Alcohol consumption (%) | 30 | 6.7 | .018b |
| Physical activity (%) | 40 | 36.7 | .500b |
| Current illnesses | |||
| Rheumatoid arthritis (%) | 0 | 100 | <.001b |
| High blood pressure (%) | 6.7 | 10 | .500b |
| Diabetes mellitus (%) | 10 | 3.3 | .306b |
| Asthma (%) | 0 | 6.7 | .246b |
| Others (%) | 3.3 | 10 | .306b |
RA: rheumatoid arthritis; SD: standard deviation.
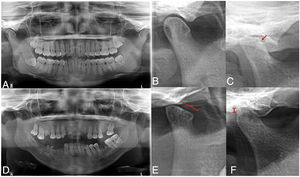
The findings from assessment of TMJ abnormalities are presented in Table 2. In accordance with the clinical assessment using the Helkimo index, patients with RA presented a prevalence of 100% of TMD, whilst only 60% of the group without RA presented with abnormalities. Mild TMD was most prevalence in the group without RA, whilst the moderate or severe TMD predominated in the patients with RA. Of the markers assessed only the presence of pain in making some type of movement was higher in the group with RA. The panoramic radiographs (Fig. 1) revealed that radiographic TMD in the patients with RA was higher (Table 2), and abnormalities were found in the osseous cortex, and there was a higher number of erosions (Fig. 1B), remodelling (flattening) of the condyles (Fig. 1C), and greater reduction or collapse of the joint space (Fig. 1F).
Temporomandibular disorders in patients with rheumatoid arthritis.
| Variable | Without RA | RA | P |
|---|---|---|---|
| Clinical disorders (Helkimo index) | |||
| Range of movement on opening (mean±SD) | .30±.95 | .56±.97 | .287a |
| Range of movement to right side (mean±SD) | .30±.95 | .96±1.67 | .063a |
| Range of movement to left side (mean±SD) | .86±1.71 | 1.4±1.88 | .257a |
| Change in joint function (mean±SD) | .96±1.44 | 1.26±1.55 | .442a |
| Presence of pain on making a movement (mean±SD) | .60±1.27 | 2.2±2.49 | .003a |
| Muscular pain (mean±SD) | .83±1.48 | 1.66±2.08 | .080a |
| Pain in TMJ (mean±SD) | .93±1.68 | 1.70±2.07 | .121a |
| Total score (mean±SD) | 4.80±6.85 | 9.60±7.68 | .013a |
| TMD diagnosis (%) | 60 | 100 | <.001a |
| Absent or mild (%) | 80 | 56.70 | .047b |
| Moderate or severe (%) | 20 | 43.30 | |
| Radiographic disorders (panoramic radiograph) | |||
| Integrity of bone cortex | |||
| Normal (%) | 84 | 38.50 | .001b |
| Changed (%) | 16 | 61.50 | |
| Size of mandibular condyles with respect to one another | |||
| Symmetrical (%) | 20 | 50 | .025b |
| Asymmetrical (%) | 80 | 50 | |
| Reduction of joint space | |||
| Normal (%) | 48 | 15.40 | .002b |
| Reduced (%) | 52 | 53.8 | |
| Collapsed (%) | 0 | 30.8 | |
| Shape of condyles | |||
| Normal (%) | 76 | 30.80 | .001b |
| Flattened (%) | 24 | 69.20 | |
| Erosions | |||
| Absent (%) | 84 | 50 | .010b |
| Present (%) | 16 | 50 | |
| Number of erosions (mean±SD) | .24±.59 | .92±1.19 | .014a |
RA: rheumatoid arthritis; TMJ: temporomandibular joint; SD: standard deviation; TMD: temporomandibular disorder.
Radiographic temporomandibular disorder in patients with rheumatoid arthritis. The panoramic radiograph revealed greater prevalence of TMD in patients with RA (C–F) compared with individuals from the group without RA (A, B), including greater prevalence of bone erosions (C), condyle deformity (E) and reduction of joint space (F).
In the cranio-cervical biomechanical analysis (Table 3), results showed that cranio-vertebral angle is higher in patients with RA compared to individuals in the control group without RA (P=.025). In accordance with the angle measurement classification, the majority of patients with RA presented with a normal position (55.6%), whilst for individuals of the group without the disease the most common position was extension (43.3%). There were no differences between the angle position (normal, extension or flexion) between the groups. The height of the hyoid triangle was also higher in the patients with RA compared to the control group (P=.032). The positive and neutral position of the hyoid bone was different for the study groups. There was a higher prevalence of the positive position in the patients with RA (P=.005) and the neutral position in individuals of the group without RA (P=.001). No differences were found in the space between C0 and C1 among the groups.
Cranio-cervical biomechanical analysis of patients with rheumatoid arthritis.
| Variable | Without RA | AR | P |
|---|---|---|---|
| Rocabado analysis | |||
| Craniovertebral angle (mean±SD) | 93.2±8.40 | 98.4±8.59 | .025a |
| Classification | |||
| Normal (%) | 40 | 55.60 | .436b |
| Extension (%) | 43.30 | 22.20 | .091b |
| Flexion (%) | 16.70 | 22.20 | .644b |
| Hyoid triangle (mean±SD) | 2.33±5.30 | 5.51±5.61 | .032a |
| Classification | |||
| Positive (%) | 50 | 85.20 | .005b |
| Negative (%) | 20 | 14.80 | .607b |
| Neutral (%) | 30 | 0 | .001b |
| C0–C1 space (mean±SD) | 5.76±1.86 | 5.81±2.35 | .932a |
| Normal (%) | 83.30 | 85.20 | .848b |
| Reduced (%) | 13.30 | 11.10 | .799b |
| Increased (%) | 3.40 | 3.70 | 1b |
AR: rheumatoid arthritis; SD: standard deviation.
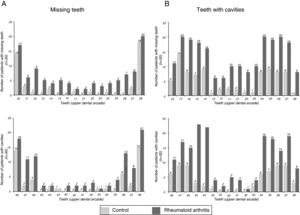
The findings referring to odontological changes in the patients with RA are presented in Table 4. The patients with RA demonstrated greater impairment in the dentition status, periodontal status, oral hygiene status and facial pain on being compared with patients of the group without RA. The number of missing teeth was higher in patients with RA (P=.001). The patients with RA had an average of 6.90 missing teeth, whilst the mean of those people in the group without RA was 3.03. Fig. 2A outlines the number of missing teeth in each group. As may be observed, the majority of missing teeth in both groups correspond to molars; however, it is clear that the patients with RA presented a greater loss of anterior teeth than the control group subjects.
Odontological changes in patients with rheumatoid arthritis.
| Variable | Without RA | AR | P |
|---|---|---|---|
| State of dentition and treatment needs(ICDAS) | |||
| Missing teeth (mean±SD) | 3.03±2 | 6.90±5.77 | .001a |
| Sealed or repaired teeth (mean±SD) | 5.96±6.25 | 3.70±4.86 | .122a |
| Sealed (mean±SD) | .30±1.11 | 0±0 | .147a |
| Repaired (mean±SD) | 5.66±6.24 | 3.70±4.86 | .179a |
| Teeth with cavities (mean±SD) | 4.90±6.55 | 13.46±5.48 | <.001a |
| Slight cavities (mean±SD) | 2.33±3.91 | 4.30±4.39 | .072a |
| Moderate cavities (mean±SD) | 1.96±3.48 | 6.83±5.25 | <.001a |
| Advanced cavities (mean±SD) | .6±1.88 | 2.33±3.45 | .019a |
| Periodontal status (mean±SD) | .77±.78 | 1.33±.94 | .015a |
| Oral hygiene status | |||
| Detritus (mean±SD) | .84±.88 | 1.66±.93 | .001a |
| Calculus (mean±SD) | .93±.71 | 1.52±.67 | <.001a |
| Oral hygiene (SOHI) (mean±SD) | 1.51±1.52 | 3.29±1.73 | <.001a |
| Classification | |||
| Excellent (%) | 30 | 0 | .005b |
| Good (%) | 16.70 | 13.30 | |
| OK (%) | 40 | 40 | |
| Bad (%) | 13.30 | 43.30 | |
| Facial pain (%) | 20 | 66.7 | <.001b |
| Intensity (mean±SD) | 1.11±2.12 | 3.86±3.12 | <.001a |
| Area of pain | |||
| Anterior (%) | 6.70 | 31 | .016b |
| Media (%) | 13.30 | 31 | .117b |
| Posterior (%) | 6.70 | 27.60 | .032b |
| Type of pain | |||
| Neuropathic (%) | 0 | 10.30 | .071b |
| Somatic (%) | 20 | 58.60 | .002b |
RA: rheumatoid arthritis; SD: standard deviation; SOHI: simplified oral hygiene index.
The number of teeth with cavities was also higher in the group with RA compared to the group without RA (P<.001). The patients with RA presented moderate and advanced levels of tooth decay which were higher than those in the group without RA. In the analysis of the decay distribution by enamel organ (Fig. 2B) we observe that the majority of decayed teeth in both groups correspond to molars. Moreover, greater prevalence of involvement is observed in superior teeth to inferior ones in the group with RA.
66.7% of the patients with RA presented with facial pain, whilst only 20% of patients in the group without RA did so. The intensity of the pain was also higher in the patients with RA (P<.001). The presence of facial pain in the anterior and posterior area and somatic type pain were more prevalent in the patients with RA when compared to the patients of the group without RA.
Regarding oral hygiene, the patients with RA had higher levels of dental bacterial plaque and calculus than the patients in the group without RA (Table 4). Combined with this, the self-report on oral hygiene habits (Table 5) revealed that a smaller number of patients with RA brush their teeth more than twice a day, the majority of them never use dental floss and they go to the dentist less than once a year.
Oral hygiene habits in patients with rheumatoid arthritis.
| Question | Without RA | RA |
|---|---|---|
| n=30 | n=30 | |
| How many times a day do you brush your teeth? | ||
| Twice or more times (%) | 90 | 66.7 |
| Once a day (%) | 10 | 33.3 |
| Almost never (%) | 0 | 0 |
| Never (%) | 0 | 0 |
| How many times a day do you use dental floss? | ||
| Never (%) | 36.7 | 56.7 |
| Occasionally, not every day (%) | 36.7 | 26.7 |
| Once a day (%) | 23.3 | 10 |
| Twice a day (%) | 3.3 | 6.7 |
| Three times a day (%) | 0 | 0 |
| When was the last time you went to the dentist? | ||
| Six months ago (%) | 60 | 13.3 |
| More than 6 months ago but less than a year ago (%) | 26.7 | 20 |
| More than a year ago (%) | 13.3 | 60 |
| I have never been (%) | 0 | 6.7 |
| Why did you go to the dentist? | ||
| For a routine check-up (%) | 43.3 | 23.3 |
| To check up on the orthodontic treatment I have (%) | 13.3 | 3.3 |
| Because I had cavities, pain or infection (%) | 20 | 46.7 |
| For other reasons (%) | 23.3 | 20 |
| I have never been (%) | 0 | 6.7 |
| Going to the dentist is ….? | ||
| Pleasant (%) | 36.7 | 6.7 |
| Unpleasant (%) | 26.7 | 30 |
| Indifferent (%) | 36.7 | 33.3 |
| I don’t know (%) | 0 | 30 |
| Do you think the problems with your teeth affect your arthritis? | ||
| Definitely yes (%) | 0 | 33.3 |
| Probably yes (%) | 0 | 33.3 |
| I’m not sure (%) | 0 | 23.3 |
| Probably not (%) | 0 | 0 |
| Definitely not (%) | 0 | 10 |
| I don’t have arthritis (%) | 100 | 0 |
| Do you think caring for your teeth is a relevant aspect for your general health? | ||
| Definitely yes (%) | 76.7 | 46.7 |
| Probably yes (%) | 6.7 | 40 |
| I’m not sure (%) | 3.3 | 13.3 |
| Probably not (%) | 13.3 | 0 |
| Definitely not (%) | 0 | 0 |
| Have you ever received advice or guidance regarding your dental hygiene? | ||
| No (%) | 20 | 6.7 |
| Yes, although I have never followed it (%) | 13.3 | 40 |
| Yes, although I only follow it at times (%) | 43.3 | 46.7 |
| Yes, I follow it (%) | 23.3 | 6.7 |
| Have you lost any teeth (which were not health teeth extractions or due to trauma)? | ||
| Yes, all or most of them (%) | 0 | 0 |
| Yes, I have only lost 1–2 teeth (%) | 40 | 29.6 |
| Yes I have lost between 3 and 6 teeth (%) | 10 | 44.4 |
| No, I have all my teeth (%) | 50 | 25.9 |
| How frequently do you think a person should go to the dentist? | ||
| Once a year, as a routine (%) | 93.3 | 53.3 |
| Only when they are in pain, have an infection or some damage to a tooth (%) | 6.7 | 36.7 |
| When someone suggests it (%) | 0 | 10 |
RA: rheumatoid arthritis.
The correlation coefficients obtained from the variables which defined the radiographic TMD, clinical TMD and oral health status measured for all study subjects (patients with RA and those without RA) are contained in Table 6. There were positive correlations between variables of the same category (e.g. the shape of the condyles and reduction of the joint space), and also variables in different categories (e.g. the shape of condyles and missing teeth). The presence of tooth decay correlates with the reduction of joint space and severity of the TMD, whilst the tooth loss correlates with flattening of the condyles. Interestingly, the level of oral hygiene correlates with the 3 radiographic variables assessed, and also with the number of cavities and periodontitis. In contrast, with the exception of the cranio-vertebral angle measurement with periodontal status, the cranio-vertical biomechanical analysis variables showed no significant correlations with any of the variables of the other categories.
Correlations between the TMD and oral health status in the study sample (n=60).
| Radiographic TMD | Clinical TMD | Oral health status | Cranio-cervical biomechanical analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduction of joint space | Condyle shape | Erosions | Total score on Helkimo index | Helkimo index interpretation | Facial pain | Cavities | Missing teeth | Periodontitis | Oral hygiene | Hyoid triangle | Craniovertebral space | C0–C1 space | ||
| Radiographic TMD | Reduction of joint space | 1.000 | .633** | .423** | .282* | .322* | .221 | .351* | .17 | .078 | .384** | .117 | −.032 | .118 |
| Condyle shape | .633** | 1.000 | .506** | .129 | .137 | .193 | .219 | .320* | .21 | .441** | .086 | .055 | .130 | |
| Erosions | .423** | .506** | 1.000 | .133 | .121 | .020 | .166 | .095 | .073 | .389** | −.039 | .071 | −.108 | |
| Clinical TMD | Total score on Helkimo index | .282* | .129 | .133 | 1.000 | .936** | .543** | .227 | .256* | .195 | .181 | .048 | .139 | .114 |
| Helkimo index interpretation | .322* | .137 | .121 | .936** | 1.000 | .570** | .271* | .239 | .176 | .203 | .056 | .197 | .099 | |
| Facial pain | .221 | .193 | .020 | .543** | .570** | 1.000 | .203 | .182 | .033 | .180 | .051 | .191 | .051 | |
| Oral health status | Cavities | .351* | .219 | .166 | .227 | .271* | .203 | 1.000 | .042 | .134 | .276* | .252 | .228 | .000 |
| Missing teeth | .17 | .320* | .095 | .256* | .239 | .182 | .042 | 1.000 | .219 | .255 | .082 | .094 | .069 | |
| Periodontitis | .078 | .21 | .073 | .195 | .176 | .033 | .134 | .219 | 1.000 | .629** | .172 | .311* | .222 | |
| Oral hygiene | .384** | .441** | .389** | .181 | .203 | .180 | .276* | .255 | .629** | 1.000 | .058 | .222 | −.107 | |
| Cranio-cervical biomechanical analysis | Hyoid triangle | .117 | .086 | −.039 | .048 | .056 | .051 | .252 | .082 | .172 | .058 | 1.000 | .320* | .343** |
| Craniovertebral angle | −.032 | .055 | .071 | .139 | .197 | .191 | .228 | .094 | .311* | .222 | .320* | 1.000 | .476* | |
| C0–C1 space | .118 | .130 | −.108 | .114 | .099 | .051 | .000 | .069 | .019 | −.107 | .343** | .476* | 1.000 | |
TMD: temporomandibular disorder.
The Spearman correlation coefficients are shown.
This study comprehensively assessed the conditions associated with the stomatognathic system in a group of patients with RA in the city of Chihuahua, Mexico and expressed the findings relating to oral health and the clinical and radiographic disorders of the TMJ. This study provides valuable data on the Mexican population which has not previously been reported and the scientific dissemination of which will impact the wider medical and odontological panorama in orofacial health of patients with RA.
The most frequent symptoms in patients with RA derive from polyarthritis of the small joints of the hands and other peripheral joints22; however, the patients present other conditions such as disorders of the stomatognathic system, which impoverish even more their quality of life and complicate treatment.23 The stomatognathic system includes the TMJ, the mouth, teeth and pharynx; it provides essential physiological functions such as breathing, chewing, salivating, swallowing, talking and gesticulating. The diseases which affect it create a diversity of sequelae and physical disorders.24 Although the TMJ as a synovial joint is included in the counting of joints ACR 66/68 (66 inflamed and 68 painful), it is not included in the DAS-2825 activity measurement tool, which is one of the most frequently used by rheumatologists today, and as a result, involvement of the TMJ is underestimated in the assessment and its effect therefore minimized. Furthermore, it is expected that strong impact (acute or chronic) of joints in the hands means that the patient is unable to use the appropriate dental hygiene technique due to lack of grip, limited arch movement and intense pain in some stages of disease activity.
The socio-demographic characteristics of the sample indicated a higher number of female patients with RA at an average age of 44.30 years. Interestingly this study showed that the occupation of the patients with RA was mostly homemaker (63.3%) and compared with the population without RA there were fewer professionals in the patient group (3.3 vs 30%; P=.006). Although our survey did not encompass information on educational level or income, the fact that the number of professionals, employed and sales representative was lower in the patients with RA infers a reduction of employment capacity and/or lower level of studies, which both lead to a lower socioeconomic level.
It is a well known fact that RA is associated with different forms of employment disability which are directly related to the severity of the disease.26 Also, the cost of RA in Mexican homes and particularly those who do not have a total health cover scheme, leads to catastrophic expenses and impoverishment principally due to the high cost of the medicines.27 Considering the above and the fact that the majority of patients in our study do not have an income, this exacerbates the difficulty in caring for other aspects associated with their health, such as orofacial disorders which were more severe in the patients with RA in our findings. These data are backed up by reports from epidemiological monitoring in the Mexican population which show that oral health problems are associated with the level of education in all age groups. For example in most of the groups people with under 9 years of schooling present with a higher number of teeth affected by dental decay.16
This study shows that the rate of clinical TMD is higher in patients with RA than in the control group and also that the disorders are more severe in the patients with RA. Previous studies using the Helkimo index have demonstrated greater TMJ abnormality rates in patients with RA compared with the population without RA,28,29 with prevalence close to those found in this study (98.6% with RA vs 80% without RA).30 In the Mexican population patients with rheumatic diseases, Aceves-Avila et al.31 determined a prevalence of 26%RM disorders (defined by the presence of pain, difficulty in opening the mouth, chewing or talking or inharmonious movements of the joints). This prevalence is lower than that found in our study population; however, the operational definition of TMD differs from ours, where abnormality in any of the parameters of the Helkimo index was interpreted as a positive diagnosis of TMD.
Other studies have revealed a higher prevalence of some subjective and objective symptoms relating to TMD in patients with RA in other population groups. Here, headache and pain from the TMJ are more common in rheumatic patients than in patients without RA.32 Relating to this, in the current study, facial pain was clearly greater in proportion and intensity in patients with RA compared to those without RA. These findings are also consistent with those reported by Bono et al.,17 who when assessing the involvement of the stomatognathic system in patients with RA found there to be a greater prevalence of pain in the TMJ, headache and pain when chewing, compared to patients without RA.
The prevalence of TMD on a radiographic level is also higher in the group with RA. These findings are in keeping with previous radiographic studies which demonstrate a higher joint compromise in patients with RA,33 particularly a higher rate of bone erosions, where prevalence of between 7%34 and 88.4%17 have been found. Furthermore, a sign of change in TMJ at radiographic level is the deformity of the condyle, which is normally oval in the axial plane. In patients with RA, the mandibular condyle, the fossa and the temporal eminence may lose their shape and appear flattened and widened.35 In this study, the majority of patients with RA presented with flattening of the condyles and had a reduced or collapsed joint space, which is suggestive of inflammatory changes and secondarily osteoarthritis.
The association between the cranio-cervical posture and the TMD has been widely researched and discussed in the literature for years, but up until now results have not been conclusive.36 In patients with RA cranio-cervical biomechanical characteristics were not previously described and neither was it established whether these characteristics differed from those of patients without the disease or if there was some association with the presence of disorders in TMJ. This study compared the cranio-cervical measurements between the patients with and without RA. Although there were some differences in some of the measurements between the groups, the majority of measurements did not correlate with the presence of any clinical or radiological manifestations of TMD.
With regard to oral health status, the results from this study indicate that patients with RA present with insufficient oral health and have more cavities, missing teeth, periodontitis and poor oral hygiene. These findings are consistent with previous reports in different population groups where the patients with RA are more likely to present with a periodontal disease and poorer oral hygiene which is manifested by a greater accumulation of bacterial plaque.10,37,38
Our reference in Mexico with respect to oral pathologies is the epidemiological monitoring system of oral pathologies (SIVEPAB for its initials in Mexican) the most recent report of which was issued in 2016.16 Although this report does not stratify by stages or other medical conditions, such as RA, it shows that 66.3% of our adult population has an insufficient oral hygiene which increases with age, and reaches up to 7.4% in groups aged 75–79 years of age. The simplified oral hygiene index average in the population studies was reported to be 1.17 which is lower than the average found in our study for the benchmark population group (1.51) and for the patients with RA (3.29). The SIVEPAB also reports that in the total population the prevalence of dental decay is reported to be 93.1%, whilst in our study we found that 50% of patients in the control group and 100% of the patients with RA presented with cavities, respectively. The 2016 report also states that 56.7% of the Mexican population presents with some type of periodontal disease whilst in the present study these values were higher for subjects without RA (66.7%) and patients with RA (83.3%).
Previous studies have demonstrated that patients with RA are more susceptible to periodontitis, but the results of individual studies continue to be controversial. Recently, Tang et al.39 reported a metaanalysis, the aim of which was to exhaustively assess the association between RA and periodontitis and which included 8 case studies and controls. According to their analysis, the prevalence of periodontitis in patients with RA varied from 15.5% to 100%, compared with that of 10% to 82.1% in the controls, finding that TA was significantly associated with a generally increased risk of periodontitis.
The possible aetiopathogenic relationship between RA and periodontitis has been specifically researched, not only regarding shared inflammatory mechanisms but also the importance of the bacterial infections in citrullination of peptides and as a result in the development of antibodies against citrullinated antibodies which are specific to RA and are linked with the pathogenesis and severity of the disease. The bacteria Porphyromonas gingivalis (P. gingivalis) is the microorganism with the greatest impact in periodontitis and is also the only bacteria which expresses the enzyme peptidyl arginine deiminase, responsible for the citrullination process. In Mexican populations it has been demonstrated that P. gingivalis occurs more frequently in patients with RA either at subgingival40 level or even in serum.41 It has recently been discovered that Aggregatibacter actinomycetemcomitans, another microorganism related to periodontal infection may also induce citrullination of proteins through leukotoxin A.42 It has been suggested that infection with P. gingivalis could accelerate the beginning and the progress of RA enabling the presentation of auto-antigens and the expression of auto-antibodies against citrullinated peptides which are specific and almost exclusive to AR.12,43
The patients with RA in our study reported more insufficient oral hygiene habits than those without RA, which correlated with the findings of the review of their oral health status. However, the majority report that they had received guidance on dental hygiene techniques and also that they considered that the care of their teeth was relevant for their overall health and for their arthritis. An incongruence is reflected here between patient perception with regard to the need for appropriate oral health and their limited real attempts to maintain it. In other words although their oral hygiene is important to them they are not able to apply the necessary measures to maintain good status. Part of the difficulty lies in the disability generated by the disease in its different stages. Economic factors are also influential here, with dental care costs most being covered by the patients. In this environment the costs derived from RA frequently override attention to other health problems considered to be less important.
This study shows the correlation between oral and TMJ abnormalities. This confirms the need for a comprehensive assessment of the stomatognathic system from the early stages of RA and subsequent assessment so that odontological treatment may be offered to the patient bringing improvements to their quality of life. Preventative dental care measures could have a huge impact in preventing deterioration of the oral cavity of patients with RA, limiting the amount of teeth lost and controlling chronic infectious sites at dental and periodontal tissue levels.
To conclude, patients with RA require comprehensive and multidisciplinary care which should include the treatment of orofacial disorders. Collaboration between odontologists and rheumatologists is required and it would be prudent to refer the patient from the earliest stages of the disease as a non-pharmacological measure. There is a need for specific educational programmes to prevent deterioration of the quality of life relating to the oral health in these patients and strategies (between patients and family members) for encouraging dental care during periods of immobility, disability and intense joint pain should be established.
Conflict of interestsThe authors have no conflict of interests to declare with respect to the publication of this article.
The researchers would like to thank Dr. Marco de la Torre and Dr. Silvia Cervantes Rodríguez for their methodological support in carrying out this research.
Please cite this article as: González-Chávez SA, Pacheco-Tena C, Campos Torres RM, Quiñonez-Flores CM, Reyes-Cordero G, Caraveo Frescas TJ. Alteraciones temporomandibulares y odontológicas en pacientes con artritis reumatoide. Reumatol Clin. 2020;16:262–271.