There is cumulative evidence in the literature supporting a potential role of faecal calprotectin (FCP) as a biomarker for gut inflammation in spondyloarthritis (SpA). However its relevance in undifferentiated SpA (USpA) is still uncertain. The aim of the current study is to assess the diagnostic significance of FCP levels in patients with differentiated and undifferentiated SpA.
Material and methodsA total of 52 differentiated SpA, 33 USpA and 50 controls could be included. For all patients, clinical evaluation, routine laboratory investigations, FCP levels, and occult blood in stool were performed. When indicated imaging and/or endoscopies were performed.
ResultsThe differentiated SpA patients were 12 (23.1%) with ankylosing spondylitis, 21 (40.4%) with psoriatic arthritis, 13 (25%) with ulcerative colitis, 5 (9.6%) with Crohn's disease (CD) and one (1.9%) with reactive arthritis. The mean FCP level in 85 patients correlated with CRP and ESR. Within the SpA group ulcerative colitis and Crohn's disease patients had increased FCP levels compared to other SpA subgroups and USpA patients (p<0.001). The mean FCP levelwas significantly higher in the SpA patients compared to USpA and controls (p<0.001).
ConclusionsElevated FCP levels may identify patients who are most likely to have SpA already in the unclassified phase of the disease. Further studies in different series of patients are needed to evaluate the potential diagnostic and prognostic roles of FCP in both differentiated and undifferentiated phases of the disease.
Existe evidencia acumulada en la literatura que respalda un papel potencial de la calprotectina faecal (FCP) como un biomarcador para la inflamación intestinal en la espondiloartritis (SpA). Sin embargo, su relevancia en SpA indiferenciada (USpA) aún es incierta. El objetivo del presente estudio es evaluar la importancia diagnóstica de los niveles de FCP en pacientes con SpA diferenciada e indiferenciada.
Material y métodosSe incluyeron un total de 52 SpA diferenciadas, 33 USpA y 50 controles. Para todos los pacientes, se realizaron evaluaciones clínicas, investigaciones de laboratorio de rutina, niveles de FCP y sangre oculta en las heces. Cuando se indicó se realizaron imágenes y/o endoscopias.
ResultadosLos pacientes con SpA diferenciada fueron 12 (23,1%) con espondilitis anquilosante, 21 (40,4%) con artritis psoriásica, 13 (25%) con colitis ulcerosa, 5 (9,6%) con enfermedad de Crohn y uno (1,9%) con artritis reactiva. El nivel medio de FCP en 85 pacientes se correlacionó con la PCR y la VSG. Dentro del grupo de SpA, los pacientes con colitis ulcerosa y enfermedad de Crohn habían aumentado los niveles de FCP en comparación con otros subgrupos de SpA y pacientes con USpA (p<0,001). El nivel medio de FCP fue significativamente mayor en los pacientes con SpA en comparación con los controles normales y USpA (p<0,001).
ConclusionesLos niveles elevados de FCP pueden identificar a los pacientes que tienen más probabilidades de tener SpA ya en la fase no clasificada de la enfermedad. Se necesitan más estudios en diferentes series de pacientes para evaluar las posibles funciones de diagnóstico y pronóstico del FCP en las fases diferenciadas e indiferenciadas de la enfermedad.
The link between bowel and joint in spondyloarthritis (SpA) has been established for several decades. A subgroup of patients with SpA develops inflammatory bowel disease (IBD) conversely; patients with IBD may develop SpA. In the 1980s, it was demonstrated that up to 50% of SpA patients have microscopic bowel inflammation, without associated gastrointestinal (GI) symptoms and can be associated with a more aggressive disease course.1 Calprotectin (CP) is a sensitive marker of neutrophilic inflammation which can be measured in serum and stools.2 Faecal calprotectin (FCP) is a calcium binding protein with antimicrobial properties; it is a member of the family of S100 leucocyte proteins secreted primarily by neutrophilic granulocytes and monocytes in response to inflammation.3,4 Gut inflammation provokes neutrophil activation with subsequent FCP release in a proportionate degree to that of inflammation. Gastrointestinal tract (GIT) inflammation, as in active IBD, releases calprotectin into body fluids including faeces.5,6 FCP remains stable in faeces for up to 5 days and its use as a non-invasive biomarker in IBD management is gaining popularity. Additionally FCP release is proportionate to the degree of GI inflammation, given that it may also have a role in monitoring disease activity as well as the responses and the effectiveness of different therapeutics used in IBD.4,7
In up to two-thirds of patients with differentiated SpA a significantly higher frequency of subclinical GI inflammation has been described compared to other autoimmune disorders and evident histopathologic gut inflammation in up to 30%–60% of cases.8,9 On the other hand extra-intestinal symptoms generally improve when the underlying GI inflammation is treated, suggesting strong associations between these two clinical entities. The mechanism by which this occurs is still not fully understood.10,11
To the best of our knowledge the clinical utility and the diagnostic performance of FCP in undifferentiated spondyloarthritis (USpA) and its role in predicting the progression of USpA to IBD and/or other types of SpA is still unclear and not extensively investigated.
The aim of the current study is to investigate the diagnostic performance of FCP in differentiated and undifferentiated SpA, in order to define its possible roles as a diagnostic biomarker, especially in patients with USpA and with possible subclinical GI inflammation. We also aim to determine the optimal cut-off value for FCP between the investigated groups of patients.
Patients and methodsConsecutive patients were included with differentiated SpA, USpA, and age and sex matched healthy controls. The patients in the differentiated SpA group had to fulfil the Assessment of SpondyloArthritis international Society (ASAS) classification criteria.12 For classifying patients with early USpA we used the European Spondylarthropathy Study Group Preliminary Criteria for the Classification of Spondylarthropathy, due to the lack of diagnostic criteria to define early “unclassified spondylarthropathy”.13 The criteria simply entail: positive family history of SpA, arthritis, psoriasis, IBD, or acute diarrhoea, alternating buttock pain, enthesitis and sacroiliitis by confirmed imaging.13 Additionally as inclusion criteria: all patients in the USpA group had to be seronegative for rheumatoid factor (RF), anti-citrullinated protein/peptide antibodies (ACPA) and antinuclear antibodies (ANA). All patients gave informed consent for their participation.
Clinical assessmentAt baseline, full history was taken regarding inflammatory low back pain, joint problems and complaints suggestive of conjunctivitis, uveitis, IBD. A complete physical examination was performed including detailed rheumatological and musculoskeletal examinations for axial joints, sacroiliac joints testing, reduced axial mobility, and chest expansion. Physical examination gave special attention to extra-articular features such as nail lesions (pits, onycholysis, longitudinal ridging and dystrophic nail changes), dactylitis, enthesitis, distal interphalangeal joint (DIP) involvement and psoriatic skin lesions in the hidden areas. For peripheral joints the pattern of arthritis, swollen joint count (SJC), tender joint count (TJC), duration of morning stiffness in minutes, Current medications were also recorded.
Laboratory investigationsFor all patients in both differentiated and undifferentiated SpA groups baseline laboratory parameters obtained at inclusion included ESR 1st hour, C-reactive protein (CRP) (mg/dl), complete blood count, complete liver and kidney function tests, urine analysis and occult blood in stool. For all patients in the USpA further immunological investigations were performed by enzyme-linked immunosorbent assay (ELISA), including anti-nuclear antibodies (ANA), IgM-rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) antibodies. None of the control subjects performed occult blood in stool or ESR or CRP.
Faecal Calprotectin (FCP) measurementQuantum Blue® Calprotectin Extended, which covering an extended range from 30 to 1000μg/g was used to estimate FCP in both groups of patients and normal controls. All subjects in the control group are healthy without any GIT symptoms before the assessment of FCP and all perceived as normal healthy individuals. Sample preparation: load a definite amount of the stool extract onto the sample loading port of the test cassette. Immediately after loading, start the timer by pressing the Enter Button of the reader. To avoid contamination of the reader's optical device, close the tray after about 30s. At the end of the incubation time, the test cassette is instantly read and the result is displayed in μg/g. Normal FCP values <50μg/g are not indicative of IBD. As recommended re-testing samples was performed in the grey zone between 30 and 70μg/g, which corresponds to the 2.5th–97.5th percentile of imprecision around the cut-off of 50μg/g. Elevated FCP values between 50 and 200μg/g can represent mild organic disease e.g. IBD in remission phase. Elevated FCP values >200μg/g are indicative of active organic disease. The manufacturer cut off value was used to assess normal from abnormal increased FCP values in all groups and the mean FCP were compared between all groups.
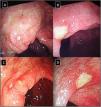
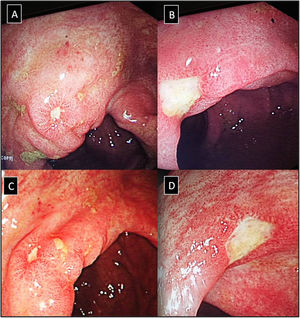
IleocolonoscopyIleocolonoscopy was performed when clinically indicated in patients in both groups to investigate suspected IBD in case of dominant GIT symptoms and signs, positive occult blood in stool and with elevated initial FCP specially when exceeding 200μg/g (Fig. 1). Biopsy samples were taken and further subjected to histopathological examination.
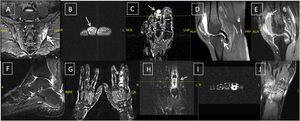
(A–D) Lower gastrointestinal endoscopy showing colonic ulceration in a patient with undifferentiated spondyloarthritis presented with acute asymmetrical oligoarticular synovitis of lower limbs and gastrointestinal symptoms highly suggestive of inflammatory bowel disease over two weeks duration. The estimated faecal calprotectin (FCP) at disease onset is 550μg/g (normal values <50μg/g).
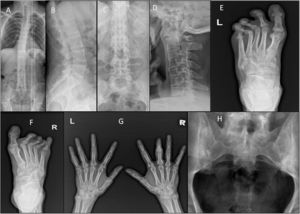
Plain radiographs were obtained of the involved joints according to standardised projections for different joints when clinically indicated in both groups (Fig. 2A–H).
(A & C) Ankylosing spondylitis: X-ray whole spine and thoracolumbar spine AP view showing diffuse syndysmophytic ankylosing giving the Bamboo spine appearance, dagger sign (ossification of interspinous ligament) with bilateral sacroilitis; (B) X-ray of the thoracolumbar spine lateral view showing Romanus lesions (Shiny Corner Sign) due to sclerosis of the vertebral body corners; (D) X-ray cervical spine lateral view showing ossification of the anterior spinal longitudinal ligaments from C3-T1; (E) psoriatic arthritis: X-ray of the left foot AP view with poly-articular erosive arthritis of the lateral 3 MTP joints with subluxation and pencil cup deformity; (F) psoriatic arthritis: X-ray of the right foot AP view showing subluxation of the middle 3 MTP joints with marginal erosions; (G) psoriatic arthritis: X-ray both hands AP view showing sausage shaped right middle finger with erosive arthritis of its PIP joint; (H) ankylosing spondylitis: X-ray of the SIJs AP view showing bilateral and symmetric sacroiliitis with subchondral bony sclerosis on both sides.
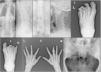
Gadolinium-enhanced MRI studies were performed when clinically indicated, in twenty-two cases in the USpA patients and twenty-six cases in the SpA group. In case of peripheral arthritis MRI studies were ordered to document the inflammatory nature of peripheral synovitis, to exclude intra-articular lesions with mono-articular presentation that can mimic arthritis and/or other atypical presentations,14 and to detect subclinical synovitis and/or enthesitis in certain patients in both groups (Fig. 3B–E).15–17 Additionally MRI was used as an additive diagnostic tool especially in the USpA group to establish the diagnosis and to document the classical features related to SpA like sacroiliitis (Fig. 3A), tenosynovitis and/or dactylitis (Fig. 3B, C, G, H, F) and enthesitis (Fig. 3D–F), the latter are important features from the diagnostic point of view. All MRI studies were performed on General Electric echo speed 1.5-T MR unit equipped with a dedicated coil according to the joint(s) scanned. MRI sequences included; sagittal, coronal, and axial, T1-weighted, spin-echo including Fat Sat.
Undifferentiated and differentiated SpA with axial and peripheral joints involvement; (A) axial STIR MRI showing bilateral Sacroiliitis with bone marrow oedema (BME), more prominent on the left side; (B, C) axial and coronal T1 post-contrast fat suppressed MRI of the left foot showing tenosynovitis of the DIP joint of the left 2nd toe with significant enhancement signifying underlying dactylitis; (D, E) reactive arthritis: sagittal T2 fat suppressed and T1 post-contrast fat suppressed MRI of the knee showing peri-entheseal bone marrow oedema around the tibial attachment of the PCL with joint effusion and enhancing synovium suggestive of synovitis; (F) undifferentiated SpA: sagittal STIR MRI of the ankle with posterior calcaneal erosions and mild oedema and thickening of the planter fascia; undifferentiated SpA: (G) coronal T1 post-contrast fat suppressed MRI of both hands with diffuse enhancement and inflammatory changes of the right middle finger (dactylitis); (H, I): undifferentiated SpA: coronal and axial T1 post-contrast fat suppressed MRI of the left hand with diffuse enhancement and tenosynovitis of the left index finger. Undifferentiated SpA (mono arthritis pattern): (J) coronal T1 post-contrast fat suppressed MRI of the left wrist with diffuse inter-carpal & CMC joint enhancement.
Statistical analyses were performed using Stata software version 15 (Stata Corp., College Station, TX). Continuous variables were expressed as mean (±SD) if normally distributed and as median (interquartile range) if not normally distributed, and categorical variable as absolute number (percentage). Differences in characteristics between cases and controls were examined using ANOVA (continuous variables) and χ2 test (categorical variables). Comparisons between patients were performed using Student t test, Mann–Whitney U, chi-square tests, or Fisher's exact test, as appropriate. A cut-off value for the FCP for the studied patients groups was estimated with receiver operating characteristic (ROC) curve analysis. The p-values were considered significant when <0.05.
Ethics: All patients gave informed written consent to be enrolled into the study according to the Declaration of Helsinki. The study was approved by the local ethical committee.
ResultsA total of 52 differentiated SpA patients, 33 USpA patients could be included, and 50 controls matched for age and sex, with a mean age (±SD) of 42.1±11.3 years, 40.8±7.6 years, and 42.1±9.2 years, respectively. There were no statistically significant differences with regard to age (p=0.803) and gender (p=0.543) between patients and controls. The median (IQR) disease duration was 60 (3–240) and 18 (1–60) months for SpA and USpA patients respectively (p<0.001). The presence of occult blood in stool was detected in 19 (36.5%) of SpA patients compared to 3 (9.1%) of USpA patients (p=0.005). A family history of SpA in first and/or second-degree relatives was observed in 29 (55.8%) of SpA patients and 13 (39.4%) of USpA patients.
Regarding endoscopy; 25 (48.1%) of the SpA patients, and only 5 (15.2%) of the USpA underwent endoscopy (p=0.005). Detailed demographic, clinical, and lines of treatment are illustrated in Table 1. The ESR and CRP levels were not significantly different between SpA en USpA groups. Laboratory findings among the studied groups of patients are presented in Table 2.
Patients’ characteristics, laboratory investigations and endoscopic findings among the studied groups of patients.
| Parameters | SPA (n=52) | USPA (n=33) | p-value | Parameters | SPA (n=52) | USPA (n=33) | p-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 42.1±11.3 | 40.8±7.6 | 0.574 | Classification of arthritis | |||
| Sex (men) | 31 (59.6%) | 21 (63.6%) | 0.037 | None | 13 (25%) | 0 | <0.001** |
| Disease duration (months) | 91.8±75.4 | 34.1±47.7 | <0.001** | Axial | 11 (21.1%) | 2 (6.1%) | |
| Age at disease onset | 34.5±9.9 | 38.7±8.3 | 0.044* | Mono-arthritis | 0 | 7 (21.2%) | |
| Swollen joint count | 2.3±2.6 | 2.7±2.2 | 0.426 | Oligo-arthritis | 21 (40.4%) | 19 (57.6%) | |
| Tender joint count | 2.3±2.6 | 2.9±2.3 | 0.285 | Polyarthritis | 7 (13.5%) | 5 (15.1%) | |
| Hand arthritis | 14 (29.8%) | 17 (51.5%) | 0.050* | Pattern of arthritis | |||
| PIP | 11 (21.6%) | 10 (30.3%) | 0.367 | None | 14 (26.9%) | 0 | 0.001** |
| DIP | 6 (11.7%) | 5 (15.1%) | 0.653 | Axial | 11 (21.1%) | 2 (6.1%) | |
| Wrist | 9 (17.6%) | 12 (36.4%) | 0.053 | Asymmetrical | 23 (44.2%) | 26 (78.8%) | |
| Knee | 15 (29.4%) | 13 (39.4%) | 0.343 | Symmetrical | 4 (7.7%) | 5 (15.2%) | |
| Ankle | 14 (27.4%) | 15 (45.5%) | 0.090 | Endoscopic findings | |||
| Feet | 13 (25.5%) | 14 (42.4%) | 0.105 | ND | 27 (51.9%) | 28 (84.8%) | 0.005* |
| Sacroiliac joint | 10 (19.6%) | 3 (9.1%) | 0.193 | Normal | 0 | 1 (3%) | |
| Hip | 11 (22%) | 2 (6.1%) | 0.051* | CD | 8 (15.4%) | 2 (6.1%) | |
| Nail changes | 14 (27.5%) | 4 (12.1%) | 0.094 | UC | 17 (32.7%) | 2 (6.1%) | |
| Sausage digit | 14 (27.4%) | 11 (33.3%) | 0.565 | Medications | |||
| Sausage toes | 11 (21.6%) | 10 (30.3%) | 0.367 | SAZ | 25 (48.1) | 21 (63.3) | 0.161 |
| Presence of skin psoriasis | 17 (34.7%) | 0 | <0.001** | CS | 13 (25) | 10 (30.3) | 0.592 |
| Iridocyclitis | 1 (1.9%) | 4 (12.1%) | 0.051* | AZA | 9 (17.3) | 4 (12.1) | 0.517 |
| Family history of SPA | 29 (55.8%) | 13 (39.4%) | 0.141 | MTX | 28 (53.8) | 26 (78.8) | 0.020 |
| Positive MRI findings | 16 (30.7%) | 15 (45.4%) | 0.170 | Anti-TNF | 34 (65.4) | 3 (9.1) | <0.001** |
USpA: undifferentiated SPA, SpA: seronegative spondylitis, PIP: proximal interphalangeal; DIP: distal interphalangeal; MRI: magnetic resonance image; SAZ: salazopyrin; TNF: tumour necrosis factor.
Laboratory findings among the studied groups of patients.
| Parameters | SpA (n=52) | USpA (n=33) | p-value |
|---|---|---|---|
| HGB (g/dl) | 11.7±1.4 | 11.5±1.6 | 0.581 |
| WBCs (×103/mm3) | 8.8±2.1 | 8.3±2.8 | 0.258 |
| PLT (×103/mm3) | 334.6±78.1 | 339.6±77.9 | 0.776 |
| ESR (1st hour) | 39.8±18.5 | 37.2±15 | 0.506 |
| CRP (mg/dl) | 4.5±4.3 | 4.6±3.6 | 0.921 |
| FCP level (μg/g) mean | 448.6±457.3 | 139.1±136.7 | <0.001** |
| Median (IQR) | 268 (22–1200) | 66 (25–345) | |
| Elevated FCP | 43 (82.7%) | 22 (66.7%) | 0.090 |
| Occult blood in stool | 19 (36.5%) | 3 (9.1%) | 0.005* |
WBCs: white blood cells, HGB: haemoglobin, PLT: platelets, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate.
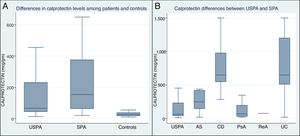
The mean FCP level (μg/g) was significantly higher in the SpA patients (448.6±457.3) compared to USpA (139.1±136.7) and to control subjects (29±15) (p<0.001), Fig. 4A. In addition, within the SpA group, the UC and CD patients displayed statistically significantly increased FCP levels compared to other SpA subgroup of patients and USpA (p<0.001), the mean differences between the subgroups of SpA patients and USpA group are illustrated in Fig. 4B.
When we considered all patients as one group (n=85), we found significant associations between FCP levels (μg/g) versus; age at onset (r=−0.22, p=0.03), ESR 1st hour (r=0.32, p=0.003), occult blood in stool (r=0.66, p<0.001), Hb level (r=−0.30, p=0.005), WBCs (r=0.24, p=0.03), platelets count (r=0.36, p<0.001) but not versus CRP.
DiscussionThere is cumulative evidence in the literature supporting that bowel inflammation in SpA group of disorders can affect the ileum as well as the colon, especially the terminal ileum. Already in 1980s, two types of GIT inflammation could be identified based on histopathological assessment, the first is acute inflammation where the epithelium is infiltrated with granulocytes yet normal mucosal architecture, and the second is a chronic pattern of inflammation with disturbed mucosal architecture and chronic lymphoplasmacytic infiltration in the lamina propria. The mucosal changes seen in the latter type bear particular resemblance to those documented in early phases of Crohn's disease (CD) bowel inflammation.1
Most of the previous studies that investigated FCP levels were performed in patients with well differentiated SpA group of disorders.2,18–24 Van Praet et al. performed ileocolonoscopy in 65 patients with axial and peripheral SpA and found that overall, 46.2% of the patients showed microscopic gut inflammation.18 The authors reported that axial SpA (axSpA) disease pattern, younger age with shorter symptom duration, progressive disease course, male gender and higher disease activity are independently associated with microscopic GIT inflammation. In another report 64 SpA patients with chronic GIT symptoms demonstrated mucosal inflammation characteristic of CD confirmed by video capsule endoscopy (CE), the latter is considered to be a superior diagnostic tool to detect small bowel mucosal disease.19 The authors found that FCP levels correlated significantly with CE results, while GIT symptoms were poor predictors of small bowel inflammation (SBI).19 Moreover elevated FCP can accurately identify SpA patients with subclinical bowel inflammation as detected by MRI and elevated levels at baseline could predict a better response to treatment.20 In another work Klingberg et al. assessed FCP in AS patients at baseline (n=204) and at 5-year of follow-up (n=164).21 The authors reported that FCP>50mg/kg was found in two-thirds of the patients at both study visits and 1.5% of the study group developed CD like lesions, the latter was predicted by higher FCP levels at baseline. While Turina et al. reported that a serum level of CP is an independent marker for radiographic spinal progression in axSpA.22 Interestingly a clinical improvement and reduction in serum CP levels was observed after an intensive exercise programme in patients with AS and in patients with non-radiographic axSpA (nr-axSpA).23 Nevertheless serum CP can be utilised as a marker for inflammation in both nr-axSpA and AS. Elevated CP correlated positively with ESR, CRP as we observed in our study as well as Bath Ankylosing Spondylitis Disease Activity Index(BASDAI), and Ankylosing Spondylitis Disease Activity Score(ASDAS) and as the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index.24
Interestingly the CP mediated inflammation does not correlate with principle effectors of the Wnt/β-catenin pathway, indicating that gut inflammation and bone fusion might be separate processes of the disease pathogenesis in AS. Of note, the Wnt/β-catenin pathway is a complex signalling pathways responsible for signal transduction which begins with proteins that pass signals into a cell through cell surface receptors.25 The Wnt signalling pathway is essential for both embryonic skeletogenesis, and also plays critical roles in homeostasis and bone health in adults. In recent years, there have been mounting data to suggest that this pathway is quite involved in osteoblastogenesis and can be unregulated by inflammation.26
In our study we found that the mean FCP level (μg/g) was significantly higher in the SpA group when compared to USpA and to normal control subjects (p<0.001). In accordance with our results, Kopylov et al.19 and Van Hoovels et al.27 reported significantly higher FCP levels in the SpA group versus the non-SpA group. Moreover in our study we demonstrated significantly higher FCP levels in UC and CD patients compared to other SpA subgroup of patients and USpA (p<0.001).
An ROC analysis was performed in our study to reveal the most diagnostic performance levels for the different FCP assays in our studied groups of patients. When the manufacturer's cut-off (50μg/g) was applied, the sensitivity was 66.7%, specificity was 17.3%, positive predictive value (PPV) 39.3%, negative predictive value (NPV) 45%, false negative rate (FNR): 33.3%, and false positive rate (FPR): 82.7%. Positive likelihood ratio (LR+) was 1.5, and negative likelihood ratio (LR−) was 0.61. The area under the curve (AUC) at this level was 0.61, 95% CI (0.48–0.73). On performing the ROC analysis to determine the optimal cut-off value of FCP for the differentiating USpA and SpA, we found that a FCP cut-off value at 64mcg/g showed 82.7% sensitivity, 45.5% specificity, 1.5 LR+, and LR− was 0.38. At this level, AUC increased to 0.71, 95% CI (0.60–0.82). In a recent work Van Hoovels et al. quantified FCP in 99 adult patients with a clinically suspected SpA out of the 99 patients the definitive diagnosis of SpA was reached in 52 patients.27 A ROC analysis was performed to reveal the diagnostic performance for the different FCP assays for SpA. The area under the curve did not differ significantly among assays, but there was a significant difference in sensitivity and specificity for SpA when the manufacturer's cut-offs of different assays were applied.27
It appears that GIT inflammation may occur in patients with SpA including the clinically evident well differentiated IBD in the form of either CD or UC and that others display a subclinical gut inflammation despite the absence of signs and symptoms of IBD. Such subclinical gut inflammation in patients with SpA, is apparently driven by intestinal dysbiosis, dysregulating the intestinal epithelial barrier, especially in human leucocyte antigen (HLA-B27) positive patients.28 Moreover a dysregulated microbiome along with the migration of T lymphocytes and other cells from gut to the joint (“gut-joint” axis) has been identified in the context of genetic background in terms of associations with alleles inside or outside the HLA system.29
In our opinion FCP may play an additive diagnostic role especially in those patients with clinical suspicion of early undifferentiated SpA that remain unclassified after initial clinical assessment, radiological and laboratory investigations. Careful history taking and clinical evaluation remains the cornerstone for assessment of SpA group of disorders even if the disease remained in the undifferentiated phase. Silent microscopic gut inflammation can be seen in around 60% of AS patients.30 Overlapping SpA features are comprehensively included in the European Spondylarthropathy Study Group preliminary criteria for the classification of differentiated SpA as well as USpA that remain unclassified.13 In early disease presentations of SpA the presence of inflammatory spinal pain and/or synovitis which is usually asymmetric and predominantly involving the lower limbs, together with at least 1 of the following: positive family history, psoriasis, IBD, urethritis, or diarrhoea, enthesitis, dactylitis or sacroiliitis as determined from radiography of the pelvic region. Given that FCP could play a diagnostic role in detecting subclinical gut inflammation especially in those patients with USpA.
The understanding of all these complex overlapping features is not only important from the diagnostic point of view but also carry important prognostic as well as therapeutic implications. The mounting evidence of subclinical autoimmune gut inflammation in classified and unclassified SpA warrants special precautions when considering the use of non-steroidal anti-inflammatory drugs (NSIADs) in these patients. It is now established that NSAIDs may lead to relapse of IBD and could provoke disease activity in both UC and CD. More seriously NSAIDs are associated with hospitalisations for severe colitis in patients with IBD.31
ConclusionsIncreased FCP levels may identify patients who are most likely to have SpA already in the unclassified phase of the disease. An elevated level of FCP in either classified or unclassified SpA could point to an underlying ongoing autoimmune gut inflammation and can give an indication to perform diagnostic ileocolonoscopy in such clinical scenario. This knowledge may also have therapeutic consequences. The use of NSAIDs seems serious and can lead to an exacerbation of a silent inflammatory bowel disease in both clinical entities whether classified or still in the unclassified phase of the disease. Further studies in different series of patients are needed to evaluate the potential diagnostic and prognostic roles of FCP in both differentiated and undifferentiated phases of the disease.
Conflict of interestThe authors declare that they have no conflicts of interest concerning this article.
The authors thank the patients who participated in the study.