To determine predictive factors for the development of lupus nephritis (LN) at the time of diagnosis of systemic lupus erythematosus (SLE).
MethodsA case-control study was carried out in a single center, 595 patients with a diagnosis of SLE without LN participated by clinical or laboratory parameters at diagnosis, they were followed for a mean of 6.8 (+4.5) years, conforming to the data of their files two groups: with NL (cases) and without NL (controls) at the end of the follow-up. Sociodemographic, clinical, serological, immunological variables and the albumin – globulin ratio (AGR), calculated as albumin/total protein-albumin at diagnosis, were compared between both groups. A univariate and multivariate analysis was carried out.
Results124 (20.8%) patients had LN during follow-up and 471 (79.2%) did not develop LN. Univariate analysis: variables significantly associated with the development of LN: smoking, oral ulcers, serositis, more than four classification criteria, abrupt onset of SLE, higher SLEDAI value, low AGR, low C3 levels, high anti-titers. –Double stranded DNA (anti-dc DNA), anti-nucleosomes and positivity of immunofluorescence in skin. Multivariate analysis: predictors of developing LN: elevated serum levels of anti-dc DNA (odds ratio (OR): 15.82; confidence interval (CI): 1.08−1.22, P < .0001), decrease in the C3 fraction (OR: 36.50; CI: 13.52–81.91, P < .0001) and the RAG < 1 (OR: 47.58; CI: 11.85−79.17, P < .0001).
ConclusionThe AGR below one was the greatest predictor of the appearance of LN, together with the low levels of C3 and high levels of anti-dc DNA antibodies, they may contribute to identifying patients with a higher risk of presenting LN.
Determinar factores predictores de desarrollo de nefritis lúpica (NL) al momento del diagnóstico del lupus eritematoso sistémico (LES).
MétodosSe realizó un estudio de casos y controles en un único centro, participaron 595 pacientes con diagnóstico de LES sin NL por parámetros clínicos o de laboratorio al diagnosticarlos, fueron seguidos durante una media de 6,8 (±4,5) años, conformándose de los datos de sus expedientes dos grupos: con NL (casos) y sin NL (controles) al final del seguimiento. Se compararon entre ambos grupos variables sociodemográficas, clínicas, serológicas, inmunológicas y la relación albumina–globulina (RAG), calculada como la albumina/proteínas totales-albumina al diagnóstico. Se efectuó un análisis univariado y multivariado.
ResultadosEn el seguimiento presentaron NL 124 (20,8%) pacientes y no desarrollaron NL 471 (79, 2%). Análisis univariado: variables asociadas significativamente al desarrollo de NL: hábito de fumar, ulceras orales, serositis, más de cuatro criterios de clasificación, inicio abrupto del LES, mayor valor de SLEDAI, baja la RAG, bajos niveles de C3, títulos elevados de anti–DNA doble cadena (anti-DNA dc), anti nucleosomas y positividad de la inmunoflurescencia en piel. Análisis multivariado: factores predictores de desarrollar NL: niveles séricos elevados de anti-DNA dc (odds ratio (OR):15, 82; intervalo de confianza (IC):1,08–1,22, P < ,0001), disminución de la fracción C3 (OR: 36, 50; IC: 13,52 – 81,91, P < ,0001) y la RAG < 1 (OR: 47,58; IC: 11,85–79,17, P < ,0001).
ConclusiónLa RAG por debajo de uno fue el mayor predictor de aparición de la NL, conjuntamente con los niveles bajos de C3 y elevados de anticuerpos anti-DNA dc, pueden contribuir a identificar pacientes con mayor riesgo de presentar NL.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect multiple organs and tissues, and the development of kidney disease is the most important predictor of morbidity and mortality. Lupus nephritis (LN) is a serious complication of the disease, affecting 25%–75% of patients, its frequency is influenced by ethnicity, gender, and age at presentation, among other factors1–3. Despite advances in the treatment of LN, a not insignificant group of patients will develop irreversible kidney damage4–6.
LN can be the first manifestation of SLE; its onset is usually within the first year of diagnosis, but it can develop over the course of the disease7,8. Early diagnosis, use of immunosuppressive agents and response to induction therapy are important factors in achieving better results in controlling the condition9–12.
When comparing the efficacy of treatments according to the time of onset of LN, whether at the beginning of the disease or during its course, the worst results have been obtained in the latter group of patients8,13,14. Other studies have attempted to identify demographic, clinical, serological and immunological differences between the groups with dissimilar results14–16. In addition, the association of serum globulin and albumin levels and the presence of immunoglobulin deposits at the dermoepidermal junction in the skin have been analysed in relation to the subsequent development of LN16–19.
The aim of this study was to determine predictors of LN development at time of SLE diagnosis.
Patients and methodsWe conducted a case-control study from the total patients diagnosed with SLE in the rheumatology department of the Hermanos Ameijeiras Hospital, Havana, Cuba, between 3 January 2005 and 31 December 2019; the patients met the American College of Rheumatology (ACR) classification criteria for SLE20. Patients were excluded if they had any of the following laboratory data for clinical LN on diagnosis of the condition: proteinuria ≥.5 g/24 h or proteinuria+++, serum creatinine ≥123.7 mol/l, estimated glomerular filtration rate ≤60 mL/min/1.73 m2, erythrocyte or leukocyte count ≥10 cells per high-power field, ≥3 granular or cellular cylinders per high-power field. Those with culture-confirmed active infection, liver dysfunction, diagnosed malignancy, pregnant women, and cases with incomplete data in their records that did not allow assessment were followed for a mean of 6.8 (±4.5) years, the data from their records were used to create two groups: one that developed clinical LN or detected through kidney biopsy (cases) and the other without clinical LN (controls) during follow-up.
The following baseline data were collected at the time of diagnosis: age, sex, ethnicity according to skin colour and anthropometric characteristics classified by three specialists as white, black or mestizo, level of education, smoking, alcohol consumption, presence of clinical symptoms such as arthritis, photosensitivity, facial erythema, discoid erythema, alopecia, mouth ulcers, fever, serositis, central nervous system (CNS) involvement, quantification of the number of classification criteria, form of presentation of the condition: abrupt (presentation of symptoms in less than 4 weeks) or insidious (more than 4 weeks until presentation of symptoms).
We also considered the values or presence of: haemoglobin, leukocytes, lymphocytes, platelets, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatinine, urea, serum total protein and albumin, albumin-to-globulin ratio (AGR) obtained by the equation AGR = albumin /(total protein – albumin), glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), glomerular filtration rate (GFR), C3 and C4 fraction, anti-double-stranded DNA (anti-dsDNA), anti-Smith (anti-Sm), anti-RNP, anti-nucleosome antibodies, anti-Ro/SSa and anti-La/SSb, lupus anticoagulant, presence of immunoglobulin deposits in immunofluorescence study on biopsy of healthy sun-exposed skin (lupus band). Disease activity was measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)21. To achieve the aim of the study, the existence of LN at follow-up was defined by the presence of one of the following two criteria:
- 1
By renal biopsy according to the classification of the International Society of Nephrology and Renal pathology22.
- 2
Presence of clinical LN: proteinuria ≥.5 g/24 h or proteinuria+++; and/or one of the following features attributable to SLE, present at ≥2 visits at least 6 months apart: proteinuria++, serum creatinine ≥123.7 μmol/l, estimated glomerular filtration rate ≤60 mL/min/1.73 m2, erythrocyte or leukocyte count ≥10 cells per high-power field, ≥3 granular or cellular cylinders per high-power field.
The time to onset of nephritis was considered to be the interval between the diagnosis of SLE and the time of LN onset.
The research project was approved by the centre’s ethics committee and scientific council, an institution of the Ministry of Public Health of the Republic of Cuba. The identity of the patients was preserved; as this was a retrospective study, informed consent was not required.
Statistical analysisThe χ2 or Fisher’s exact test were used to compare between groups for qualitative variables, as appropriate. For quantitative variables, the Student’s t-test for independent samples or the Mann-Whitney U test was used, according to the normality of the population between the groups. A multivariate binary logistic regression (BLR) was performed to identify predictors of LN. In the results of the final BLR model, odds ratios (OR) and confidence intervals (CI) were shown for each predictor variable, as well as statistical significance (P-value). Hypothesis tests with a P < .05 were considered statistically significant. ROC curves were performed for LN predictor variables and the area under the curve (AUC) was estimated. SPSS version 20.0 was used to analyse the data.
ResultsOf the 768 patients seen with a diagnosis of SLE, 148 were excluded as they had LN at disease onset (19.3%), 10 due to active infection, 8 due to liver dysfunction, 3 due to malignant disease, 2 due to pregnancy and 2 due to incomplete records, leaving 595 patients included in the study.
Of the patients 595 had no signs of LN at SLE diagnosis; 124 (20.8%) developed LN during follow-up and 471(79.2%) remained without nephritis. The mean duration of follow-up was 6.8 (±4.5) years and the mean time to developing LN was 14.7(±11.3) months. The cumulative incidence of LN was 35.4%. Of the cases included, 93.1% were female, with a female:male ratio of 13.5:1.
Nephropathy was diagnosed by biopsy in 122 cases; 67 of them (55.7%) were class IV, 21 (16.9%) class II, 19 (15.3%) class III, and 15 (12.1%) class V. It was not possible to perform biopsy in 2 patients due to coagulation disorders and it was imperative to initiate treatment; these 2 patients met the laboratory criteria indicated in the method for determining the existence of LN.
Table 1 shows that the age distribution was similar in both groups, as was the predominance of female sex, and that black ethnicity was significantly associated with the onset of LN. A set of variables presented higher values or frequencies in the group that developed LN, such as smoking, abrupt onset of the disease, SLEDAI values, anti-dsDNA, anti-nucleosomes, all with significant differences, as did low levels of C3 and AGR < 1.
Characteristics at diagnosis of SLE patients who did and who did not develop lupus nephritis at follow-up (univariate analysis).
| Variables | SLE with nephritis n = 124 | SLE without nephritis n = 471 | P |
|---|---|---|---|
| Age in years (SD) | 34.1 (11.3) | 33.7 (12.0) | .738 |
| Female sex | 116 (93.5%) | 438 (93.0%) | .506 |
| Ethnicity | |||
| White | 81 (65.3%) | 306 (64.9%) | .177 |
| Black | 22 (17.7%) | 47 (10.1%) | .021 |
| Mestizo | 21 (17%) | 118 (25%) | .019 |
| Smoking | 49 (39.5%) | 85 (18.0%) | <.001 |
| Arthritis | 98 (79%) | 320 (67.9%) | .010 |
| Photosensitivity | 77 (62.1%) | 325 (69.0%) | .089 |
| Facial erythema | 85 (68.5%) | 307 (65.2%) | .277 |
| Fever | 78 (62.9%) | 245 (52.0%) | .019 |
| Alopecia | 72 (58.1%) | 207 (43.9%) | .003 |
| Mouth ulcers | 58 (46.8%) | 146 (31.0%) | <.001 |
| Serositis | 38 (30.6%) | 80 (17.0%) | <.001 |
| Discoid erythema | 15 (12.1%) | 97 (20.6%) | .019 |
| CNS manifestations | 15 (12.1%) | 40 (8.5%) | .145 |
| ACR criteria, more than 4 | 81 (65.3%) | 179 (38.0%) | <.001 |
| Abrupt onset of disease | 8 (6.5%) | 0 (.0%) | <.001 |
| SLEDAI | 12.1 ± 3.5 | 9.3 ± 4.13 | <.001 |
| Serum creatinine (μmol/l) | 76 ± 13 | 79 ± 12 | <.001 |
| Total protein (g/l) | 74 ± 5.9 | 78 ± 6.8 | <.001 |
| Serum albumin (g/l) | 36 ± 3.5 | 38 ± 3.2 | <.001 |
| AGR < 1 | 110 (88.7%) | 66 (14.0%) | <.001 |
| AGR | .805 ± .198 | 1.095±.289 | <.001 |
| GPT (U/l) | 32.5 ± 10.7 | 32.3 ± 9.1 | .913 |
| GOT (U/l) | 28.4 ± 10.3 | 30.0 ± 9.8 | .072 |
| C3 (g/l) | .7 ± .3 | 1.1±.3 | <.001 |
| C4 (g/l) | .14 ± .1 | .15±.1 | .336 |
| Anti-nucleosome (U/l) | 10.8 ± 62.6 | .17±.38 | <.001 |
| Anti-dsDNA (U/mL) | 109.6 ± 84.1 | 20.1 ± 42.8 | <.001 |
| Anti-Smith (U/mL) | .96 ± 9.05 | .15±.36 | .054 |
| Anti-SSA/Ro (U/mL) | .20 ± .4 | .20 ± .4 | .983 |
| Anti-SSB/La (U/mL) | .08 ± .3 | .06±.2 | .468 |
| Anti-RNP (U/mL) | .18 ± .39 | .17±.37 | .747 |
| Lupus anticoagulant positive | 10 (8.1%) | 49 (10.4%) | .428 |
| Positive immunofluorescence of healthy skin | 95 (76.6%) | 144 (30.6%) | <.001 |
ACR: American College of Rheumatology; AGR: Albumin-to-globulin ratio; CNS: Central Nervous System; ds: double stranded; SD: Standard Deviation; SLEDAI: Systemic lupus erythematosus disease activity index; GOT: Glutamic Oxaloacetic Transaminase; GPT: Glutamic Pyruvic Transaminase.
Other variables compared between groups with a significance level of P < .001 were alopecia, oral ulcers and serositis, having more than 4 ACR classification criteria, serum creatinine, total protein, serum albumin, C3, and positive immunofluorescence of healthy skin.
All the other variables analysed were not statistically different between the two groups.
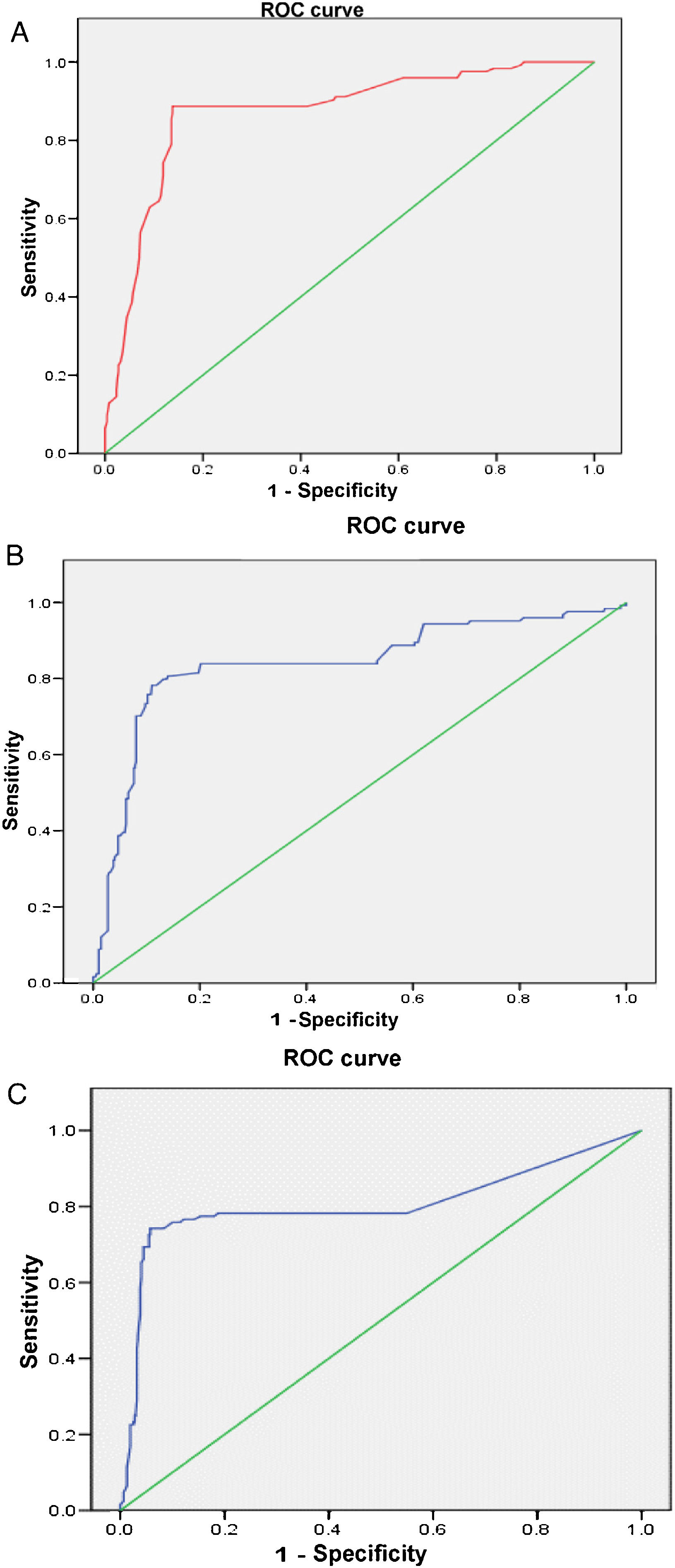
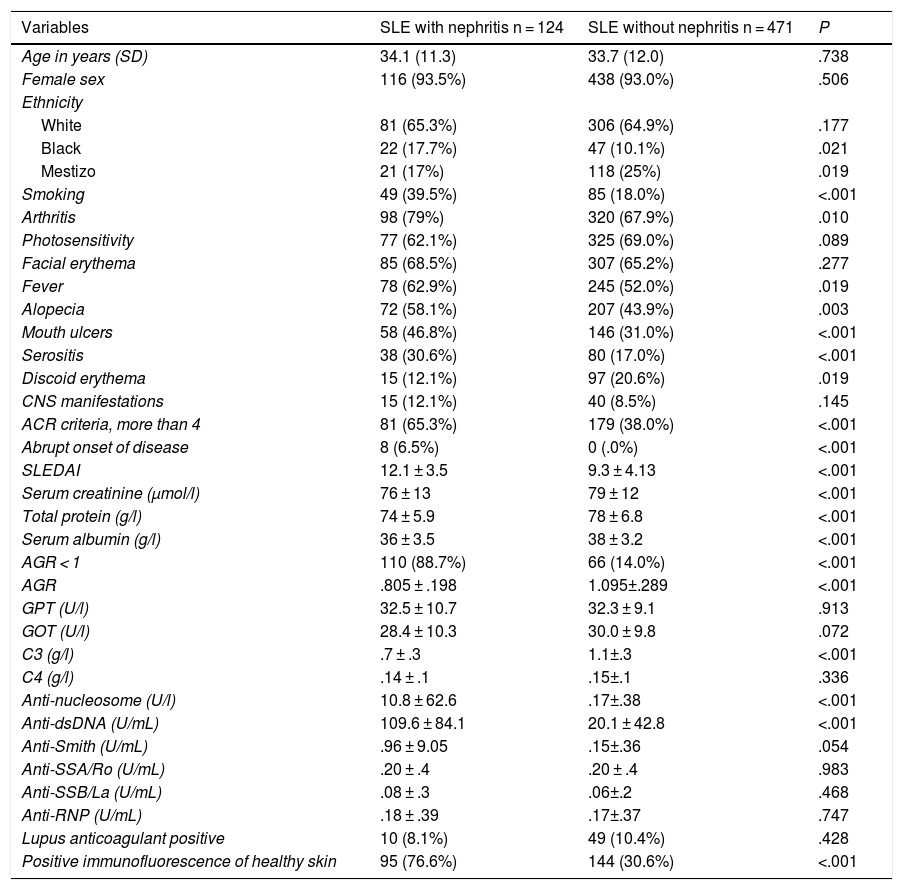
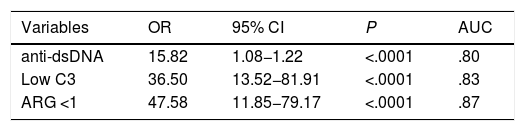
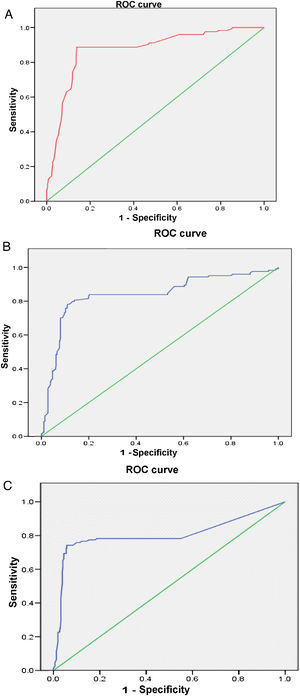
The multivariate analysis (Table 2) shows the variables that were predictors of LN, and included those that were significant in the univariate analysis; the AUC values of the ROC curves of the predictors, shown in Fig. 1 (Fig. 1A–C), were also included in the Table, highlighting an AGR below unity as the main predictor of LN. The rest of the variables that had been significant in the univariate analysis were not associated with the development of LN in the multivariate analysis.
Predictors of the development of lupus nephritis (multivariate analysis).
| Variables | OR | 95% CI | P | AUC |
|---|---|---|---|---|
| anti-dsDNA | 15.82 | 1.08−1.22 | <.0001 | .80 |
| Low C3 | 36.50 | 13.52−81.91 | <.0001 | .83 |
| ARG <1 | 47.58 | 11.85−79.17 | <.0001 | .87 |
AGR: Albumin-to-globulin ratio; AUC: Area under the curve; CI: Confidence Interval; ds: double stranded; OR: odds ratio.
This study reaffirms the usefulness of the AGR value at disease onset as a predictor of risk for developing LN during the course of SLE. Patients with values lower than one have a higher prognosis of developing LN over the course of the disease; this could be explained by a higher level of globulins within the total proteins, closely linked to the elevated autoantibodies necessary for kidney involvement to be expressed22.
The AGR has been used as a prognostic indicator in other diseases such as cancer23, but there are few reports of its use in SLE in this regard and these are from an Asian population16. Therefore we consider these results a contribution from a different population, in this case of Hispanic and African descent.
The study includes a large number of patients, and as it deals with cases from a single hospital, it may ensure greater uniformity of results in a population that is very ethnically mixed between whites, blacks, and mestizos, which inevitably entails a bias that is difficult to control when defining it, since in our setting it is not uncommon to see people of white skin colour with a close black relative. Nevertheless, black patients were significantly associated with the development of LN, which confirms the greater severity of the disease in people of African descent, which has been repeatedly published24,25.
The cumulative incidence of LN, which varies among different populations, may be due to sociodemographic, environmental and immunogenetic differences, and to the different inclusion criteria used; there are reports in Caucasian (29.1%), Hispanic (60.6%) and African American (68.9%) patients studied in the United States and a study in 391 Korean patients reported a frequency of 34.8%. A multinational research study involving countries in the Americas, Europe, and Asia, with a total of 1827 patients, reported a frequency of LN at follow-up of 38.3%16,24–26. The cumulative incidence established in our study (35.4%) is among the reported values, but lower than the published value in African American and Hispanic patients studied in the United States and that of the Latin American GLADEL cohort. We consider that this can be attributed to the different genetic characteristics of the Cuban population24,27.
Smoking was associated with the subsequent development of nephritis, which could be related to a greater presence of anti-dsDNA antibodies that have a pathogenic relationship with the development of LN28. This is a modifiable variable, which should be considered from the onset of the disease and should be addressed by educating patients and family members. The association of pleurisy, higher disease activity values, as well as a higher number of criteria at diagnosis and abrupt onset, were variables that were associated with the subsequent presence of LN and are results reported in the literature24.
Low C3 levels were another variable of high prognostic value for the subsequent development of LN. This finding suggests the activation of the alternative complement pathway for damage to occur in LN. Previous studies have shown the association between this variable and the presence of anti-dsDNA antibodies with LN29,30.
The study has its limitations. It is a retrospective research study in which only variables present at diagnosis were considered, and therefore there were no evaluation points at follow-up that could have added other variables, such as treatments and responses to treatments, which may influence the development of LN. In relation to antimalarials, studies in a Latin American population with various ethnic groups conducted by Pons-Estel et al. in the framework of the GLADEL multinational cohort found that the use of these drugs was associated with less kidney disease24,27.
In conclusion, we show in the Cuban population that a low AGR present at diagnosis of SLE is associated with the risk of developing LN during the course of the disease; moreover, it is a relatively low-cost test that, together with other indicators, such as low C3 levels and elevated anti-dsDNA antibodies, can help identify patients at increased risk of developing LN from the time of diagnosis. It is true that C3, anti-dsDNA, albumin levels and/or AGR may be affected by subclinical nephropathy and may already be marking the presence of kidney disease rather than predicting its development. This is a plausible hypothesis, like that established when autoantibodies are detected in serum before the disease is clinically expressed29, but we also consider that determining their presence means we can design preventive strategies to avoid the clinical expression of nephritis on the one hand and of disease on the other. We can also, within these actions, programme patient monitoring to detect clinical or laboratory signs of nephritis promptly and initiate early treatment which will benefit the long-term prognosis of the disease.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Estévez del Toro M, Varela Ceballos I, Chico Capote A, Kokuina E, Sánchez Bruzón Y, Casas Figueredo N. Factores predictores del desarrollo de nefritis lúpica después del diagnóstico de lupus eritematoso sistémico. Reumatol Clin. 2022;18:513–517.