Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). We assessed tofacitinib efficacy and safety in the Latin American (LA) subpopulation of global Phase 3 and long-term extension (LTE) studies.
Materials and methodsData from LA patients with RA and inadequate response to disease-modifying antirheumatic drugs (DMARDs) were pooled across five Phase 3 studies. Phase 3 patients received tofacitinib 5 or 10mg twice daily (BID), adalimumab or placebo; patients in the single LTE study received tofacitinib 5 or 10mg BID; treatments were administered alone or with conventional synthetic DMARDs. Efficacy was reported up to 12 months (Phase 3) and 36 months (LTE) by American College of Rheumatology (ACR) 20/50/70 response rates, Disease Activity Score (DAS)28-4(erythrocyte sedimentation rate [ESR]) and Health Assessment Questionnaire-Disability Index (HAQ-DI). Incidence rates (IRs; patients with event/100 patient-years) of adverse events (AEs) of special interest were reported.
ResultsThe Phase 3 studies randomized 496 LA patients; the LTE study enrolled 756 LA patients from Phase 2 and Phase 3. In the Phase 3 studies, patients who received tofacitinib 5 and 10mg BID showed improvements vs placebo at Month 3 in ACR20 (68.9% and 75.7% vs 35.6%), ACR50 (45.8% and 49.7% vs 20.7%) and ACR70 (17.5% and 23.1% vs 6.9%) responses, mean change from baseline in HAQ-DI (−0.6 and −0.8 vs −0.3) and DAS28-4(ESR) score (−2.3 and −2.4 vs −1.4). The improvements were sustained up to Month 36 in the LTE study. In the Phase 3 studies, IRs with tofacitinib 5 and 10mg BID and placebo were 7.99, 6.57 and 9.84, respectively, for SAEs, and 3.87, 5.28 and 3.26 for discontinuation due to AEs. IRs of AEs of special interest in tofacitinib-treated LA patients were similar to the global population.
ConclusionIn Phase 3 and LTE studies in LA patients with RA, tofacitinib demonstrated efficacy up to 36 months with a manageable safety profile up to 60 months, consistent with the overall tofacitinib study population.
Tofacitinib es un inhibidor oral de la quinasa Janus para el tratamiento de la artritis reumatoide (AR). Este análisis evaluó la eficacia y la seguridad de tofacitinib en la subpoblación Latinoamericana (LA) de los estudios fase 3 y de extensión a largo plazo (ELP).
Materiales y métodosSe agruparon datos de pacientes de Latinoamérica con AR y una respuesta inadecuada a agentes modificadores de la enfermedad (DMARD) de 5 estudios fase 3. Los pacientes en estos estudios recibieron tofacitinib 5 o 10mg/2 veces al día (bid), adalimumab o placebo; los pacientes en el estudio de seguridad recibieron tofacitinib 5 o 10mg/bid; los tratamientos se administraron en monoterapia o con DMARD sintéticos convencionales. La eficacia se reporta hasta 12 (fase 3) y 36 meses (ELP) mediante las tasas de respuesta del Colegio Americano de Reumatología (ACR) 20/50/70, el índice de actividad de la enfermedad (DAS)28-4 ESR (tasa de sedimentación globular [ESR]) y el índice de discapacidad del cuestionario de evaluación de la salud (HAQ-DI). Se reportan las tasas de incidencia (IR: pacientes con evento/100 pacientes/año) de eventos adversos (EA) de interés especial.
ResultadosLos estudios fase 3, incluyeron 496 pacientes de LA, el ELP reclutó 756 pacientes de fase 2 y fase 3. En los estudios de fase 3, los pacientes que recibieron tofacitinib 5 y 10mg/bid presentaron mejorías vs placebo al mes 3 en las respuestas ACR20 (68,9% y 75,7% vs 35,6%), ACR50 (45,8% y 49,7% vs 20,7%) y ACR70 (17,5% y 23,1% vs 6,9%), en cambio, desde el valor basal en el escore HAQ-DI (−0,6 y −0,8 vs −0,3) y en el escore DAS28-4(ESR) (−2,3 y −2,4 vs −1,4); estas mejorías fueron sostenidas hasta el mes 36, último mes de evaluación en el estudio de ELP. En los pacientes con tofacitinib 5 o 10mg/bid y placebo, las tasas de incidencia de SAE fueron de 7,99, 6,57 y 9,84, mientras que la incidencia de descontinuaciones por EA fueron de 3,87, 5,28 y 3,26, respectivamente. Las IR de EA de interés especial en pacientes de LA fueron similares a la población global.
ConclusiónEn los pacientes de LA con AR de estudios fase 3 y ELP, tofacitinib demostró eficacia hasta por 36 meses con un perfil de seguridad manejable hasta por 60 meses, en los pacientes de LA con AR, datos consistentes con el de la población global de los estudios de tofacitinib.
Rheumatoid arthritis (RA) is a chronic and debilitating autoimmune disease. A conservative RA prevalence rate of 0.4% has been estimated for Latin America (LA).1
RA management in LA differs from that in Europe or the US because of various challenges, e.g. delays in patient referral to rheumatologists; limited access to cost-effective medication and resources; difficulty accessing public health systems; and a lack of public policies and education surrounding RA.1 Regarding the clinical profile of RA, LA differs from other regions in terms of genetic and demographic factors.2 Infectious diseases and tuberculosis (TB) are also more prevalent in LA vs Europe and the US, and need to be taken into consideration when selecting RA therapies in this region.3,4
Current prescribing patterns are similar across LA countries, with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) prescribed as first-line treatment, followed by biologic DMARDs (bDMARDs) for patients with an inadequate response.1 However, there is still an unmet need for alternative RA therapies, as globally 20–30% of patients treated with bDMARDs still have active disease,5 and differences exist between guidelines from LA countries and those from the US and Europe regarding bDMARD recommendations.6
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. Phase 3, randomized controlled studies have demonstrated the efficacy and safety of tofacitinib 5 and 10mg twice daily (BID) as monotherapy or in combination with csDMARDs.7–12 Tofacitinib efficacy was maintained up to 96 months, with a manageable safety profile, in global long-term extension (LTE) studies.13
Since there are differences in epidemiology and treatment guidelines between LA and other regions, and little published information surrounding tofacitinib treatment in LA, this analysis assessed the efficacy and safety of tofacitinib in the LA subpopulation of Phase 3 and LTE global studies in patients with RA.
Materials and methodsPatientsThis analysis included data from patients with RA enrolled in global Phase 3 and LTE studies of tofacitinib in Brazil, Chile, Colombia, Costa Rica, Dominican Republic, Mexico, as well as Argentina (LTE only; patients enrolled from Phase 2 studies) and Venezuela (Phase 3 only). Inclusion and exclusion criteria have been previously reported for the global studies.7–11,14 Patients were ≥18 years old with a diagnosis of moderate to severe active RA based on the American College of Rheumatology (ACR) 1988 criteria. Key exclusion criteria included: serious chronic or recurring infections; active or inadequately treated latent TB; history of recurrent herpes zoster (HZ); hepatitis B or C; or other opportunistic infections and evidence or history of malignancy (except adequately treated or excised non-metastatic basal or squamous cell cancer of the skin or cervical carcinoma in situ), or lymphoma/lymphoproliferative disease.
Study designsData from the following five Phase 3, double-blind, randomized controlled studies of 6–24 months in duration were pooled into a single data set for this analysis: ORAL Step (NCT00960440)7; ORAL Scan (NCT00847613)10; ORAL Solo (NCT00814307)8; ORAL Sync (NCT00856544)9; ORAL Standard (NCT00853385).11
The Phase 3 RA population included patients who previously had an inadequate response to either methotrexate (ORAL Standard, ORAL Scan), ≥1 bDMARD or csDMARD (ORAL Sync, ORAL Solo), or ≥1 Tumor Necrosis Factor inhibitor (TNFi; ORAL Step). Patients were randomized to receive tofacitinib 5mg BID, tofacitinib 10mg BID or placebo as either monotherapy (ORAL Solo), or in combination with background methotrexate (ORAL Scan, ORAL Step, and ORAL Standard) or csDMARDs (ORAL Sync). ORAL Standard also included an active control arm of adalimumab on background methotrexate; data are not presented due to sample size (n=24). In Phase 3 studies with ≥6 months’ duration (ORAL Sync, ORAL Standard, ORAL Scan), placebo patients with <20% decrease in tender/swollen joints were advanced to tofacitinib at Month 3. All remaining placebo patients advanced to tofacitinib at Month 6. In ORAL Step and ORAL Solo, all placebo patients were advanced to tofacitinib at Month 3. Stable background arthritis therapy was permitted and included: nonsteroidal anti-inflammatory drugs, selective cyclooxygenase-2 inhibitors, opioids, acetaminophen, and/or low-dose oral corticosteroids (≤10mg prednisone or equivalent per day).
The second data set in this analysis was the LTE study population. The open-label LTE study (ORAL Sequel, NCT00413699) enrolled eligible patients who had previously participated in a qualifying randomized Phase 1, Phase 2, or Phase 3 index study of tofacitinib14; however, no Phase 1 study patients were from Latin America. All data retrieved up to and including April 10, 2013 were included in the LTE analysis (data collection/analyses ongoing; database unlocked). All LA patients enrolling from Phase 2 and Phase 3 studies initiated treatment in the LTE studies with tofacitinib 5 and 10mg BID, respectively. Patients received tofacitinib either as monotherapy or in combination with background csDMARDs (mainly methotrexate). Baseline values for LTE studies were those of the index study for patients enrolling within 14 days of index study participation; for other patients, baseline was the start of the LTE study. Dose adjustments of both tofacitinib and concomitant RA treatments were permitted during the LTE study. The total daily dose (TDD) average of tofacitinib was calculated by adding all doses received by each patient from the first dose to last dose, then dividing by the number of days a dose was received. The TDD average was used to assign the LTE dose: TDD 0–15 was considered tofacitinib 5mg BID, and TDD ≥15 was considered tofacitinib 10mg BID.
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines, and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each investigational study center. All patients provided written informed consent.
Efficacy and safety endpointsEfficacy endpoints included ACR response rates (ACR20/50/70), mean disease activity score (DAS28)-4(erythrocyte sedimentation rate [ESR]), and mean change from baseline in health assessment questionnaire-disability index (HAQ-DI). Efficacy data were reported up to 12 and 36 months for the Phase 3 and LTE studies, respectively (the small sample size post-36-months precluded analysis of efficacy variables beyond this time point).
All available safety data were presented, including data up to 24 and 60 months from the Phase 3 and LTE studies, respectively. Safety endpoints included reporting of adverse events (AEs), serious AEs (SAEs), discontinuations due to AEs, and mortality cases. Incidence rates (IRs; patients with event/100 patient-years) of AEs of special interest are reported for SAEs; discontinuations due to AEs; serious infection events (SIEs); opportunistic infections (OIs; excluding TB); TB; all HZ (serious and non-serious); serious HZ; malignancies (excluding non-melanoma skin cancer [NMSC]); lymphoma/lymphoproliferative disorders; major adverse cardiovascular events (MACE); and all-cause mortality (within 30 days of the last study drug dose). Malignancy events were adjudicated by a blinded, independent adjudication committee. MACE and all deaths were adjudicated by a blinded, independent external Cardiovascular Safety Endpoint Adjudication Committee (CVSEAC) for all Phase 3 studies, and for all events after February 2009 in the LTE study.
Statistical analysisAll efficacy and safety analyses were based on observed cases (i.e. no imputation) of the full analyses set, which included all patients who were randomized and received ≥1 dose of study treatment (tofacitinib or placebo). Due to differences in sample size between groups, all analyses were descriptive in nature and general trends were described. No statistical significance was calculated; therefore, all differences alluded to in the results section refer to numerical differences only; 95% confidence intervals (CIs) are presented.
IRs (patients with events/100 patient-years) for AEs of special interest were calculated by exposure and dose. IRs were based on the number of patients with an event and total exposure time censored at time of event, death, or withdrawal from the study; 95% CIs for IRs were based on maximum likelihood estimation.
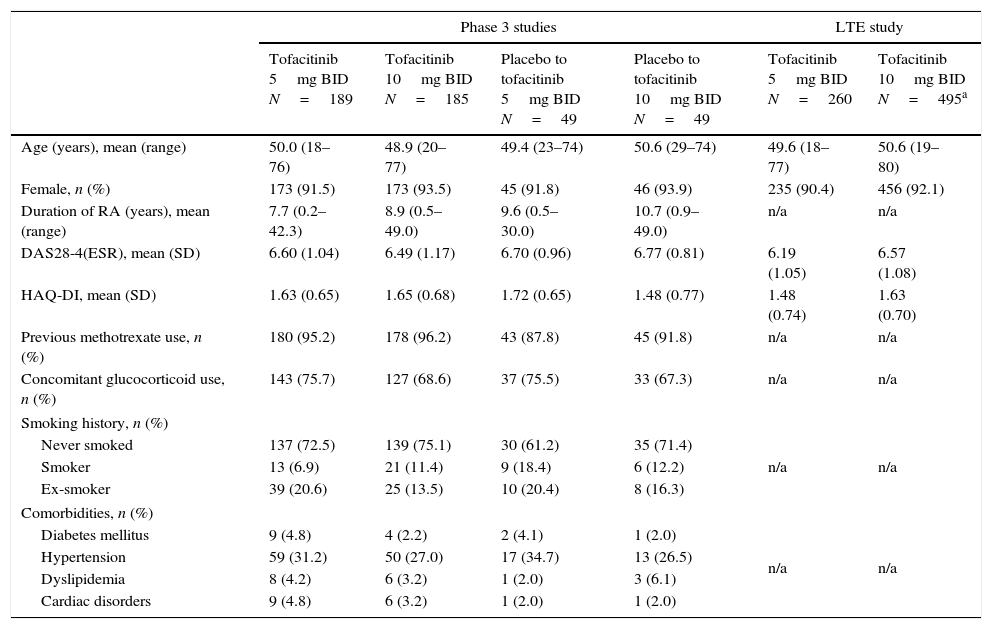
ResultsPatientsIn the Phase 3 studies, a total of 472 LA patients were randomized to receive tofacitinib 5mg BID (n=189), tofacitinib 10mg BID (n=185), placebo advanced to tofacitinib 5mg BID (n=49), or placebo advanced to tofacitinib 10mg BID (n=49); of these, 157 (83.1%), 152 (82.2%), 42 (85.7%), and 37 (75.5%) patients, respectively, completed the studies. In the LTE study, a total of 756 LA patients were assigned to receive tofacitinib 5mg BID (n=260) or tofacitinib 10mg BID (n=496). Patient demographics and baseline characteristics were similar among treatment groups in both Phase 3 and LTE studies (Table 1). The majority of patients (in Phase 3 and LTE studies, respectively) were from Brazil (n=177 [37.5%] and n=237 [31.3%]), Mexico (n=119 [25.2%] and n=202 [26.7%]), Chile (n=63 [13.3%] and n=128 [16.9%]), and Colombia (n=77 [16.3%] and n=97 [12.8%]).
Baseline demographics and disease characteristics of the LA subpopulation by treatment sequence.
| Phase 3 studies | LTE study | |||||
|---|---|---|---|---|---|---|
| Tofacitinib 5mg BID N=189 | Tofacitinib 10mg BID N=185 | Placebo to tofacitinib 5mg BID N=49 | Placebo to tofacitinib 10mg BID N=49 | Tofacitinib 5mg BID N=260 | Tofacitinib 10mg BID N=495a | |
| Age (years), mean (range) | 50.0 (18–76) | 48.9 (20–77) | 49.4 (23–74) | 50.6 (29–74) | 49.6 (18–77) | 50.6 (19–80) |
| Female, n (%) | 173 (91.5) | 173 (93.5) | 45 (91.8) | 46 (93.9) | 235 (90.4) | 456 (92.1) |
| Duration of RA (years), mean (range) | 7.7 (0.2–42.3) | 8.9 (0.5–49.0) | 9.6 (0.5–30.0) | 10.7 (0.9–49.0) | n/a | n/a |
| DAS28-4(ESR), mean (SD) | 6.60 (1.04) | 6.49 (1.17) | 6.70 (0.96) | 6.77 (0.81) | 6.19 (1.05) | 6.57 (1.08) |
| HAQ-DI, mean (SD) | 1.63 (0.65) | 1.65 (0.68) | 1.72 (0.65) | 1.48 (0.77) | 1.48 (0.74) | 1.63 (0.70) |
| Previous methotrexate use, n (%) | 180 (95.2) | 178 (96.2) | 43 (87.8) | 45 (91.8) | n/a | n/a |
| Concomitant glucocorticoid use, n (%) | 143 (75.7) | 127 (68.6) | 37 (75.5) | 33 (67.3) | n/a | n/a |
| Smoking history, n (%) | ||||||
| Never smoked | 137 (72.5) | 139 (75.1) | 30 (61.2) | 35 (71.4) | n/a | n/a |
| Smoker | 13 (6.9) | 21 (11.4) | 9 (18.4) | 6 (12.2) | ||
| Ex-smoker | 39 (20.6) | 25 (13.5) | 10 (20.4) | 8 (16.3) | ||
| Comorbidities, n (%) | ||||||
| Diabetes mellitus | 9 (4.8) | 4 (2.2) | 2 (4.1) | 1 (2.0) | n/a | n/a |
| Hypertension | 59 (31.2) | 50 (27.0) | 17 (34.7) | 13 (26.5) | ||
| Dyslipidemia | 8 (4.2) | 6 (3.2) | 1 (2.0) | 3 (6.1) | ||
| Cardiac disorders | 9 (4.8) | 6 (3.2) | 1 (2.0) | 1 (2.0) | ||
All demographic data were unavailable for one patient, and country data were unavailable for a second patient in the tofacitinib 10mg BID group of the LTE study.
Twice daily (BID), disease activity score (DAS), erythrocyte sedimentation rate (ESR), health assessment questionnaire-disability index (HAQ-DI), Latin American (LA), long-term extension (LTE), not available (n/a), standard deviation (SD).
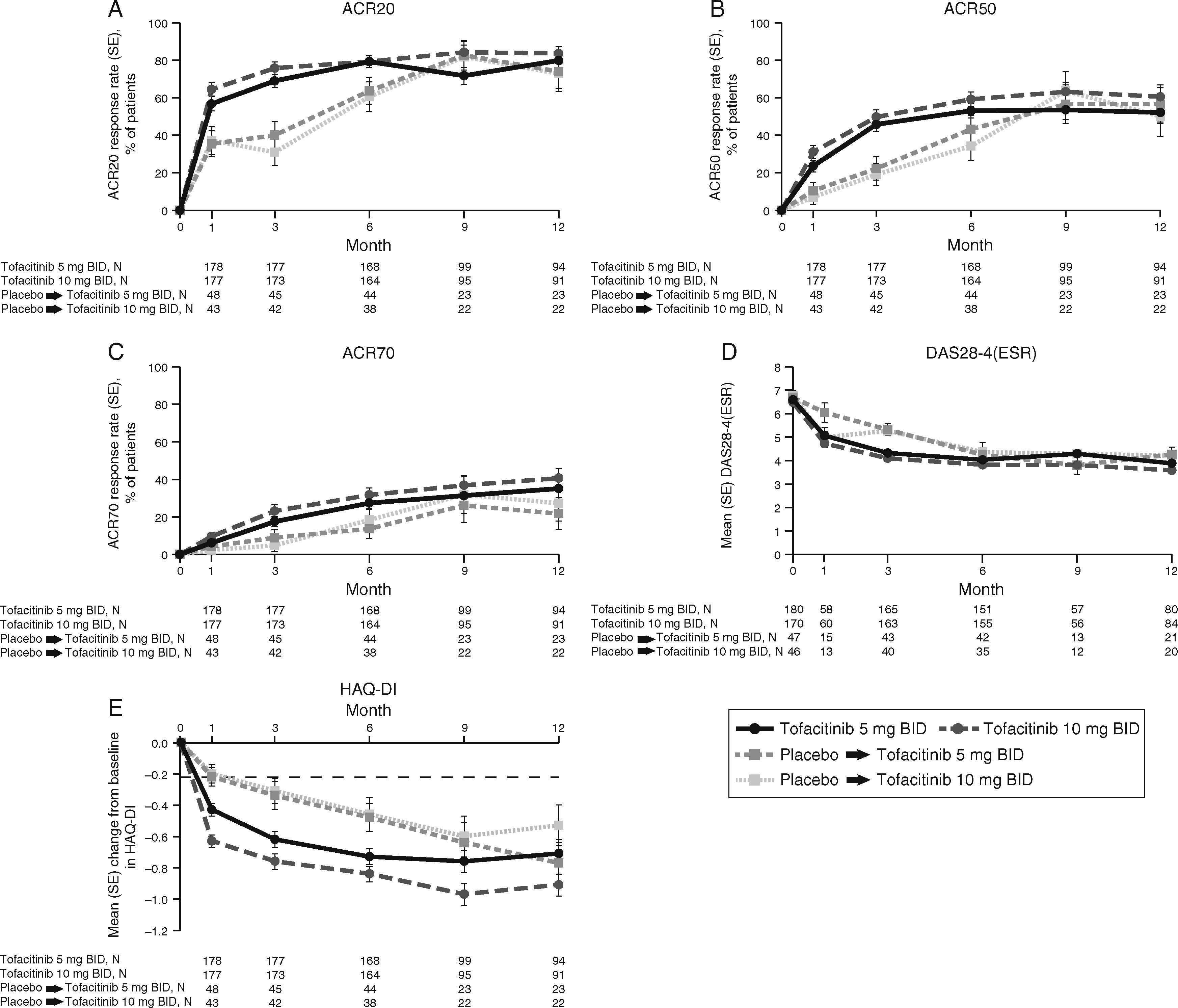
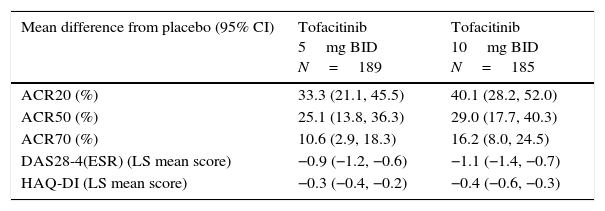
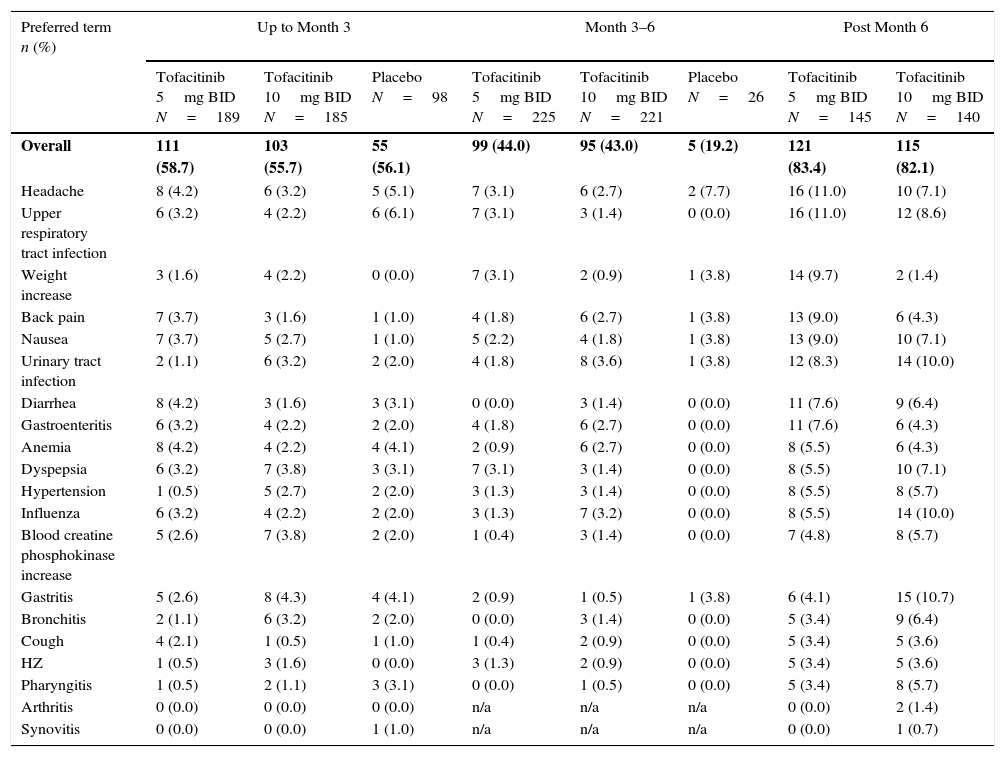
LA patients treated with both tofacitinib doses showed greater ACR20/50/70 response rates vs placebo at Month 3 (Table 2; Fig. 1). Differences in ACR response rates were maintained up to 12 months for both tofacitinib doses; similar response rates were seen by Month 9 and maintained up to Month 12 in placebo patients who advanced to tofacitinib (Fig. 1).
Difference from placebo for efficacy measures at Month 3 in patients receiving tofacitinib 5 and 10mg BID.
| Mean difference from placebo (95% CI) | Tofacitinib 5mg BID N=189 | Tofacitinib 10mg BID N=185 |
|---|---|---|
| ACR20 (%) | 33.3 (21.1, 45.5) | 40.1 (28.2, 52.0) |
| ACR50 (%) | 25.1 (13.8, 36.3) | 29.0 (17.7, 40.3) |
| ACR70 (%) | 10.6 (2.9, 18.3) | 16.2 (8.0, 24.5) |
| DAS28-4(ESR) (LS mean score) | −0.9 (−1.2, −0.6) | −1.1 (−1.4, −0.7) |
| HAQ-DI (LS mean score) | −0.3 (−0.4, −0.2) | −0.4 (−0.6, −0.3) |
All efficacy analyses are based on the full analysis set without imputation; patient numbers (N) for each outcome at Month 3 are different depending upon the number of patients with evaluable outcomes.
American College of Rheumatology (ACR), twice daily (BID), confidence interval (CI), disease activity score (28 joints) (DAS28), erythrocyte sedimentation rate (ESR), health assessment questionnaire-disability index (HAQ-DI), least squares (LS), long-term extension.
Phase 3 study pooled efficacy data for normal approximation to A) ACR20, B) ACR50, C) ACR70 response rates (SE), D) mean DAS28-4(ESR) scores per visit, and E) mean change from baseline in HAQ-DI per visit. Full analysis set, no imputation. Dashed line in Panel E represents MCID (reduction in HAQ-DI score ≥0.22). American College of Rheumatology (ACR), twice daily (BID), disease activity score (28 joints) (DAS28), erythrocyte sedimentation rate (ESR), health assessment questionnaire-disability index (HAQ-DI), minimum clinically important difference (MCID), standard error (SE).
Mean DAS28-4(ESR) scores were lower in both tofacitinib groups vs placebo at Month 3 (Table 2); improvements were maintained up to 12 months (Fig. 1). By Month 6, a similar reduction in DAS28-4(ESR) score was observed in placebo advanced patients and was maintained up to Month 12 (Fig. 1). Changes from baseline in mean DAS28-4(ESR) scores were greater in both tofacitinib groups vs placebo at Month 3.
At Month 1 and Month 3, mean change from baseline in HAQ-DI was greater for patients treated with either tofacitinib dose vs placebo-treated patients (Table 2; Fig. 1). Improvements (reductions) in HAQ-DI from baseline were maintained up to 12 months with both tofacitinib doses (Fig. 1). By Month 1, tofacitinib-treated patients reported improvements greater than the minimum clinically important difference (MCID) for HAQ-DI (reduction in HAQ-DI score ≥0.22 points).
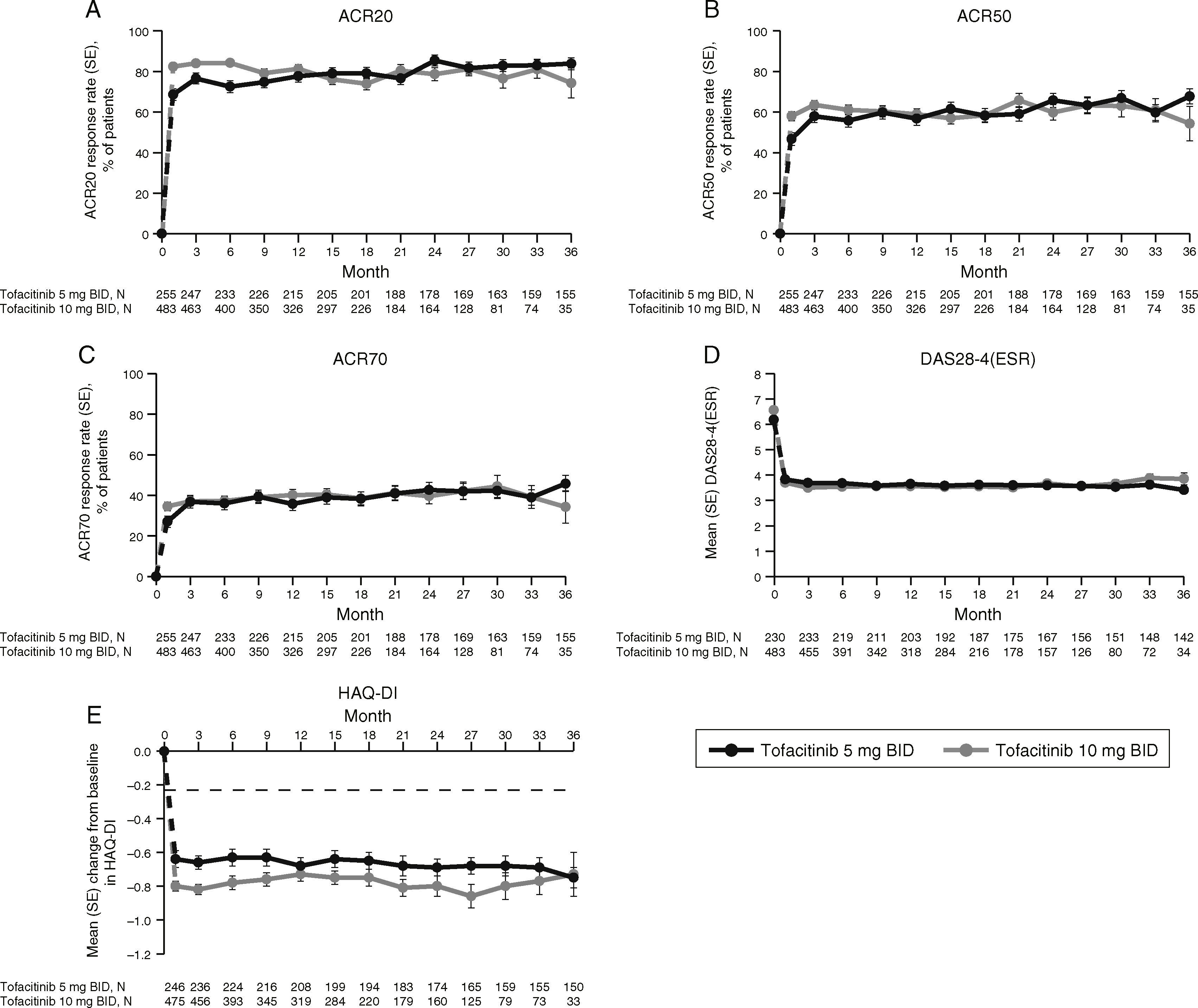
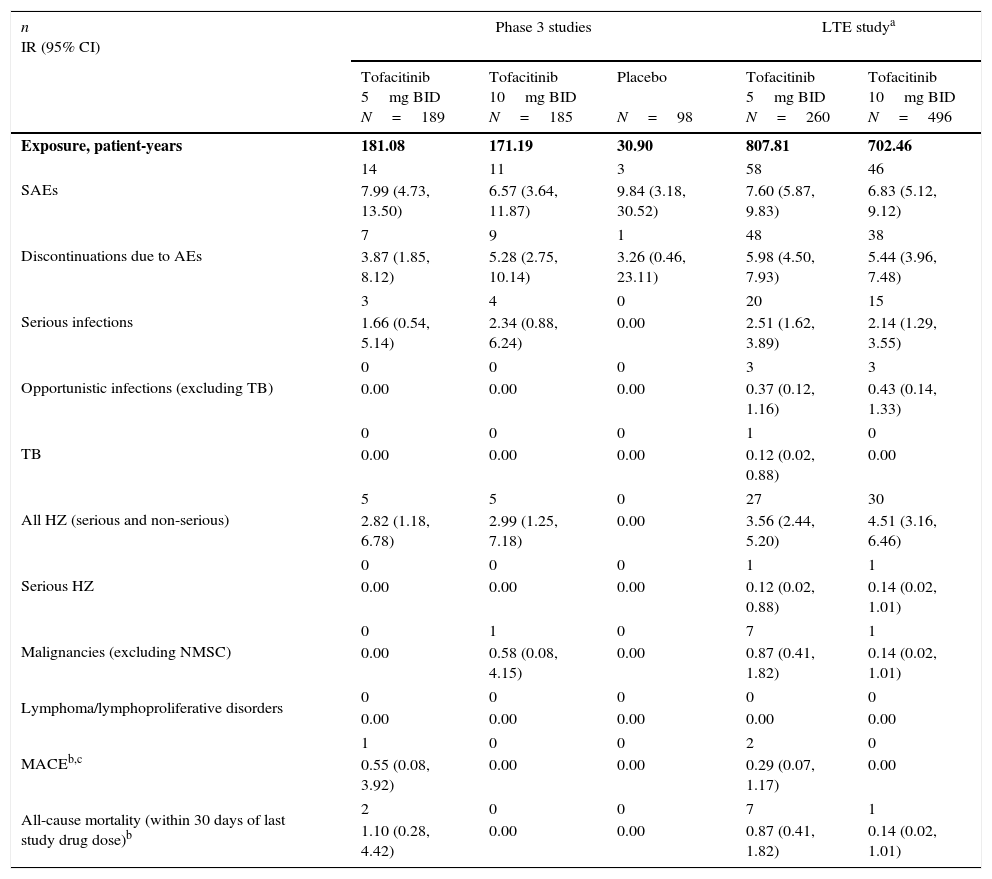
LTE studyImprovements in ACR response rates (Fig. 2), DAS28-4(ESR) (Fig. 2), and HAQ-DI (Fig. 2) were maintained up to 36 months in the LTE study (Fig. 2). Efficacy results were similar between tofacitinib doses, and improvements in HAQ-DI remained greater than the MCID for both doses (Fig. 2).
LTE study efficacy for normal approximation to A) ACR20, B) ACR50, C) ACR70 response rates (SE), D) mean DAS28-4(ESR) scores, and E) mean change from baseline in HAQ-DI per visit. Full analysis set, no imputation. Dashed line in Panel E represents MCID (reduction in HAQ-DI score ≥0.22). American College of Rheumatology (ACR), twice daily (BID), disease activity score (28 joints) (DAS28), erythrocyte sedimentation rate (ESR), health assessment questionnaire-disability index (HAQ-DI), long-term extension (LTE), minimum clinically important difference (MCID), standard error (SE).
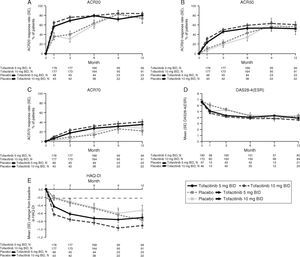
The percentages of LA patients with AEs were similar across treatment groups up to Month 3 and post Month 6 (Table 3). Between Months 3 and 6, a smaller percentage of placebo-treated patients had AEs (19.2%) vs tofacitinib 5 and 10mg BID patients (44.0% and 43.0%, respectively). Overall, the most common AEs were headache, upper respiratory tract infection, urinary tract infection, and nausea (Table 3). Discontinuations due to study-drug-related AEs (investigator-determined) occurred in four (2.1%) and seven (3.8%) patients treated with tofacitinib 5 or 10mg BID, respectively, and one (2.0%) and one (2.0%) patients who advanced from placebo to tofacitinib 5 or 10mg BID, respectively. Two patients died during the Phase 3 studies (pneumonia and cardiorespiratory arrest; tofacitinib 5mg BID).
Most frequent AEs in the Phase 3 studies reported by preferred term (reported in ≥5% of patients in any treatment group).
| Preferred term n (%) | Up to Month 3 | Month 3–6 | Post Month 6 | |||||
|---|---|---|---|---|---|---|---|---|
| Tofacitinib 5mg BID N=189 | Tofacitinib 10mg BID N=185 | Placebo N=98 | Tofacitinib 5mg BID N=225 | Tofacitinib 10mg BID N=221 | Placebo N=26 | Tofacitinib 5mg BID N=145 | Tofacitinib 10mg BID N=140 | |
| Overall | 111 (58.7) | 103 (55.7) | 55 (56.1) | 99 (44.0) | 95 (43.0) | 5 (19.2) | 121 (83.4) | 115 (82.1) |
| Headache | 8 (4.2) | 6 (3.2) | 5 (5.1) | 7 (3.1) | 6 (2.7) | 2 (7.7) | 16 (11.0) | 10 (7.1) |
| Upper respiratory tract infection | 6 (3.2) | 4 (2.2) | 6 (6.1) | 7 (3.1) | 3 (1.4) | 0 (0.0) | 16 (11.0) | 12 (8.6) |
| Weight increase | 3 (1.6) | 4 (2.2) | 0 (0.0) | 7 (3.1) | 2 (0.9) | 1 (3.8) | 14 (9.7) | 2 (1.4) |
| Back pain | 7 (3.7) | 3 (1.6) | 1 (1.0) | 4 (1.8) | 6 (2.7) | 1 (3.8) | 13 (9.0) | 6 (4.3) |
| Nausea | 7 (3.7) | 5 (2.7) | 1 (1.0) | 5 (2.2) | 4 (1.8) | 1 (3.8) | 13 (9.0) | 10 (7.1) |
| Urinary tract infection | 2 (1.1) | 6 (3.2) | 2 (2.0) | 4 (1.8) | 8 (3.6) | 1 (3.8) | 12 (8.3) | 14 (10.0) |
| Diarrhea | 8 (4.2) | 3 (1.6) | 3 (3.1) | 0 (0.0) | 3 (1.4) | 0 (0.0) | 11 (7.6) | 9 (6.4) |
| Gastroenteritis | 6 (3.2) | 4 (2.2) | 2 (2.0) | 4 (1.8) | 6 (2.7) | 0 (0.0) | 11 (7.6) | 6 (4.3) |
| Anemia | 8 (4.2) | 4 (2.2) | 4 (4.1) | 2 (0.9) | 6 (2.7) | 0 (0.0) | 8 (5.5) | 6 (4.3) |
| Dyspepsia | 6 (3.2) | 7 (3.8) | 3 (3.1) | 7 (3.1) | 3 (1.4) | 0 (0.0) | 8 (5.5) | 10 (7.1) |
| Hypertension | 1 (0.5) | 5 (2.7) | 2 (2.0) | 3 (1.3) | 3 (1.4) | 0 (0.0) | 8 (5.5) | 8 (5.7) |
| Influenza | 6 (3.2) | 4 (2.2) | 2 (2.0) | 3 (1.3) | 7 (3.2) | 0 (0.0) | 8 (5.5) | 14 (10.0) |
| Blood creatine phosphokinase increase | 5 (2.6) | 7 (3.8) | 2 (2.0) | 1 (0.4) | 3 (1.4) | 0 (0.0) | 7 (4.8) | 8 (5.7) |
| Gastritis | 5 (2.6) | 8 (4.3) | 4 (4.1) | 2 (0.9) | 1 (0.5) | 1 (3.8) | 6 (4.1) | 15 (10.7) |
| Bronchitis | 2 (1.1) | 6 (3.2) | 2 (2.0) | 0 (0.0) | 3 (1.4) | 0 (0.0) | 5 (3.4) | 9 (6.4) |
| Cough | 4 (2.1) | 1 (0.5) | 1 (1.0) | 1 (0.4) | 2 (0.9) | 0 (0.0) | 5 (3.4) | 5 (3.6) |
| HZ | 1 (0.5) | 3 (1.6) | 0 (0.0) | 3 (1.3) | 2 (0.9) | 0 (0.0) | 5 (3.4) | 5 (3.6) |
| Pharyngitis | 1 (0.5) | 2 (1.1) | 3 (3.1) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 5 (3.4) | 8 (5.7) |
| Arthritis | 0 (0.0) | 0 (0.0) | 0 (0.0) | n/a | n/a | n/a | 0 (0.0) | 2 (1.4) |
| Synovitis | 0 (0.0) | 0 (0.0) | 1 (1.0) | n/a | n/a | n/a | 0 (0.0) | 1 (0.7) |
Adverse event (AE), twice daily (BID), herpes zoster (HZ), not available (n/a), standard deviation (SD).
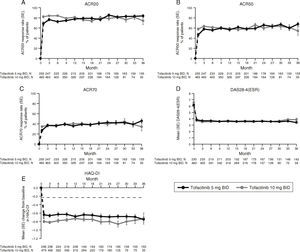
SAEs were reported in a total of 10 patients up to Month 3, seven patients between Month 3 and 6, and 13 patients post Month 6. The most common class of SAEs was infections and infestations. IRs for SAEs and discontinuations due to AEs were similar (overlapping and wide 95% CIs) between treatment groups (Table 4). IRs for SIEs and HZ (serious and non-serious) were similar (overlapping and wide CIs) for both tofacitinib groups; no SIEs or HZ cases were reported for placebo. One malignancy case (breast cancer; tofacitinib 10mg BID) and one MACE (CVSEAC-adjudicated; tofacitinib 5mg BID) were reported. No OIs, TB, serious HZ, or lymphoma were reported (Table 4).
Incidence rates (number of patients with event/100 patient-years) for AEs of special interest by treatment group.
| n IR (95% CI) | Phase 3 studies | LTE studya | |||
|---|---|---|---|---|---|
| Tofacitinib 5mg BID N=189 | Tofacitinib 10mg BID N=185 | Placebo N=98 | Tofacitinib 5mg BID N=260 | Tofacitinib 10mg BID N=496 | |
| Exposure, patient-years | 181.08 | 171.19 | 30.90 | 807.81 | 702.46 |
| SAEs | 14 | 11 | 3 | 58 | 46 |
| 7.99 (4.73, 13.50) | 6.57 (3.64, 11.87) | 9.84 (3.18, 30.52) | 7.60 (5.87, 9.83) | 6.83 (5.12, 9.12) | |
| Discontinuations due to AEs | 7 | 9 | 1 | 48 | 38 |
| 3.87 (1.85, 8.12) | 5.28 (2.75, 10.14) | 3.26 (0.46, 23.11) | 5.98 (4.50, 7.93) | 5.44 (3.96, 7.48) | |
| Serious infections | 3 | 4 | 0 | 20 | 15 |
| 1.66 (0.54, 5.14) | 2.34 (0.88, 6.24) | 0.00 | 2.51 (1.62, 3.89) | 2.14 (1.29, 3.55) | |
| Opportunistic infections (excluding TB) | 0 | 0 | 0 | 3 | 3 |
| 0.00 | 0.00 | 0.00 | 0.37 (0.12, 1.16) | 0.43 (0.14, 1.33) | |
| TB | 0 | 0 | 0 | 1 | 0 |
| 0.00 | 0.00 | 0.00 | 0.12 (0.02, 0.88) | 0.00 | |
| All HZ (serious and non-serious) | 5 | 5 | 0 | 27 | 30 |
| 2.82 (1.18, 6.78) | 2.99 (1.25, 7.18) | 0.00 | 3.56 (2.44, 5.20) | 4.51 (3.16, 6.46) | |
| Serious HZ | 0 | 0 | 0 | 1 | 1 |
| 0.00 | 0.00 | 0.00 | 0.12 (0.02, 0.88) | 0.14 (0.02, 1.01) | |
| Malignancies (excluding NMSC) | 0 | 1 | 0 | 7 | 1 |
| 0.00 | 0.58 (0.08, 4.15) | 0.00 | 0.87 (0.41, 1.82) | 0.14 (0.02, 1.01) | |
| Lymphoma/lymphoproliferative disorders | 0 | 0 | 0 | 0 | 0 |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| MACEb,c | 1 | 0 | 0 | 2 | 0 |
| 0.55 (0.08, 3.92) | 0.00 | 0.00 | 0.29 (0.07, 1.17) | 0.00 | |
| All-cause mortality (within 30 days of last study drug dose)b | 2 | 0 | 0 | 7 | 1 |
| 1.10 (0.28, 4.42) | 0.00 | 0.00 | 0.87 (0.41, 1.82) | 0.14 (0.02, 1.01) | |
Exposure for MACE events in the LTE study was 685.50 patient-years for tofacitinib 5mg BID, and 698.54 patient-years for tofacitinib 10mg BID.
Adverse event (AE), twice daily (BID), confidence interval (CI), cardiovascular safety endpoint adjudication committee (CVSEAC), herpes zoster (HZ), incidence rate (IR), long-term extension (LTE), major adverse cardiovascular event (MACE), non-melanoma skin cancer (NMSC), serious adverse event (SAE), tuberculosis (TB).
IRs for safety events of special interest were generally consistent between tofacitinib doses, although IRs were numerically greater for malignancies and mortality with tofacitinib 5mg BID vs tofacitinib 10mg BID; however, only a small number of events were reported for either dose (Table 4). No cases of TB or MACE were reported with tofacitinib 10mg BID; for tofacitinib 5mg BID, IRs for TB and MACE were 0.12 and 0.29, respectively. No cases of lymphoma were reported.
Eleven patients died during the LTE study: nine patients receiving tofacitinib 5mg BID (hepatic failure and sepsis [one patient]; gallbladder cancer; cerebrovascular accident; sudden death; sepsis and pneumonia [one patient]; cardiorespiratory arrest; respiratory failure and chronic obstructive pulmonary disease [one patient]; gastrointestinal necrosis, respiratory arrest, cardiac arrest, sepsis and appendicitis [all in one patient]; and multi-organ failure) and two patients receiving tofacitinib 10mg BID (cardiogenic shock, multi-organ failure and pneumonia [all in one patient]; and synovial sarcoma and metastases to lung).
DiscussionThis analysis has demonstrated the efficacy of tofacitinib in reducing the signs and symptoms of RA and improving physical function in LA patients with RA. Although formal statistical comparisons were not performed due to the relatively small LA subpopulation sample size, across Phase 3 studies, the LA subpopulation reported generally similar improvements in ACR20/50/70, HAQ-DI and DAS28-4(ESR) vs the global population.7–11 Furthermore, improvements in efficacy endpoints were similar when comparing LTE data between the LA and global populations.14 Although data collection and analyses are ongoing for the LTE study, it was felt that including long-term data in the current analysis would add to the overall profile of efficacy and safety of tofacitinib in the LA subpopulation.
Demographic data from the LA RA subpopulation showed some differences vs the global population.7–11 The mean age of patients in the LA subpopulation (48.9–50.6 years) was slightly lower than the global population excluding LA patients (52.5–53.8 years) but with a higher proportion of female patients (92.0% vs 81.3%); consistent with previous demographic analyses in LA patients.15,16 Although no substantial differences were seen in the clinical response of patients in the LA and global populations, the observed disparity in age and gender may contribute to small differences in the response in the LA population.
Unlike many bDMARDs, which require subcutaneous or intravenous administration, tofacitinib is administered orally. This may be particularly beneficial in LA where many RA patients do not have easy access to the resources needed for subcutaneous or intravenous administration. This study has demonstrated that tofacitinib could provide an effective oral alternative in LA patients with RA.
Evaluation of the safety profile of tofacitinib in LA was important, particularly due to the epidemiological differences between LA and other global populations, and there are many LA countries where TB is endemic and there is increased mortality due to infection.3,4 The most frequently reported AEs in the Phase 3 studies were comparable between LA and global populations7–11: headache, upper respiratory and urinary tract infections, and nausea. The most commonly reported system organ class of AEs during tofacitinib treatment in the LA subpopulation was infections and infestations – consistent with reports from other LA studies with bDMARDs.17,18 IRs of SAEs were similar between the LA subpopulation and both the global population19 and bDMARDs in the Mexican biologics register.17 Thirteen patients died during the Phase 3 or LTE studies; further details have been reported by Cohen et al.20
Overall, the safety profile of tofacitinib was similar between LA and global populations. Lymphoma has been reported with an IR (95% CI) of 0.06 (0.3, 0.13) in the global population.19 No cases of lymphoma were reported in the LA subpopulation of tofacitinib studies up to April 2013. However, it must be acknowledged that the global population included a larger number of patients, with greater and longer total tofacitinib exposure vs the LA subpopulation.
IRs for SIEs were comparable between LA and global populations.19,20 The incidence of SIEs in tofacitinib-treated patients in global studies had similar or lower IRs to various bDMARDs.21 Given the similarity of LA and global IRs for SIEs, these trends are likely to be comparable in LA patients treated with tofacitinib or bDMARDs.
The risk of TB with immunosuppressant therapy varies directly with the background TB rate in the underlying population.22 Nevertheless, despite the high prevalence of TB in some LA countries,23 the incidence of TB was low in LA patients with RA treated with tofacitinib and consistent with that of the tofacitinib global population.24 Although, it is recommended that patients from all countries are screened for TB before initiating tofacitinib treatment, as is already the case for bDMARDs.25,26
No OIs were reported in the LA subpopulation in Phase 3 trials, and the IR for OIs in the LTE study was similar vs the global tofacitinib studies.24 Similar to tofacitinib, OIs have been reported with TNFi treatments27; and treatment with bDMARDs has been reported to increase the risk of infections and infestations compared with csDMARDs in Mexican and Brazilian patients.17,18
The IRs for all HZ (serious and non-serious) in the LA subpopulation were generally comparable with the global population28 but was higher than reports on csDMARDs and TNFi therapies from a large US multi-institutional collaboration29 and the British Society for Rheumatology Biologics Register.30 Although relatively low incidences of OIs, serious HZ, TB, and malignancies (excluding NMSC) were reported in LA patients, it will be important to carefully monitor AEs in LA patients treated with tofacitinib, as is already recommended in Brazilian and Mexican guidelines for other immunosuppressant therapies.25,26
This analysis was limited by reliance on pooled data across Phase 3 studies, which provided a heterogeneous patient population, including different study designs and methodology. The placebo group had fewer patients and less exposure than tofacitinib, and direct comparison of tofacitinib vs placebo was not continued in the LTE study. No formal statistical analysis was performed between treatment groups due to the small sample size; thus, conclusions are based on descriptive analyses and 95% CIs only. Additionally, at later time points in the LTE study the patient population is small, and is likely to be made up of patients who show good tolerability for tofacitinib, therefore results should be interpreted with caution.
Due to limited treatment options and lack of access to resource and specialists, there is still an unmet need for new RA therapies for LA patients who have shown an inadequate response to other therapies. These data suggest that in the LA subpopulation tofacitinib 5 and 10mg BID are efficacious, with a manageable safety profile; these data are consistent with the global population. Therefore, this analysis supports the use of tofacitinib as an oral alternative to bDMARDs for the treatment of LA patients with RA.
Ethical disclosuresProtection of human subjects and animals in researchThis study involved human patients. The following ethics statement is currently included in the methods section:
All studies were conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice Guidelines, and were approved by the Institutional Review Boards and/or Independent Ethics Committees at each investigational study center. All patients provided written informed consent.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestThis study was funded by Pfizer Inc. Medical writing support was provided by Alice Palmer, PhD, of Complete Medical Communications and funded by Pfizer Inc.
SC Radominski has received consulting fees, speaking fees and/or honoraria from Amgen, AstraZeneca, Bristol-Myers Squibb, Celltrion, GlaxoSmithKline, Janssen, Pfizer Inc, Roche and Sanofi. MH Cardiel has received research grants, consultancy and speakers’ fees from Pfizer Inc, Roche, Bristol Myers Squibb, UCB, Astellas, Infinity, Lilly, Merck, and Janssen. G Citera has received research grants, consultancy and speaker's fees from Pfizer Inc, Bristol Myers Squibb, and AbbVie. A Goecke has received consultancy fees from AbbVie, BMS, GlaxoSmithKline and Roche, and has received payment for clinical studies from Abbott, BMS, Celltrion, Centocor, Janssen, Medimmune, Pfizer Inc, Roche and Sanofi. JJ Jaller has no conflicts of interest to declare. ABV Lomonte has received research grants from Pfizer Inc. P Miranda has received consultancy fees from Pfizer Inc and payment for clinical studies from Roche, Pfizer Inc, Abbott, Medimmune, BMS, HGS, Sanofi, Janssen, and Centocor. P Velez has no conflicts of interest to declare. D Xibillé has received consultancy fees from Pfizer Inc and payment for clinical studies from AstraZeneca, BMS, GlaxoSmithKline, Janssen and Pfizer Inc. K Kwok and R Rojo are employees and shareholders of Pfizer Inc. EG García was an employee of Pfizer Inc at the time of the analysis.
The authors would like to thank the patients, investigators and study teams involved in the studies included in this analysis. This study was funded by Pfizer Inc. Medical writing support was provided by Alice Palmer, PhD, of Complete Medical Communications and funded by Pfizer Inc.