Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by symmetric polyarthritis that can lead to joint deformity, disability, and osteoporosis. We aimed to evaluate whole hand and regional BMD in RA patients compared to controls. In addition, we evaluated the BMD of dominant versus non-dominant hands in healthy subjects. We included adult female and male RA patients and control subjects matched by age, sex, and BMI. BMD (g/cm2) was measured by DXA in lumbar spine (LS), whole hand, and three regions of interest: carpus, metacarpal bones, and phalanges. Results: 44 control subjects (49.5±11.8 y) and 60 with RA (52.7±12.7 y) were included. Significant lower BMD in RA patients was found in LS (−8.7%), dominant whole hand (−9.5%), carpus, metacarpal bones, and phalanges, and non-dominant whole hand (−8.7%), metacarpal bones, and phalanges compared to controls. A significant positive correlation was found between LS and whole-hand BMD (dominant r=.63, non-dominant r=.67). Finally, the whole hand, metacarpal bones, and carpus BMD measurements were significantly higher in the dominant hand compared to the non-dominant hand without differences in the phalangeal ROI. In conclusion, hand BMD was significantly lower in RA patients compared to control subjects and there was a significant correlation with LS BMD. We demonstrated that BMD measurements of the whole-hand, and different ROI (carpus, metacarpal bones, and phalanges) by DXA would be an easily reproducible technique to evaluate bone loss. In addition, the whole hand, metacarpal bones and carpus BMD measurements were significantly higher in the dominant hand compared to the non-dominant hand without differences in the phalanges.
La artritis reumatoide (AR) es una enfermedad autoinmune crónica caracterizada por poliartritis simétrica que puede provocar deformidad e incapacidad articular y osteoporosis. Nuestro objetivo fue evaluar la DMO de manos completa y por regiones en los pacientes con AR en comparación con los controles. Se incluyeron pacientes adultos de ambos sexos con AR, y sujetos controles de edad, sexo e IMC similar. La DMO se midió por DXA en columna lumbar (CL), manos completas y 3 regiones de interés: carpo, metacarpianos y falanges. Resultados: se incluyeron 44 sujetos control (49,5±11,8 años) y 60 con AR (52,7±12,7 años). Se encontró una DMO significativamente más baja en los pacientes con AR en CL (−8,7%), mano completa dominante (−9,5%) y mano completa no dominante (−8,7%) en comparación con los sujetos controles. Se encontró una correlación positiva significativa entre la CL y la DMO de la mano completa (dominante, r=0,63; no dominante, r=0,67). Finalmente, la DMO de la mano completa, los huesos metacarpianos y el carpo fueron significativamente más altos en la mano dominante en comparación con la mano no dominante sin diferencias en la región de las falanges. En conclusión, la DMO de la mano fue significativamente menor en los pacientes con AR en comparación con los sujetos controles, y hubo una correlación significativa con la DMO de la CL. Demostramos que las mediciones de la DMO de toda la mano y diferentes ROI (carpo, huesos metacarpianos y falanges) por DXA serían una técnica fácilmente reproducible para evaluar la pérdida ósea. Además, la DMO de la mano completa, los huesos metacarpianos y el carpo fueron significativamente más altos en la mano dominante en comparación con la mano no dominante.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by symmetric polyarthritis that can lead to joint deformity and disability. Chronic inflammation in RA has a detrimental effect on bone mass even in premenopausal women and can lead to focal or generalized osteoporosis.1–4 Moreover, several other factors, such as female sex, age, corticosteroid therapy, disease activity itself, and immobility due to pain, among others increase bone loss in RA patients.5,6 An increase in trabecular and cortical bone involvement and risk of vertebral and non-vertebral fractures have been reported in RA patients.7,8 A high incidence of clinical fragility fractures in postmenopausal Spanish women with RA was recently described where the incidence of major osteoporotic fracture was 3.55 per 100 patient-year in RA patients and 0.72 in controls (HR: 2.6) being the previous fracture the more relevant risk factor for fractures (HR: 10.37).9 In addition, RA is characterized by lower lean mass, muscle strength and increased prevalence of sarcopenia.10,11
Hand periarticular osteopenia is an early radiographic sign of RA. This bilateral and symmetrical bone loss at the hand could appear despite clinical improvement and good control of inflammation through treatment.12
Despite digital X-ray radiogrammetry using conventional X-ray is a validated technique for the evaluation of hand BMD,13–15 dual-energy X-ray absorptiometry (DXA) from the lumbar spine (LS) and femoral neck (FN) is the gold standard imaging technique used for the diagnosis and monitoring of osteoporosis.16
Whole-hand BMD assessment by DXA (Lunar GE) showed to be more sensitive than conventional radiology for measuring disease-related bone damage in early active RA.17 Hand BMD by DXA (Lunar GE) was validated for estimating BMD in healthy (n=88) and osteoarthritic women (n=46) and potentially to be used for mass screening.18 A previous report carried out in 10 early arthritis and 17 healthy women showed lower cortical BMD at metacarpal bones, especially after menopause using an adapted technique in HologicQDR-4500.19 However, there is no specific software in Hologic devices and there are no unique standardized technique and reference values to compare hands. Recently, a study exploring the differences between dominant and non-dominant hands BMD by DXA was reported.20
The main purpose of our study was to evaluate the hand BMD including the whole-hand, carpus, metacarpal bone and phalanges in RA patients compare to controls. In addition, we evaluated dominant and non-dominant hand BMD.
Subjects and methodsStudy populationWe performed a cross-sectional study in RA patients (n=60)>18 years who fulfilled the RA classification criteria of the 2010 American College of Rheumatology/European League Against Rheumatism (ACR-EULAR).21 As a control group (CG), we included 44 healthy volunteers matched by age, gender, and body mass index (BMI). Exclusion criteria for both groups were pregnancy, participants with a recent history (last 12 months) of immobilization, or those who had a disease or were receiving drugs that could affect bone metabolism (anticonvulsants, bisphosphonates, denosumab, teriparatide, raloxifene, and hormonal replacement therapy, except for corticosteroids).
RA disease activity was assessed according to DAS-28 including erythrocyte sedimentation rate (ESR), the number of tenders and swollen joints (28 joints) and a visual analogue scale and Health Assessment Questionnaire Disability Index) (HAQ-I).22,23
The study (1MED486) was approved by the Ethics Committee of the School of Medicine, Rosario National University (Argentina) under the Declaration of Helsinki. All participants gave written informed consent and were identified by a number to keep their identities confidential.
Lumbar spine BMDWeight and height were measured using a mechanical scale (ROMA scale, Argentina). BMD (g/cm2) was measured by DXA (Hologic Discovery Wi, Hologic Inc. Bedford, MA) on the LS (L1-L4) according to manufacturer recommendations. Quality control was performed by the daily assessment of a phantom spine. All scans were performed by the same certified physician, with a coefficient of variation<1%.
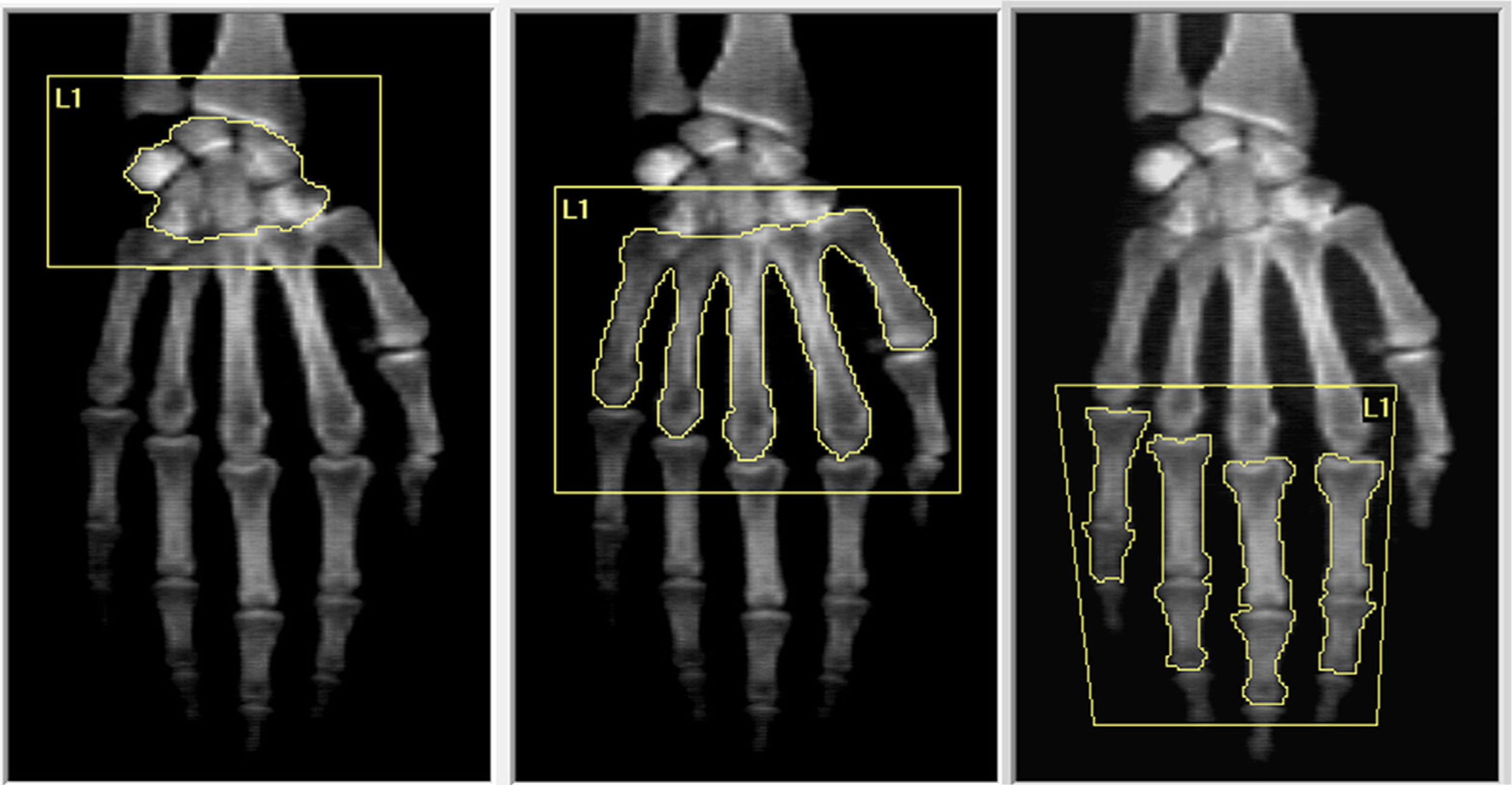
DXA hands assessmentDespite there being a specific software for hands in Lunar GE devices, there is no software available in Hologic equipment, which is widely distributed around the world. Therefore, we developed a technique to analyze the whole-hand and three regions of interest (ROI): carpus, metacarpal bones and phalanges in Hologic Discovery Wi devices using the lumbar spine software. To measure hands BMD, both hands of each subject were placed palm-down on the table with the fingers extended, as described by Deodhar et al.24 We measure carpus as one, the five metacarpal bones and the two proximal phalanges of fingers excluding the thumb and sesamoid bones (Fig. 1). All determinations were made by the same bone densitometry-certified physician.
Statistical analysisThe distribution of the data was evaluated with the Shapiro–Wilk test and the differences between groups were analyzed using the Student's t-test or the Mann–Whitney test, as appropriate. Pearson correlation test and linear regression were used to analyze LS and hand BMD. Data were expressed as mean±SD and p<0.05 was considered significant.
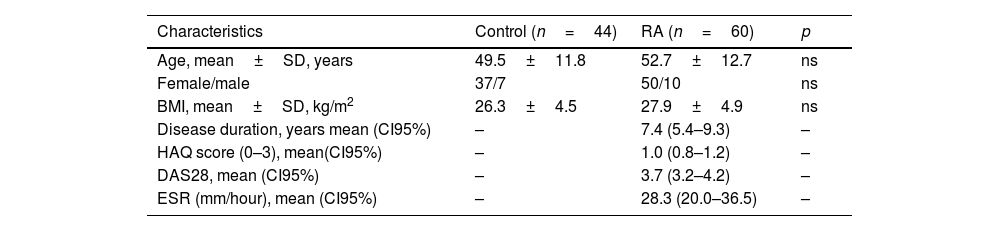
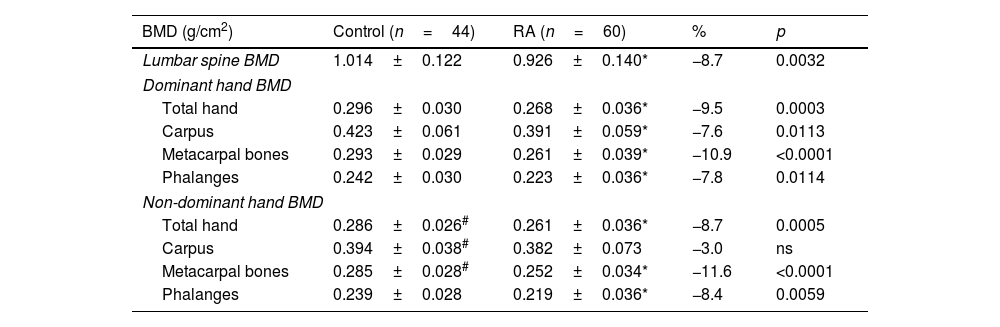
ResultsA total of 44 control subjects (37 female and 7 male, 49.5±11.8 y) and 60 with RA (50 female and 10 male; 52.7±12.7 years) were included in this study (Table 1). Significant lower BMD in RA patients was found in LS (−8.7%), dominant whole-hand (−9.5%), carpus, metacarpal bones, and phalanges, and non-dominant whole-hand (−8.7%), metacarpal bones, and phalanges (Table 2).
Clinical characteristics of the included subjects.
| Characteristics | Control (n=44) | RA (n=60) | p |
|---|---|---|---|
| Age, mean±SD, years | 49.5±11.8 | 52.7±12.7 | ns |
| Female/male | 37/7 | 50/10 | ns |
| BMI, mean±SD, kg/m2 | 26.3±4.5 | 27.9±4.9 | ns |
| Disease duration, years mean (CI95%) | – | 7.4 (5.4–9.3) | – |
| HAQ score (0–3), mean(CI95%) | – | 1.0 (0.8–1.2) | – |
| DAS28, mean (CI95%) | – | 3.7 (3.2–4.2) | – |
| ESR (mm/hour), mean (CI95%) | – | 28.3 (20.0–36.5) | – |
Differences in the lumbar spine and hands BMD between RA patients and control subjects.
| BMD (g/cm2) | Control (n=44) | RA (n=60) | % | p |
|---|---|---|---|---|
| Lumbar spine BMD | 1.014±0.122 | 0.926±0.140* | −8.7 | 0.0032 |
| Dominant hand BMD | ||||
| Total hand | 0.296±0.030 | 0.268±0.036* | −9.5 | 0.0003 |
| Carpus | 0.423±0.061 | 0.391±0.059* | −7.6 | 0.0113 |
| Metacarpal bones | 0.293±0.029 | 0.261±0.039* | −10.9 | <0.0001 |
| Phalanges | 0.242±0.030 | 0.223±0.036* | −7.8 | 0.0114 |
| Non-dominant hand BMD | ||||
| Total hand | 0.286±0.026# | 0.261±0.036* | −8.7 | 0.0005 |
| Carpus | 0.394±0.038# | 0.382±0.073 | −3.0 | ns |
| Metacarpal bones | 0.285±0.028# | 0.252±0.034* | −11.6 | <0.0001 |
| Phalanges | 0.239±0.028 | 0.219±0.036* | −8.4 | 0.0059 |
The intra-assay coefficient of variability (CV) (n=10 for each region) of whole-hand, carpus, metacarpal bones and phalanges BMD were 1.2%, 0.9%, 2.8% and 2.1% and the inter-assay CV (n=10) were: 2.7%, 2.2%, 4.3% and 3.7% respectively. Also, the minimal detectable change for each region was calculated as 1.96×√2×SEM according to Bland and Altman (1988)25 based on duplicate DXA measures: 0.007, 0.010, 0.012, and 0.008g/cm2, respectively.
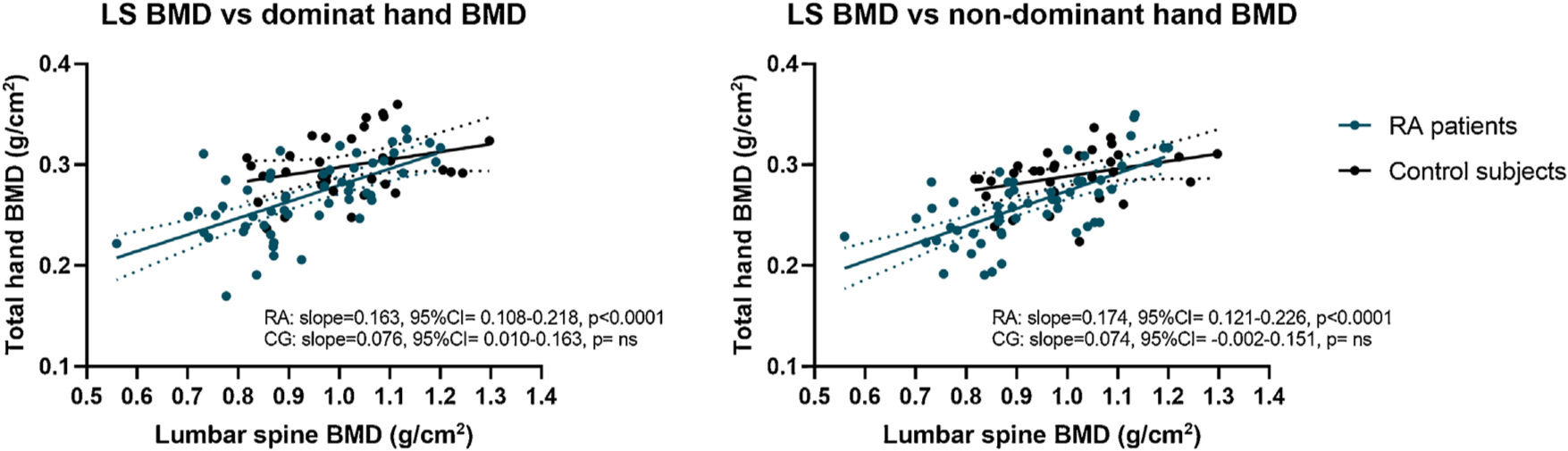
A significant positive correlation was found between LS BMD and whole-hand BMD in both hands only in RA patients (dominant r=0.63, non-dominant r=0.67; p<0.0001 each) without correlation in control subjects. Linear regression of LS BMD and whole-hand BMD in controls and RA patients revealed a significant difference from zero only in RA patients (Fig. 2). Moreover, there were significant differences between LS BMD and hands slope (p<0.05).
In addition, a negative correlation between dominant and non-dominant hand BMD and age were found in RA patients (dominant: r: −0.32, p=0.0148, non-dominant: r: −0.28, p=0.0302) without correlation with disease duration and disease activity parameters (HAQ, DAS28 and ESR).
Further, we analyzed differences between dominant and non-dominant hands BMD in control subjects as an estimation of dominance differences. More than 93% (n=41) of the participants were right-handed. The whole-hand, metacarpal bones and carpus BMD were significantly higher in the dominant hand compared to the non-dominant hand without differences in the ROI phalanges (Table 1). The difference between the dominant and non-dominant sides were: whole-hand BMD 0.009g/cm2 (95%CI 0.014–0.004, p=0.0007), carpus 0.030g/cm2 (95%CI 0.045–0.015, p=0.0003) and metacarpal bones 0.009g/cm2 (95%CI 0.014–0.004, p=0.0003).
DiscussionRA is a chronic inflammatory disease that involves the joints and bones. There is an increased prevalence of bone fragility fracture in RA patients compared to the general population.8 As hand periarticular osteopenia is an early radiographic sign of RA,12 the evaluation of BMD in the hands could be an early study with clinical value. Therefore, we evaluate a technique to measure whole-hand BMD and also three regions carpus, metacarpal bone and phalanges in RA patients compare to controls in Hologic equipment. This technique using a modified lumbar spine software in a Hologic device was previously described by Deodhar et al.24 but they use an aluminium step wedge resembling the bone thicknesses for bone mineral content (BMC) measurement. More recently, Castañeda et al. (2007)26 and Llorente et al. (2019)19 also used a modified technique in Hologic device using the forearm software to juxta-articular BMD at metacarpophalangeal joints and metacarpal bones, respectively.
We found significantly lower BMD in RA patients in dominant whole-hand (−9.5%), carpus, metacarpal bones, and phalanges, and non-dominant whole-hand (−8.7%), metacarpal bones, and phalanges. Our result adds additional evidence to a previous report who evaluated cortical BMD by DXA in 10 early arthritis and 17 healthy volunteers.19 Furthermore, we found similar coefficients of variation compared to Llorente I et al. (2020): 2.25%, 2.91%, 2.85%, and 2.07% for metacarpal-2, metacarpal-3, metacarpal-4, and mean metacarpal-second to fourth, respectively and similar to Brownbill and Ilich (2022) for total hand (0.7%).18 In addition, the minimal detectable change at the different anatomical locations analyzed ranged were 0.006g/cm2 for whole-hand and from 0.007 to 0.022g/cm2 for metacarpal bones in healthy controls (n=16) and 0.009g/cm2 for whole-hand and from 0.005 to 0.010g/cm2 for metacarpal bones in early arthritis (n=22).26
Moreover, we found a significant positive correlation between LS BMD and whole-hand BMD in both hands only in RA patients without correlation in control subjects. Finally, we found a negative correlation between dominant and non-dominant hand BMD and age in RA patients without correlation with disease duration and disease activity parameters (HAQ, DAS28 and ESR). Probably the initial DAS28 (3.7) and HAQ (1.0) values indicating a low/moderate disease activity and adequate treatment are responsible, at least in part, for this lack of association. However, other studies found the disease activity (among others: age, previous fracture, parental hip fracture, years since menopause, erosions, and cumulative dose of glucocorticoids) as a risk factor for major osteoporotic fractures.9 In fact, the inflammatory state in the early stages of RA mediated by proinflammatory interleukins and TNF-α has been described to activate and differentiate osteoclasts leading to bone loss by different mechanisms (RANK/RANKL/OPG and Wnt/DKK1/sclerostin pathways).4 Further, anti-citrullinated protein antibodies and anti-carbamylated protein antibodies are independently associated with severe trabecular bone loss and increased risk of fracture in the next 10 years.4,27
Previous studies showed that cortical thickness of the metacarpal bones by X-ray reflects BMD in patients with RA showing a good correlation with LS and FN BMD.28,29 However, the measurements in this study were performed manually and it was not suitable as a predictive marker for major osteoporotic fractures, including vertebral, hip, humeral, and wrist fractures.30
Further, hand BMD by DXA was able to detect the increase in BMD in RA patients under 12 months under denosumab treatment.31 Another study evaluates the relationship between BMD by DXA and the second metacarpal head microarchitecture by high-resolution peripheral quantitative computed tomography (HR-pQCT).32 HR-pQCT can provide a detailed quantitative assessment of periarticular cortical and trabecular bone loss, however, has a higher cost and is not available for clinical use. Finally, a report indicates that periarticular BMD by DXA seems not to be useful as diagnostic due to a strong overlap of BMD values between healthy controls, established RA and early arthritis patients.33 Despite the areas closest to the joint surface are more prone to BMD loss early, the use of small ROIs and a periarticular measurement could not be accurate. Moreover, the authors indicated that the use of hand DXA without considering factors involved in bone loss as age, sex and menopause does not improve the diagnosis in RA patients.
Also, we found differences between dominant and non-dominant hands BMD in the whole-hand, metacarpal bones, and carpus without differences in the ROI of phalanges. These data are consistent with a recent report which evaluated differences between dominant and non-dominant feet and hands BMD and bone mineral content (BMC).20 The authors evaluated a total of 42 subjects (11 men and 31 women) with a mean age of 43.82±9.91 using 2 different approaches, the whole region (feet or hands) and 2 specific ROIs and found higher BMD and BMC in the dominant hand in comparison with the non-dominant hand without significant differences in the foot. Despite our difference between dominant and non-dominant whole-hand BMD was similar to data reported by Abdala R et al.20 we can observe differences in the total hand BMD absolutes values which is assumed as a difference in the densitometer used (Lunar Prodigy or Hologic Discovery Wi). On the other hand, Deodhar et al. reported that hand dominance had no significant effect on hand BMC.24
The limitations of our study include the need for technical training for hand and ROI positioning and, there is no available specific software to measure hands and their different ROI in Hologic equipment. Secondly, the DXA measurements might be influenced by the presence of synovitis or bone erosions characteristics of RA. However, this non-invasive technique is easily adaptable and reproducible, safe as the radiation effective dose is small with an acceptable and comparable coefficient of variability.
In conclusion hand BMD was significantly lower in RA patients compared to control subjects and there was a significant correlation with LS BMD. In addition, the whole-hand, metacarpal bones and carpus BMD were significantly higher in the dominant hand compared to the non-dominant hand without differences in the ROI phalanges. We demonstrated that BMD measurements of the whole-hand, and different ROI (carpus, metacarpal bones and phalanges) by DXA would be an easily reproducible technique to evaluate the bone loss. Nevertheless, more data are necessary to consider as a screening tool for early bone loss in patients with RA. A negative correlation between hand BMD and age in RA patients was found without correlation with disease duration and disease activity parameters (HAQ, DAS28 and ESR). A study with a large sample considering the disease duration and other parameters for bone involvement would be necessary.
Authors’ contributionsStudy design: MLB and LRB. Patient and data acquisition: MLB, AR, BAPE, NJQ, MJ, and GB. Data analysis: MLB, AR and LRB. Data interpretation and drafting of manuscript: MLB, and LRB. All authors read and approved the final manuscript.
Ethics approval informationEthics Committee of the School of Medicine, Rosario National University (Argentina) (1MED486).
Funding statementThis work was supported by a Pan American League of Associations for Rheumatology (PANLAR) Award to MLB and Rosario National University to LRB.
Conflict of interestsAll authors declare that they have no conflict of interest.