The use of immunosuppressive therapies in COVID-19 infection is a recently raised topic which comes to fill an unmet need in the management of the patients.1 Intriguingly, not only COVID-19 but also SARS and MERS CoVs – all members of the Betacoronavirus genus – associate to an increased risk of respiratory distress syndrome. Already in patients with SARS-CoV, the development of respiratory failure was thought to be the consequence of a vigorous innate immune response, while effectiveness of tocilizumab in COVID-19 infected patients also supports the participation of a cytokine storm in severe phenotypes.1,2 A factor underlying this explosive response could be the capacity of betacoronaviruses to invade immune-competent cells, particularly macrophages, thereby hijacking the major drivers of innate immune responses. Nonetheless, targeting pro-inflammatory cytokines is neither the sole nor the first-line immunomodulatory approach in combating the infection. As represented in the figure, complex virus-host cell interactions providing opportunities for therapeutics should be regarded (Fig. 1).
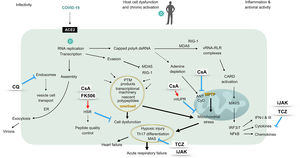
The graphic represents a pathogenic model of 2019-nCoV considering different virus-host cell interactions. The virus entry, replication, assembly and shedding indicating infectivity are shown at the left, while the right side displays the innate antiviral response characterized by an interferon signature. The center of the figure represents intracellular events unchained by the presence of the virus, driving cell and mitochondrial stress and eventually ending in hypoxic damage. The sites of action of different immunomodulatory drugs are marked. ACE2: angiotensin converting enzyme 2, ANT: adenine nucleotide translocation, CARD: caspase activation and recruitment domains, CQ: chloroquine, CyD: cyclophilin D, ER: endoplasmic reticulum, FK506: tacrolimus, HSR: heat shock response (also unfolded-protein response of the cytosol), IFN: interferon, IRF: interferon regulatory factor, iJAK: inhibitor of Janus kinases, MAS: macrophage activation syndrome, MAVS: mitochondrial antiviral proteins, MDA5: melanoma differentiation-activated protein 5, mtUPR: mitochondrial unfolded-protein response, NFκB: transcriptional activator kappa B, polyA: polyadenylated, PTM: postranslational modifications, RIG-1: retinoic acid inducible gene 1, RLR: RIG-1-like receptors, Th: T helper lymphocytes, TCZ: tocilizumab, vRNA: viral RNA.
Betacoronaviruses replicate and carry out transcriptional activities at the cell cytosol, where the viral genome is detected by RIG-1 like receptor (RLR) helicases. Upon binding of vRNA, RLR activate mitochondrial antiviral proteins (MAVS). These in turn trigger phosphorylation of transcription factors and gene expression of interferons and cytokines, which are pivotal for an effective antiviral response.3 Mitochondrial function is thus essential for the antiviral defense, while these organelles also need to provide for the increased energetic needs of infected cells. This fact points to mitochondrial failure as the mechanism unchaining severe forms of COVID-19 infection.4 In brief, infected cells are exposed to an overload of nascent polypeptides, transcriptional machinery and by-products of helicases activation, altogether jeopardizing maintenance of protein folding and triggering cell and mitochondrial stress.5,6 In addition, COVID-19 genome polyadelnylation at the cytosol could waste adenine deposits and challenge mitochondrial permeability transition pore (MPTP). Ultimately, mitochondrial proteostasis collapse would drive caspases activation and irreversible cell damage. According to available literature, calcineurin inhibitors could confer protection from these pathogenic processes. Briefly, these compounds help restore the unfolded-protein response (UPR) at the cytosol, and may in this way rescue cells from necrosis.7 In addition, upon targeting cyclophilin D, cyclosporin A inhibits MPTP opening, activates mitochondrial UPR (mtUPR) and prevents mitochondrial failure.8,9 Moreover, through this mechanism, cyclosporin A has shown cardioprotective effects in patients with myocardial infarction.10
Of interest, there is a subtype of clinically amyopathic dermatomyositis (CADM) identified by the presence of antibodies against melanoma differentiation activated protein 5 (MDA5), which is an RLR helicase and also the putative cytoplasmic receptor for COVID-19. Patients with MDA5 syndrome are prone to the development of rapidly progressive interstitial pneumonia and refractory respiratory failure. Even though MDA5 syndrome is a rare condition, its resemblance with the clinical features of CoV infections cannot go unnoticed. Notably, critically ill MDA5+ CADM patients can be rescued when a calcineurin inhibitor is administered early in the course of respiratory failure.11
Finally, it should be emphasized that cyclosporin A has shown remarkable antiviral activities in a variety of RNA viruses, including the family of betacoronavirus, which employ cyclophilins as chaperones and nuclear factor of activated T cells (NFAT) as a major signaling pathway.12,13
On the whole, we suggest that COVID-19 deadly action on host cells including pneumocytes and T lymphocytes, results from their failure to adapt to cell and mitochondrial stress, while dysfunctional macrophages remain as virus reservoir at the target tissue. According to this model, cyclosporin A could confer protection upstream of the cytokine storm in COVID-19 infected patients, a hypothesis which it is planned to be tested in a randomized clinical trial in the coming weeks.
All authors have contributed to the conception of the manuscript, have revised it critically, have approved the final version and agree to be accountable for all aspects of the work.