To describe the frequencies of fibromyalgia syndrome (FMS) in various rheumatic diseases; rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and Behçets disease (BD) patients and to study the relation to clinical manifestations and quality of life (QoL).
Patients and methods160 patients (50 RA, 50 SLE, 30 SSc and 30 BD) and matched corresponding healthy controls were included. Disease activity was assessed using disease activity score in 28 joints (DAS28) for RA, SLE Disease Activity index (SLEDAI), modified Rodnan skin score for SSc and BD Current Activity Form (BDCAF). The QoL was also recorded. Severity in FMS cases was estimated using the revised Fibromyalgia Impact Questionnaire score.
ResultsIn the RA, SLE, SSc and BD patients, FMS was found in 14%, 18%, 6.67% and 3.33% respectively compared to 2.1%, 3%, 3.3% and 0% in their corresponding controls. In RA patients, DAS28 was significantly higher in those with FMS (p=0.009) and significantly correlated with both Widespread Pain Index (WPI) (p=0.011) and Symptom Severity (SS) scale (p=0.012). The QoL scale in those with FMS was significantly worse (62.3±7.9) compared to those without (71.7±14.4) (p=0.023). In SLE patients, The WPI and SS both significantly correlated with the presence of thrombosis (r=0.28, p=0.049 and r=0.43, p=0.002 respectively). The SS scale tended to correlate with the SLEDAI (r=0.28, p=0.05). In BD patients, BDCAF and WPI significantly correlated (p=0.03).
ConclusionFibromyalgia syndrome is more frequent in rheumatic diseases, could be related to the disease activity in RA and BD patients and to thrombosis in SLE and affected the QoL in RA.
Describir las frecuencias del síndrome de fibromialgia (SFM) en los pacientes de diversas enfermedades reumáticas; artritis reumatoide (AR), lupus eritematoso sistémico (LES), esclerosis sistémica (ES) y enfermedad de Behçet (EB), y estudiar su relación con las manifestaciones clínicas y la calidad de vida (CV).
Pacientes y métodosSe incluyó en el estudio a 160 pacientes (50 AR, 50 LES, 30 ES y 30 EB) y a los controles sanos emparejados. La actividad de la enfermedad se evaluó utilizando las escalas Disease Activity Score en 28 articulaciones (DAS28) para AR, SLE Disease Activity Index (SLEDAI), Rodnan modificada para ES y BD Current Activity Form (BDCAF). También se registró la CV. La severidad en los casos de SFM se estimó utilizando la escala Fibromyalgia Impact Questionnaire revisada.
ResultadosEn los pacientes de AR, LES, ES y EB se encontró SFM en el 14, el 18, el 6,67 y el 3,33%, respectivamente, en comparación al 2,1, el 3, el 3,3 y el 0% en sus controles correspondientes. En los pacientes con AR, la clasificación DAS28 fue significativamente superior en aquellos con SFM (p=0,009), guardando una correlación significativa con las escalas Widespread Pain Index (WPI) (p=0,011) y Symptom Severity (SS) (p=0,012). La escala CV en aquellos pacientes con SFM fue considerablemente peor (62,3±7,9) en comparación con aquellos que no presentaban dicho síndrome (71,7±14,4) (p=0,023). En los pacientes de LES, ambas escalas, WPI y SS, guardaron una correlación significativa con la presencia de trombosis (r=0,28, p=0,049, y r=0,43, p=0,002 respectivamente). La escala SS tendió a guardar una relación con la escala SLEDAI (r=0,28, p=0,05). En los pacientes con EB, las escalas BDCAF y WPI guardaron una correlación significativa (p=0,03).
ConclusiónEl síndrome de fibromialgia es más frecuente en las enfermedades reumáticas y podría guardar relación con la actividad de la enfermedad en los pacientes de AR y EB, y con la trombosis en los pacientes de LES, afectando a la CV en la AR.
Fibromyalgia syndrome (FMS) is defined by the presence of generalized pain, fatigue, unrefreshed sleep, multiple somatic symptoms and cognitive problems.1 Pain and inflammation in patients with inflammatory arthritis play a role in the development and course of FMS.2 Perhaps the most important role of the rheumatologist is to confirm the diagnosis and determine if the patient has a co-morbid rheumatic condition that should be treated. If FMS is complicating another rheumatic disease, specific management of FMS may improve overall health outcomes.3
Rheumatic diseases are characterized by chronic pain and as many as 15–30% of patients also have associated FMS.4 As these rates are much higher than the prevalence of FMS in the general population (2%), it seems that the pain accompanying chronic rheumatic diseases is also capable of triggering FMS.5 As concomitant FMS is a common clinical problem in rheumatic diseases, its recognition is important for their optimal management. Increased pain, physical limitations, and fatigue may be interpreted as increased activity of these diseases.6 The association of systemic lupus erythematosus (SLE) and FMS may pose a clinical diagnostic dilemma as both share many symptoms.7 The superimposed pain of FMS may lead to the prescription of higher doses of corticosteroids or biologic agents.6
In one study on systemic sclerosis (SSc) patients, the frequency of FMS was reported to be 2%.8 There are little published data on the relationship between FMS and Behçets disease (BD). FMS is a common and important clinical problem that may represent an additional factor that worsens pain and physical limitations in BD patients. An increased awareness of this possible coexistence may contribute more accurate management of BD.9
The aim of the present work was to describe the frequencies of FMS in various rheumatic diseases; rheumatoid arthritis (RA), SLE, SSc and BD patients and to study the relation of FMS to the clinical manifestations, laboratory features, disease activity and/or damage as well as the quality of life (QoL).
Patients and methodsThe study included 160 patients; 50 with RA, 50 with SLE, 30 with SSc and another 30 with BD. All patients were consequently recruited from those attending the Rheumatology outpatient clinic and department, Faculty of Medicine, Cairo University Hospital. Patients were included when they fulfilled their corresponding classification criteria; 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria10 for RA, Systemic Lupus International Collaborating Clinics (SLICC) classification criteria11 for SLE, 2013 ACR/EULAR classification criteria12 for SSc patients and the International Study Group criteria for BD.13 Apparently healthy volunteers (n=141) were included as control groups who were age and sex matched for each disease; they were 48 control for RA patients, 33 for SLE, 30 for SSc and 30 for the BD patients. All controls were recruited from the hospital staff members and employees and relatives of the patients were not considered to avoid familiar aggregation. The study was performed in accordance with the Declaration of Helsinki, and all patients gave written consent for enrollment in the study.
All patients were subjected to full history taking and physical examination. Relevant laboratory and radiological investigations were done. The following disease activity indices and score were considered: disease activity score in 28 joints (DAS28)14 and health assessment questionnaire II (HAQII)15 for RA patients; SLE Disease Activity index (SLEDAI)16 and SLICC/ACR damage index17 for SLE patients, modified Rodnan skin score (mRss)18 and systemic sclerosis disease severity19 for SSc patients and BD Current Activity Form (BDCAF)20 for BD patients. The QoL scale21 was assessed for all the patients. The 2010 ACR preliminary diagnostic criteria for FMS was applied to all the patients and control22 and those with FMS were assessed for severity using the revised Fibromyalgia Impact Questionnaire (FIQR) score.23
Statistical analysisData were analyzed using the computer program, SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) version 15. Data were described in terms of range, mean±SD, median, frequencies (number of cases) and percentages when appropriate. Comparison of quantitative variables between the study groups was done using Mann Whitney U test for independent samples. For comparing categorical data, Chi square (χ2) test was performed. Comparison among more than 2 groups was by ANOVA. Spearman's correlation analysis was used for detection of the relation between 2 variables. p-Value <0.05 was considered statistically significant.
ResultsThe characteristic features of the RA patients with and without FMS are presented in Table 1. The controls were matched in age (39.6±14 years) (p=0.1) and sex (F:M 7:1) (p=0.7). The frequency of FMS in the RA patients was 14% while in their corresponding control was 2.1% (1/48 subjects). The mean FIQR score of the 7 RA patients with FMS was 104.4±23.9. The WPI component of FMS significantly correlated with the DAS28 (r=0.36, p=0.01) and negatively with the QoL scale (r=−0.39, p=0.004) and the SS scale correlated with the DAS28 (r=0.35, p=0.012), HAQII (r=0.39, p=0.006) and negatively with the QoL (r=−0.36, p=0.01).
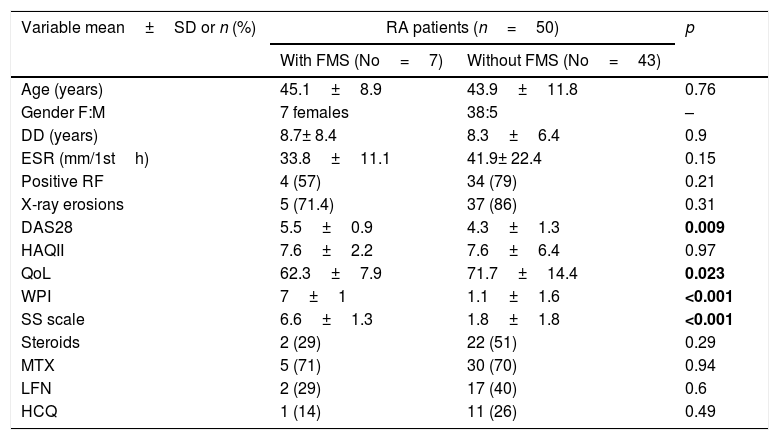
Demographic features, investigations, disease activity, functional status, quality of life and medications used in rheumatoid arthritis (RA) patients with and without fibromyalgia syndrome (FMS).
| Variable mean±SD or n (%) | RA patients (n=50) | p | |
|---|---|---|---|
| With FMS (No=7) | Without FMS (No=43) | ||
| Age (years) | 45.1±8.9 | 43.9±11.8 | 0.76 |
| Gender F:M | 7 females | 38:5 | – |
| DD (years) | 8.7± 8.4 | 8.3±6.4 | 0.9 |
| ESR (mm/1sth) | 33.8±11.1 | 41.9± 22.4 | 0.15 |
| Positive RF | 4 (57) | 34 (79) | 0.21 |
| X-ray erosions | 5 (71.4) | 37 (86) | 0.31 |
| DAS28 | 5.5±0.9 | 4.3±1.3 | 0.009 |
| HAQII | 7.6±2.2 | 7.6±6.4 | 0.97 |
| QoL | 62.3±7.9 | 71.7±14.4 | 0.023 |
| WPI | 7±1 | 1.1±1.6 | <0.001 |
| SS scale | 6.6±1.3 | 1.8±1.8 | <0.001 |
| Steroids | 2 (29) | 22 (51) | 0.29 |
| MTX | 5 (71) | 30 (70) | 0.94 |
| LFN | 2 (29) | 17 (40) | 0.6 |
| HCQ | 1 (14) | 11 (26) | 0.49 |
RA: rheumatoid arthritis, FMS: fibromyalgia syndrome, DD: disease duration, ESR: erythrocyte sedimentation rate, RF: rheumatoid factor, DAS28: disease activity score 28, HAQII: Health Assessment Questionnaire II, WPI: widespread pain index, SS scale: symptoms severity scale, QoL: quality of life, MTX: methotrexate, LFN: leflunomide, HCQ: hydroxychloroquine. Results are either expressed as number (percent) or as mean±SD. Bold values are significant at p<0.05.
The characteristic features of the SLE patients with and without FMS are presented in Table 2. The controls were matched in age (29.9±7.1 years) and similarly were all females. The frequency of FMS in the SLE patients was 18% while in their corresponding control was 3% (1/33 subjects). The mean FIQR score of the 9 SLE patients with FMS was 94.2±13.9. The WPI and SS scale both significantly correlated with the presence of thrombosis (r=0.28, p=0.049 and r=0.43, p=0.002 respectively). The SS scale tended to correlate with the SLEDAI (r=0.28, p=0.05).
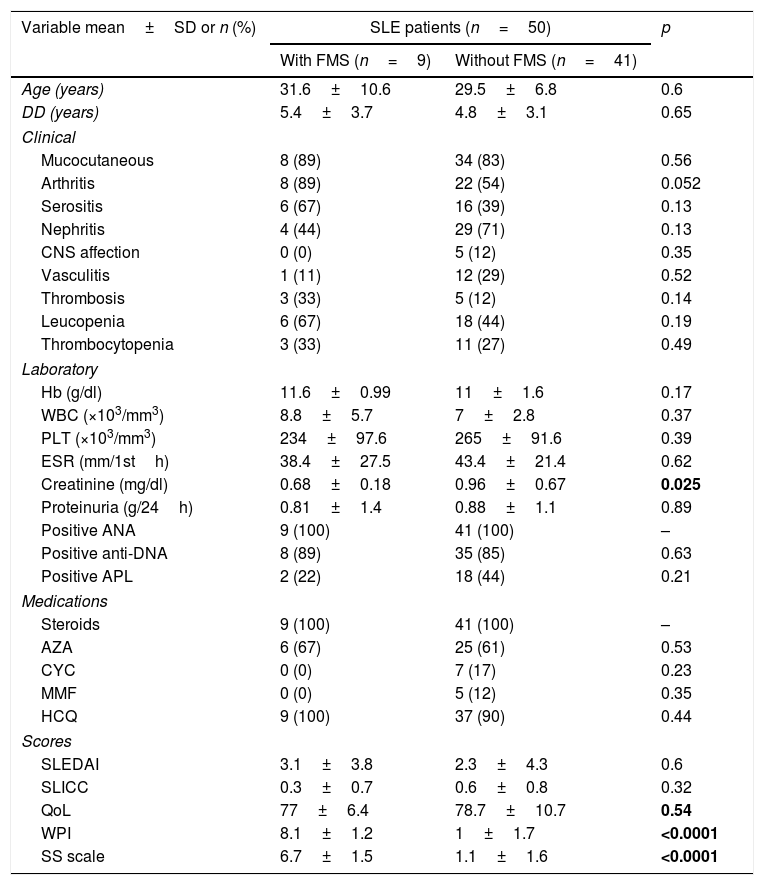
Demographic features, clinical manifestations, laboratory investigations, disease activity, damage, quality of life and medications used in systemic lupus erythematosus (SLE) patients with and without fibromyalgia syndrome (FMS).
| Variable mean±SD or n (%) | SLE patients (n=50) | p | |
|---|---|---|---|
| With FMS (n=9) | Without FMS (n=41) | ||
| Age (years) | 31.6±10.6 | 29.5±6.8 | 0.6 |
| DD (years) | 5.4±3.7 | 4.8±3.1 | 0.65 |
| Clinical | |||
| Mucocutaneous | 8 (89) | 34 (83) | 0.56 |
| Arthritis | 8 (89) | 22 (54) | 0.052 |
| Serositis | 6 (67) | 16 (39) | 0.13 |
| Nephritis | 4 (44) | 29 (71) | 0.13 |
| CNS affection | 0 (0) | 5 (12) | 0.35 |
| Vasculitis | 1 (11) | 12 (29) | 0.52 |
| Thrombosis | 3 (33) | 5 (12) | 0.14 |
| Leucopenia | 6 (67) | 18 (44) | 0.19 |
| Thrombocytopenia | 3 (33) | 11 (27) | 0.49 |
| Laboratory | |||
| Hb (g/dl) | 11.6±0.99 | 11±1.6 | 0.17 |
| WBC (×103/mm3) | 8.8±5.7 | 7±2.8 | 0.37 |
| PLT (×103/mm3) | 234±97.6 | 265±91.6 | 0.39 |
| ESR (mm/1sth) | 38.4±27.5 | 43.4±21.4 | 0.62 |
| Creatinine (mg/dl) | 0.68±0.18 | 0.96±0.67 | 0.025 |
| Proteinuria (g/24h) | 0.81±1.4 | 0.88±1.1 | 0.89 |
| Positive ANA | 9 (100) | 41 (100) | – |
| Positive anti-DNA | 8 (89) | 35 (85) | 0.63 |
| Positive APL | 2 (22) | 18 (44) | 0.21 |
| Medications | |||
| Steroids | 9 (100) | 41 (100) | – |
| AZA | 6 (67) | 25 (61) | 0.53 |
| CYC | 0 (0) | 7 (17) | 0.23 |
| MMF | 0 (0) | 5 (12) | 0.35 |
| HCQ | 9 (100) | 37 (90) | 0.44 |
| Scores | |||
| SLEDAI | 3.1±3.8 | 2.3±4.3 | 0.6 |
| SLICC | 0.3±0.7 | 0.6±0.8 | 0.32 |
| QoL | 77±6.4 | 78.7±10.7 | 0.54 |
| WPI | 8.1±1.2 | 1±1.7 | <0.0001 |
| SS scale | 6.7±1.5 | 1.1±1.6 | <0.0001 |
SLE: systemic lupus erythematosus, FMS: fibromyalgia syndrome, DD: disease duration, SLEDAI: systemic lupus erythematosus disease activity index, SLICC: Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index, WPI: widespread pain index, SS scale: symptoms severity scale, QoL: quality of life. Hb: hemoglobin, WBCs: white blood cells, PLT: platelet, ESR: erythrocyte sedimentation rate, ALT: alanine transferase, AST: aspartate transferase, ANA: antinuclear antibodies, DNA: deoxyribonucleic acid, APL: antiphospholipid. AZA: azathioprine, CYC: cyclophosphamide, MMF: mycophenolate mofetil, HCQ: hydroxychloroquine. Bold values are significant at p<0.05.
The study included 30 SSc patients with a mean age of 39.3±13.04 years (range 21–71 years). They were 26 females and 4 males (F:M 6.5:1) and the mean disease duration was 6.6±6.1 years (1–30 years). The 30 control were matched in age (37.4±15 years) and sex (F:M=6.5:1). All the SSc patients had Raynaud's phenomenon, 80% had pitting ulcers, 40% arthritis, interstitial lung disease in 36.7% and pulmonary artery hypertension in 16.7%. 90% of the patients had gastrointestinal manifestations and only 2 developed gangrene. The mean erythrocyte sedimentation rate was 41.1±24.2mm/1sth, hemoglobin content 11.8±1.6g/dl, WBC count 7.6±2.3 ×103cell/mm3, platelet count 292±117 ×103cell/mm3 and the antinuclear antibodies positive in 86%. The mean mRss was 20.23±8.04 (range 6–35) and the SSc disease severity scale 6.67±2.26 (range 3–11). The QoL scale was 72.9±10 (range 50–87). 25 (83%) patients received steroids, 3 received methotrexate, 9 received azathioprine (AZA), 2 received cyclophosphamide (CYC) and 90% received vasodilators. The mean WPI was 1.9±2.2 (range 0–9) and SS score 1.7±2.3 (range 0–9). Two (6.67%) female patients had FMS while only one (3.3%) of the control had FMS. The WPI and SS scale did not correlate with any of the studied parameters.
The study included 30 BD patients with a mean of 37.4±11.4 years (range 21–69 years) They were 6 females and 24 males (F:M=1:4) and the mean disease duration was 12.1±8.4 years (1.5–33 years). The 30 control were matched in age (35.8±9 years) and sex (F:M 1:4). All the patients had oral ulcers, genital ulcers in 93%, skin lesions in 70%, uveitis in 47%, deep venous thrombosis in 30%, CNS involvement in 20% and pulmonary aneurysm was present in 2 patients. The mean ESR was 19.3±15.1mm/1sth, hemoglobin content 13.3±1.4g/dl, WBC count 8.9±2.7 ×103cell/mm3 and platelet count 248±73.6 ×103cell/mm3. The mean BDCAF score was 2.3±1.4 (range 0–5) and the QoL 71.7±14.5. 29 patients received steroids, 11 received AZA, 3 received CYC, 7 received cyclosporine A, 3 received infliximab, 26 (86.7%) received colchicine and 7 received oral anticoagulation. The WPI ranged from 0–4 with a mean of 0.67±1.09 and SS score ranging from 0 to 9 with a mean of 1.67±2.26. Only one (3.33%) female patient had FMS and none of the control had FMS. Both the WPI and SS scale significantly correlated with the BDCAF (r=0.4, p=0.03 and r=0.48, p=0.008 respectively)
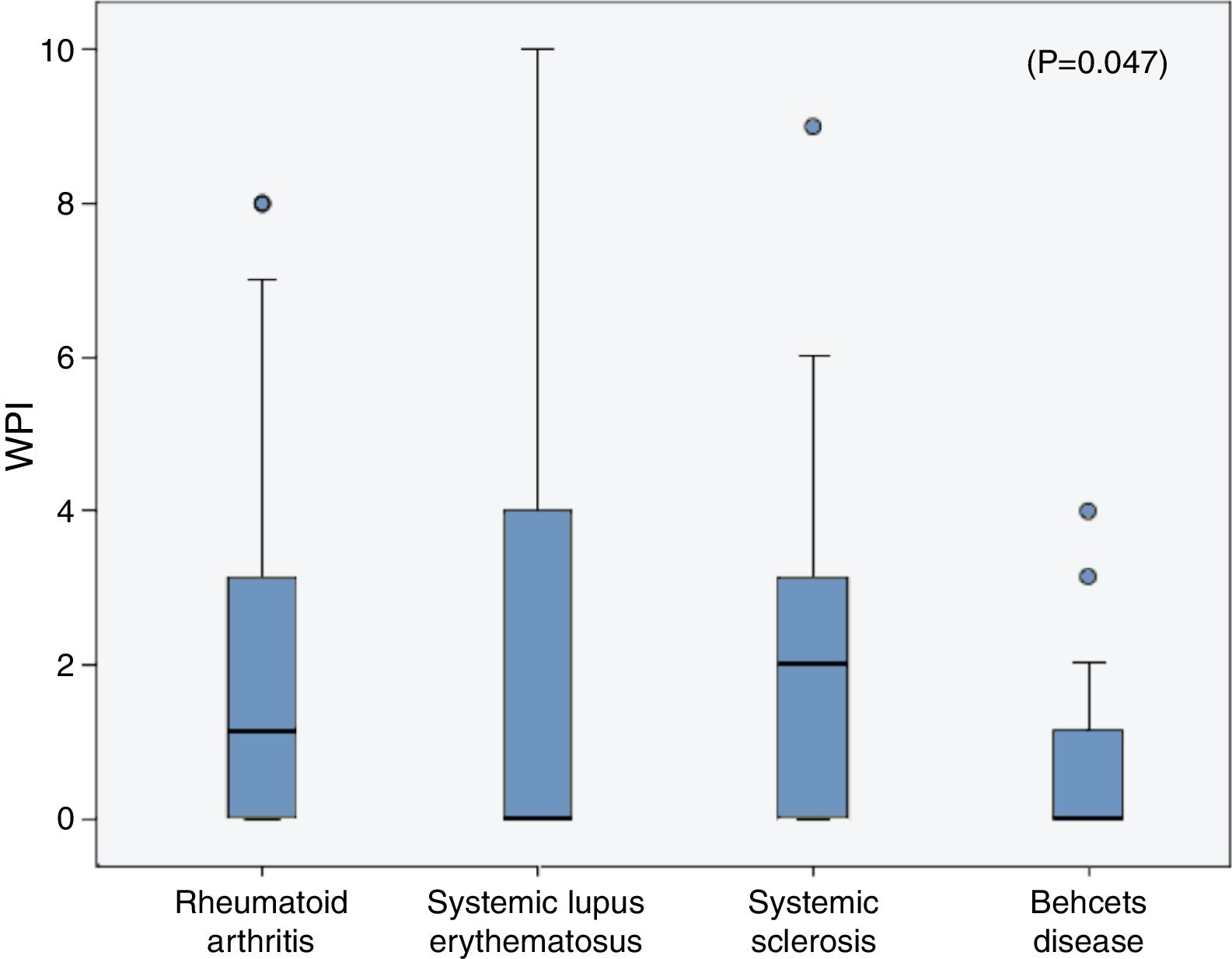
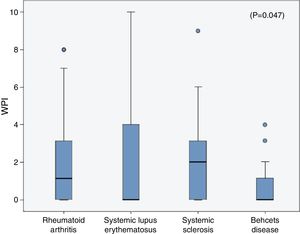
The frequencies of FMS in the rheumatic diseases were as follow; 14% in RA, 18% in SLE, 6.67% in SSc and in 3.33% of the BD patients and all were higher than the frequencies in their corresponding control (2.1%, 3%, 3.33% and 0% respectively). On comparing the WPI among the rheumatic diseases patients, the mean was significantly higher in the SLE patients (2.3±3.2) compared to that in the RA (1.96±2.6), SSc (1.9±2.2) and BD (0.7±1.1) patients (p=0.047) (Fig. 1), although the age and sex could not be unified among the diseases. The SS scale was comparable among the different rheumatic diseases (p=0.43).
DiscussionIn clinical practice, the co-expression of FMS and a rheumatologic disease deserves special attention as FMS may go unrecognized especially when it develops after the disease or more commonly when it is misdiagnosed as an autoimmune disorder.24 Concomitant FMS could influence the interpretation of the disease activity and QoL. Considerations of the FMS component in the management of rheumatologic diseases increase the likelihood of the success of the treatment.6
In the present study, the frequency of FMS in the RA patients was 14%. Similarly, the prevalence of FMS in RA was reported to be 12–17%.25–29
In the present study there was no significantly difference in the age or disease duration between RA patients with and without FMS. This is in agreement to the results of another study.30 In the present study, rheumatoid factor positivity was comparable between those with and without FMS. This result is similar to that of previous studies.25,27,28,30,31 Erosive changes in x-rays occurred in 86% of RA patients without FMS as compared to 71.4% of those with and the difference was not significant. In accordance to the present results, articular erosions tended to occur more frequently in RA patients without FMS.27 It has been suggested that FMS may act as a protective trait in RA patients, possibly by alerting the physician more rapidly to onset of flare. Hence it was proposed that the association between RA and FMS does not appear to be a marker of worse prognosis, but rather an accidental relation that may provide these patients some protection against joint destruction.31 The DAS28 is a strong predictor of physical capacity and radiologic progression. Therefore, the possibility that FMS affects the interpretation of this score may have important implications and misclassification of disease activity may lead to an unnecessary change in the therapy of RA.27 In this study, the mean DAS28 was significantly higher in RA with FMS than those without. Similarly, DAS28 was significantly higher when FMS was associated to RA.27,28 Functional assessment in the current study using the HAQII score revealed a similar result in the RA with and without FMS. However, the QoL was significantly worse in RA with FMS. These results came in accordance with previous studies.25,27–29,31 The medications received by those with and without FMS were comparable, yet those with FMS tended to be less treated.
In this work, the frequency of FMS in SLE was 18%. Comparable frequencies were reported.32–34 A lower prevalence of FMS in SLE has been presented by others.35–37 Ethnic differences may contribute to the differences found in the co-existence of FMS and SLE.36
Regarding the age and disease duration, comparison between the SLE patients with and without FMS yielded no significant difference. This was concomitant with the results of previous studies.33,36,37 In the present study, there was no statistical difference between those with and without FMS with respect to the clinical manifestations and disease activity or damage. The same finding was present in previous study.36,37 However, thrombosis tended to be increased in those with FMS and together with the significant correlation found between the WPI and SS scale with the presence of thrombosis throws light on the importance of considering subclinical thrombosis in this vulnerable subgroup of SLE patients. Interestingly, a hypercoagulable state has been reported in FMS patients demonstrated by increased markers of coagulation activation and increased blood viscosity due to the generation of soluble fibrin monomer. Moreover, in FMS patients an associated hereditary defect in coagulation regulatory proteins has been suggested.38 The QoL scale was not significantly different between the SLE patients with and without FMS. In contrast, impairment of health related QoL among SLE patients with FMS has been described.36 In terms of treatment, there were no significant differences between the SLE patients with and without FMS. Similar findings were reported.33,37
The current study only 2 (6.67%) SSc patients had FMS. Similarly, FMS has been found in 2% of SSc patients and was not different from that in the healthy control.8
Only one BD patients (3.33%) had FMS. The studies on the relationship between FMS and BD are limited. However, in a Turkish study39 FMS was reported in 9.2% of BD patients and was found to be 18% in another.9 This discrepancy from the current results could be attributed to using different classification criteria for diagnosis, ethnic differences and the female predominance of their study cohorts. Female predominance is a well-known feature of FMS possibly due to hormone-related mechanisms.40 Again, in disharmony to the present findings, a Korean study revealed FMS in 37.1% of BD patients.24 In this study, the BDCAF score significantly correlated with WPI and SS scale. In disagreement, tender points did not correlate with the ESR or disease activity.24
A larger scale longitudinal study is recommended to confirm the presented results and to detect the impact of treatment on the associated FMS. The significance of this study is boosted by the fact that it was among the first to investigate the prevalence of FMS in patients with SSc. Also, adds to the limited insights on the relation of FMS to BD. The clinical significance of the association between FMS and the presence of thrombosis in SLE patients has to be considered. It is novel to present the relative prevalence of FMS in different Egyptian rheumatic diseases patients and to throw light on the association with disease activity in RA and BD as well as thrombosis in SLE. The impact of FMS on the QoL in RA patients requires special attention.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestNone declared.