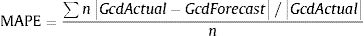Glucocorticoids are associated with serious side effects related to dosing and time of use. Unfortunately, there is no standard method for determining glucocorticoid exposure, especially in patients undergoing long-term treatment.
ObjectiveThe aim of this work was to create a free and easy-to-use web application to calculate, in a systematic way, the total cumulative dose of corticosteroids.
MethodsThe total cumulative dose is calculated as the sum of all periods of treatment with different doses of corticosteroids, and is expressed as the equivalent dose of prednisone in mg. Glucocorticoid doses during periods in which the available information is missing or incomplete are estimated by systematic assumptions.
ResultsA simulation exercise using standard patterns of steroid use in polymyalgia rheumatica, and giant cell arteritis showed that even when the period of no information reached 50% of the time, the accuracy of the calculator had a mean absolute percentage error (MAPE)<7%.
ConclusionThis tool simplifies and standardizes the glucocorticoids cumulative dose calculation, thereby minimizing bias in the assessment of glucocorticoid cumulative dose.
Los glucocorticoides se asocian con efectos secundarios graves, relacionados con dosis y tiempo de uso. Desafortunadamente, no existe un método estándar disponible para determinar el nivel de exposición a glucocorticoides en tratamientos prolongados.
ObjetivoCrear una aplicación web gratuita y fácil de usar para calcular, de forma sistematizada, la dosis acumulada de glucocorticoides.
MétodosLa dosis acumulada se calcula como la suma de todos los períodos de tratamiento con diferentes dosis, y se expresa como la dosis equivalente de prednisona en mg. La dosis durante los períodos en los que la información no está disponible o está incompleta se estima mediante asunciones sistematizadas.
ResultadosUn ejercicio de simulación utilizando patrones estándar de uso de esteroides en la polimialgia reumática y la arteritis de células gigantes demostró que, incluso cuando el período de ausencia de información alcanzaba el 50% del tiempo, la precisión de la calculadora tenía un porcentaje de error medio absoluto (MAPE)<7%.
ConclusiónEsta herramienta simplifica y estandariza el cálculo de la dosis acumulativa de glucocorticoides, minimizando el sesgo del cálculo.
Glucocorticoids are a cornerstone of effective therapy for many inflammatory and autoimmune human disorders.1–5 Despite their undoubted beneficial therapeutic properties, the use of these compounds is associated with many well-known harmful adverse events,6 which constitute the main limitation preventing their widespread use. Most glucocorticoid-related adverse events are tied to dosing and treatment duration, two variables that determine the cumulative dose and, consequently, patient exposure to these compounds.7 It has been demonstrated that glucocorticoid-related adverse events can be mitigated by certain actions; for example, by using less potent agents or by administering them at the lowest dose and over the shortest time frame possible.8,9 Consequently, correctly assessing the cumulative dose of glucocorticoids is particularly important to determine the risk of suffering undesirable effects.
Due to long-term use and frequent dose variations in response to changes in disease activity, information about glucocorticoids is often inaccurately and incompletely recorded in patient medical records. Glucocorticoid exposure assessments are often carried out by physicians during daily clinical practice using non-systematized estimations. This makes it particularly difficult to conduct inter-study comparisons of the side effects caused by glucocorticoids, or even to analyze the influence of glucocorticoid treatments on the side effects caused by other concomitant medications. Although several tools have been designed to assess the cumulative dose of glucocorticoids,10,11 as of yet no consensus has emerged on how best to standardize the assessment of glucocorticoid exposure when using daily clinical practice data.
The aim of this work is to provide an easy-to-use method to standardize the assessment of the cumulative dose of glucocorticoids using information obtained from patients’ daily clinical practice records. We believe that the use of this tool will reduce bias in the assessment of glucocorticoid exposure that currently exists between different studies.
MethodsThis section presents our general reasoning, including a description of the assumptions made in cases of incomplete information, underlying this project. To facilitate the use of this tool, the version 2 of a free software application based on this proposal (available both in English and Spanish and designated as CORTISER+) has been developed (https://cortiser.ser.es) (see supplementary text). This calculator has been developed by members of the Research Unit of the Spanish Society of Rheumatology: two methodologists and a statistician, and by three clinical rheumatologists with the aim of being used for the calculation of the accumulated dose of glucocorticoids in the ARTESER project, a retrospective registry of patients with giant cell arteritis (GCA) supported by the Spanish Society of Rheumatology (https://www.ser.es/arteser/). The source code of the calculator belongs to the Spanish Society of Rheumatology and is available upon reasoned request.
Calculator descriptionIn this proposal, oral, intravenous (iv), intramuscular (im) and intra- and peri-articular (ia) doses of glucocorticoids were calculated separately. The total cumulative dose was calculated by adding the partial doses of the different iv-, im- and ia-administered glucocorticoids to the partial doses produced by each of the different periods during which the patient received glucocorticoids orally. Finally, the cumulative dose is expressed as the total prednisone-equivalent dose in mg (Table 1).12
To estimate the dose of iv, im and ia steroids, information about the strength of each bolus, the number of boluses, and the start and end dates of each treatment must be entered into the computer application. The application then simply multiplies the strength by the number of boluses, and transforms the result into an equivalent dose of mg of prednisone.
Oral glucocorticoids are calculated using information regarding the strength of the daily dose, the type of compound prescribed and the start and end dates of treatment. The total days of treatment are calculated as the difference between the end date and the start date of treatment. In the optimal situation, in which all information is available, the application simply multiplies the dose administered daily by the number of days of treatment, transforming the result into an equivalent dose mg of prednisone, as detailed in Table 1.12 However, information regarding dosage, days of treatment, or both, of oral glucocorticoids is often incomplete or unavailable in medical records. The proposed method incorporates assumptions to estimate the cumulative dose of glucocorticoids, when:
- 1)
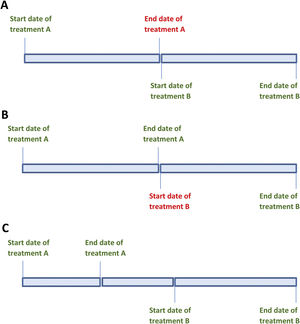
The strength of the daily dose and treatment start date are known, but the end date is not. In this situation, the end date will be estimated as the day before the start date of the next known treatment period (Fig. 1A). To calculate the cumulative dose, the number of days between the known start date and the estimated end date is calculated and then multiplied by the daily dose. Any known data must be included in the application, while leaving the unknown data blank.
- 2)
The strength of the daily dose and the end date of the treatment period are known, but not the start date. The start date is estimated as the day after the known end date of the previous period (Fig. 1B). To calculate the cumulative dose for this period, the number of days between the known end date and the estimated start date is calculated and multiplied by the daily dose. Only known data should be entered into the calculator.
- 3)
The start and end doses of glucocorticoids differ and the variations in dosing during that period are unknown (Fig. 1C). To calculate the cumulative dose for this period, the average between the initial and end doses of glucocorticoids is multiplied by the duration of the period.
To correctly use the calculator, one should adhere to the following recommendations:
- 1)
The date of glucocorticoids treatment start is essential. If investigators do not know this date, the date from which the cumulative dose was assessed should be utilized.
- 2)
The end date of glucocorticoids treatment is also mandatory. If the investigator does not know this date, the last known date glucocorticoids were used or the date the cumulative dose assessment ended should be utilized.
- 3)
When the patient takes glucocorticoids on alternate days or at different doses on consecutive days, the strength of the daily dose for that period should be assumed to be equal to the average of two consecutive days.
- 4)
Periods when the patient is not taking glucocorticoids should be included as daily dose=0. This allows for a distinction between periods when the patient was not on glucocorticoid treatment and those when it was unknown whether the patient was receiving them.
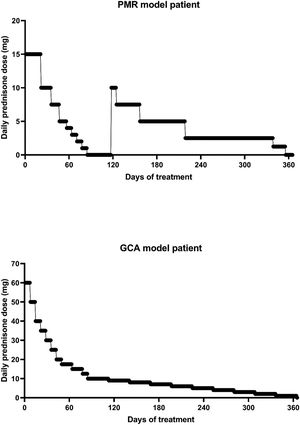
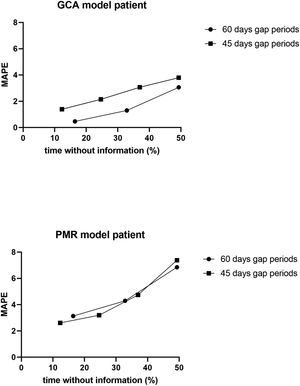
To determine the reliability of the calculator, a simulation exercise was performed. Two standard patterns of glucocorticoid tapering commonly used for the treatment of patients with polymyalgia rheumatica (PMR) and GCA were used as the gold standard, beginning with 15 and 60 mgs of prednisone, respectively (Fig. 2). Both glucocorticoid tapering regimens were extended over one year, and a relapse was included in the PMN patient model. In both standard glucocorticoid tapering schemes, 1–4 non-overlapping periods of 45 days and 1–3 non-overlapping periods of 60 days’ duration were randomly eliminated, and the total cumulative dose was assessed with the calculator in each situation. The simulations were repeated 20 times and the data are expressed as the mean absolute percentage error (MAPE) in accordance with the following formula:
Gcd=glucocorticoids cumulative dose.
ResultsResults of simulation exercise with two standard patterns of glucocorticoid tapering, one for PMR and other for GCA are shown in Fig. 3. In both reduction patterns and in all situations involving unknown information, the mean difference between the predicted and actual value of the cumulative glucocorticoid dose was less than 7%, even in those circumstances in which the period without information reached 50%. As expected, the shorter the period and the shorter the time without information, the more accurate was the result obtained by the calculator.
Dot-plot showing the results of the calculator in a simulation exercise involving two standard one-year glucocorticoid tapering schemes: one in PMR and the other in GCA. In both schemes, 1–4 non-overlapping periods of 45 days’ duration, as well as 1–3 non-overlapping periods of 60 days’ duration, were randomly eliminated, and the total cumulative dose was assessed with the calculator for each situation. The simulation exercise was repeated 20 times and the data are expressed as the mean absolute percentage error (MAPE) with respect to the total % of time without information.
The main aim of this proposal was to provide an easy-to-use tool to standardize the assessment of the cumulative dose of glucocorticoids. In simulation exercises, the calculator showed a mean error between the predicted and actual value of the cumulative glucocorticoid <7%, even in those circumstances in which the time periods without information reached 50%. This calculator can help minimize the bias that non-standardized assessments of long-term glucocorticoid are prone to when evaluating the side effects of these compounds, both in daily clinical practice and in clinical research.
The chronicity of glucocorticoid use and the variations in glucocorticoid dosing over time due to changes in the inflammatory process explain why information on these compounds is often incompletely or inaccurately recorded in medical records, specifically oral administration. While there are many rheumatological conditions in which the cumulative dose of steroids have been examined as a confounder of comorbidities and response to treatment,10,11,13,14 and though some researchers have proposed their own tools,15–21 there is no standardized method for calculating the cumulative dose of glucocorticoids. This lack of standardization means that data on cumulative doses of glucocorticoids are not comparable between studies. Simulation results show that our calculator provides an average difference between the forecasted value and the actual value of<7%, even when the amount of steroids consumed by the patient is unknown, which is often where more than 50% of the time. Recently, our calculator was used to assess the cumulative dose of glucocorticoids in the ARTESER project, a retrospective register sponsored by the Spanish Society of Rheumatology that included 1675 GCA patients (https://www.ser.es/arteser/).
The main limitation of this tool is that when no specific information is available in the clinical histories (mainly concerning oral administration), it becomes necessary to make assumptions about doses and exposure times to glucocorticoids based on the available data. However, since these assumptions follow a systematic procedure, it is possible to compare cumulative doses of glucocorticoids individually among patients, as well as globally among studies, when using this tool.
ConclusionWe believe that widespread use of this calculator may help minimize the bias that afflicts non-standardized assessments of cumulative glucocorticoid doses in both clinical practice and clinical trials.
Authors’ contributionsN.M.-P., J.T.-S. and F.D.-G. designed the method and application, tested the application and wrote the final version of the manuscript. M.G.-R. conducted the literature search. F.S.-A. participated in the statistical study. C.M. and J.L. tested the application and wrote the preliminary version of the manuscript. All authors discussed, corrected and approved the final version of the manuscript.
FundingThis application is supported by Roche. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The author(s) received no specific funding for this work.
Conflict of interestsThe authors state that there are no competing financial interests.