Considering the increased fracture risk in early breast cancer patients treated with aromatase inhibitors (AI), we assessed the impact of a preventive intervention conducted by a specialized osteoporosis unit on bone health at AI treatment start.
Material and methodsRetrospective cohort of postmenopausal women who started treatment with AI after breast cancer surgical/chemotherapy treatment and were referred to the osteoporosis unit for a comprehensive assessment of bone health. Bone densitometry and fracture screening by plain X-ray were performed at the baseline visit and once a year for 5 years.
ResultsThe final record included 130 patients. At AI treatment start, 49% had at least one high-risk factor for fractures, 55% had osteopenia, and 39% osteoporosis. Based on the baseline assessment, 79% of patients initiated treatment with bisphosphonates, 88% with calcium, and 79% with vitamin D. After a median of 65 (50–77) months, 4% developed osteopenia or osteoporosis, and 14% improved their densitometric diagnosis. Fifteen fractures were recorded in 11 (8.5%) patients, all of them receiving preventive treatment (10 with bisphosphonates). During the follow-up period, patients with one or more high-risk factors for fracture showed a greater frequency of fractures (15% vs. 3%) and experienced the first fracture earlier than those without high-risk factors (mean of 99 and 102 months, respectively; P=0.023).
ConclusionsThe preventive intervention of a specialized unit at the start of AI treatment in breast cancer survivors allows the identification of patients with high fracture risk and may contribute to preventing bone events in these patients.
Evaluar el impacto de la intervención preventiva de una unidad de osteoporosis en supervivientes de cáncer de mama que inician un tratamiento con inhibidores de la aromatasa (IA).
Material y métodosEstudio retrospectivo en mujeres posmenopáusicas con cáncer de mama precoz que iniciaron un tratamiento con IA tras la cirugía y/o quimioterapia, derivadas a la unidad de osteoporosis para una evaluación de la salud ósea, incluyendo densitometrías óseas y búsqueda sistemática de fracturas mediante Rx al inicio del tratamiento y anualmente durante 5 años.
ResultadosSe incluyeron 130 pacientes. Al inicio del tratamiento con IA el 49% tenía al menos un factor de riesgo alto para fracturas, el 55% osteopenia y el 39% osteoporosis. Tras la evaluación inicial, el 79% de las pacientes inició un tratamiento con bifosfonatos, el 88% con calcio y el 79% con vitamina D. Tras una mediana de 65 (50-77) meses, el 4% desarrolló osteopenia u osteoporosis y el 14% mejoró el diagnóstico densitométrico. Se registraron 15 fracturas en 11 (8,5%) pacientes, todas ellas en tratamiento preventivo. Durante el seguimiento, las pacientes con ≥1 factores de riesgo altos registraron una mayor frecuencia de fracturas (15 vs. 3%) y un menor tiempo hasta la primera fractura (media de 99 vs. 102 meses; p=0,023).
ConclusionesLa intervención preventiva de una unidad de osteoporosis al inicio del tratamiento con IA en supervivientes de cáncer de mama permite identificar pacientes con un elevado riesgo de fracturas y puede contribuir a la prevención de eventos óseos en estas pacientes.
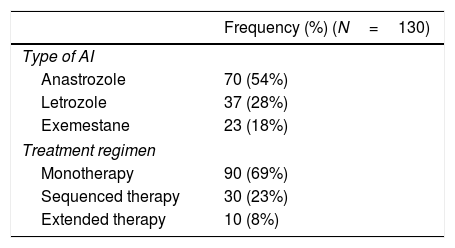
Breast cancer is the malignancy with the greatest incidence worldwide and contributes remarkably to cancer-associated mortality in women; in Spain, 27,747 new cases were diagnosed in 2015.1 However, breast cancer has higher survival rate than other types of cancer, regardless of the patient's age at diagnosis.2 Hormone therapy, used after surgical removal of early-stage hormone receptor-positive tumors, has shown to significantly reduce the relapse rate.3,4 In the last decade, treatment with aromatase inhibitors (AI) in postmenopausal women has emerged as an alternative to the traditional hormone therapy based solely on tamoxifen. AI can be introduced in three treatment regimens, with no proven advantage of one strategy over the others5,6: (a) as monotherapy (5-year treatment with AI only), (b) as sequenced therapy (2 years of tamoxifen treatment followed by 3 years of AI treatment, or vice versa), and (c) as extended therapy (5 years of tamoxifen treatment followed by 5 years of AI treatment).
One of the major drawbacks of AI treatment is the increased rate of bone loss, which has been estimated to be 1–2% yearly in the healthy population and may reach up to 5% during treatment with anastrozole.7,8 The AI-associated bone loss leads to an increased fracture risk: while postmenopausal breast cancer survivors have a 15% greater risk compared with healthy population, women treated with AI have 30% greater risk of fracture than the age-adjusted healthy population.9–11 The fracture incidence varies depending on the AI used: a 5.8% incidence has been reported with letrozole treatment,12 a 6.8% incidence with exemestane treatment,13 and a 11% incidence with anastrozole.14 Furthermore, the treatment approach regimen also seems to influence the fracture risk, which is significantly greater in sequential therapy although no differences between monotherapy and extended therapy have been found.15
In addition to AI treatment, breast cancer survivors are exposed to other factors associated with fracture risk such as demographical and clinical characteristics (aging, low weight, and previous fractures), and cancer treatment (use of corticosteroids and, to a lesser extent, chemotherapy and radiotherapy).16–18 Based on bone mineral density (BMD) measures and the presence of risk factors, various criteria have been proposed to assess the fracture risk in breast cancer survivors and to guide a preventive intervention during AI treatment.18–21 However, data regarding the efficacy of this intervention in real-life practice are limited, and the assessment of bone health in patients elected for AI treatment is uncommon in routine clinical practice.
In this study, we investigate the impact of a monographic bone health assessment on fracture risk in a retrospective cohort of breast cancer survivors treated with AI. In our center, patients were assessed in an osteoporosis-specialized unit, using the densitometric cutoffs proposed by the National Osteoporosis Foundation (NOF) and considering the optimized risk factor list available at the time for risk assessment and decision-making on preventive treatment prescription.
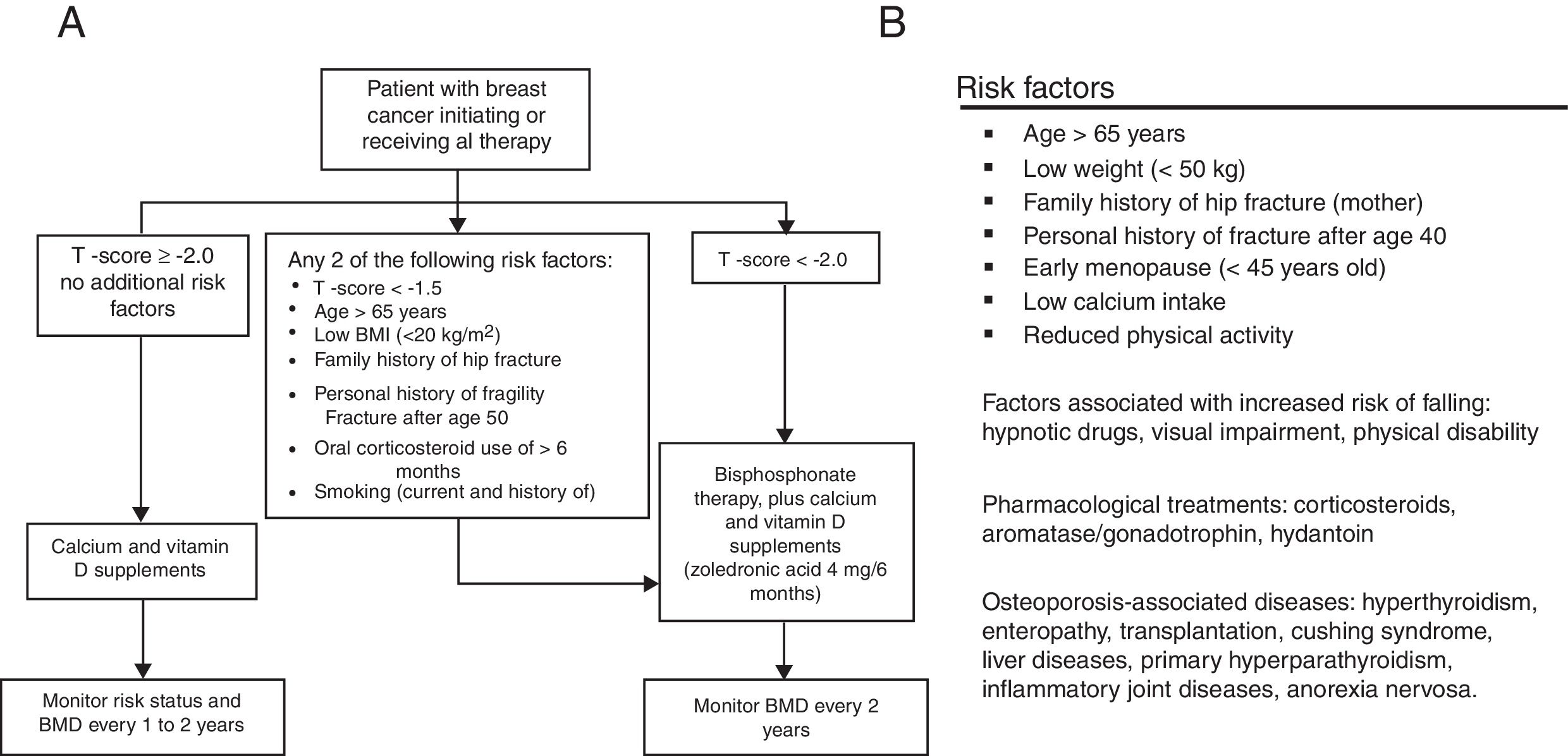
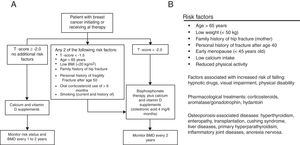
MethodsStudy design and populationThis study is based on a retrospective record of postmenopausal women, survivors to a non-metastatic breast cancer, who initiated adjuvant treatment with AI and were referred to the Osteoporosis Unit of the Basurto University Hospital (Spain) between January 2006 and December 2010 for bone health assessment. Only patients with surgically removed hormone receptor-positive cancers treated with AI for at least 3 years were considered for the study. Patients diagnosed with osteomalacia or imperfect osteogenesis were excluded from the record. Patients signed an informed consent before their clinical data were transferred, and the study protocol was approved by the independent ethics committee of our center. At the first assessment visit (baseline), scheduled at the beginning of AI treatment, all patients were provided with written recommendations on dietary and lifestyle habits for fracture prevention (adapted from Ruiz et al., 2004, see Supplementary Material).22 At baseline, a preventive treatment was established following the T-score cutoffs recommended by the NOF in 200319,20: T-scores <−1.5 and <−2.0 for patients with and without other risk factors, respectively. The risk factors considered in the assessment included two different sources: when available, the algorithm proposed by Hadji et al.18 was used whereas, before its publication, the risk factor list proposed by the Spanish Society of Rheumatology21 was considered (Fig. 1). After the baseline assessment, patients were followed-up every 6 months for 5 years. Densitometric controls were performed every 2 years.
Baseline variables and measurementsData collected at baseline included demographic characteristics (i.e. age, weight, and height), lifestyle habits potentially associated with bone loss (i.e. smoking history and alcohol consumption), risk factors for fractures, and the presence of comorbidities. Tumor characterization included the date of diagnosis, the stage, the presence of hormone and Her-2 receptors, and adjuvant treatment with chemotherapy and/or radiotherapy. The presence of osteopenia/osteoporosis was determined by a central bone densitometry (dual energy X-Ray absorptiometry [Electric Lunar DPX-NT, General Electric Healthcare, United Kingdom]) of the hip, femur, and column (from L1 to L4), performed after starting treatment with AI and every 2 years during the follow-up. The lowest T-score in any of the assessed areas was compared with the mean T-score of overall women aged between 20 and 40 years. Normality was considered when the patient's T-score was ≥−1 standard deviations (SDs) with respect the reference population mean. Accordingly, osteopenia was considered when the T-score decreased from −1.1 to −2.4 SDs, and osteoporosis when the T-score decreased −2.5 or more SDs with respect the reference population mean. The presence of an osteoporotic fracture on X-ray films was also considered for osteoporosis diagnosis. The screening of low-energy fractures was performed by dorsal and lumbar plain X-ray when a new risk factor for osteoporotic fractures was identified in a follow-up visit. Vertebral fractures were considered when the X-ray revealed a ≥20% deformity, according to the classification of Genant et al.23
EndpointsThe study endpoints included the presence of a fracture not present at the beginning of AI treatment, and a new diagnosis of osteopenia or osteoporosis during the follow-up. Only image-confirmed fractures (i.e. X-ray, computerized axial tomography, or magnetic resonance image) due to bone fragility were considered. Accident- or sport-related fractures were excluded from the analysis. In addition to the diagnosed fractures, we used column X-ray (thoracolumbar spine, lateral view) to investigate the presence of fractures in all patients with risk factors, and those who referred back pain and had not been previously explored by X-ray. Osteopenia or osteoporosis diagnosis was established by bone densitometry as described for the baseline visit.
Statistical analysisNormally-distributed quantitative variables were described as the mean and SD, whereas variables showing a skewed distribution were described as the median and the interquartile range (IR), defined by 25 and 75 percentiles. Qualitative variables were described as frequency and percentage. The prevalence of high-risk and moderate-risk factors for fracture was calculated according to the consensus statement of the Spanish Society of Rheumatology.16 The incidence of fractures was described as cumulative survival (using the Kaplan–Meier estimate), and cumulative frequency and percentage for the overall population and for patients with moderate- or high-risk factors, according to the updated consensus of the Spanish Society of Rheumatology.16 The proportion of patients with fractures or newly diagnosed osteoporosis/osteopenia in each group was compared using the chi-square test with a significance α-level of 0.05. All analyses were performed using the IPSS statistical package (IBM SPSS Statistics for Windows, Version 20. Armonk, NY).
ResultsPatient characteristicsBetween January 2006 and December 2010, 209 breast cancer survivors were referred to the osteoporosis unit for bone health assessment upon beginning AI treatment. Of them, 166 met all inclusion criteria and none of the exclusion criteria but 36 rejected entering the study, leading to a final sample of 130 women with a mean (SD) age of 62.3 (8.5) years. The tumor was at stage T1 in 72 (56%) patients, and 76 (60%) patients were node-negative. All tumors were hormone receptor positive (94% had estrogen receptors and 89% progesterone receptors), and 12 (9%) were Her-2 positive. Eighty-three (64%) patients received adjuvant treatment with chemotherapy after surgical excision of the tumor.
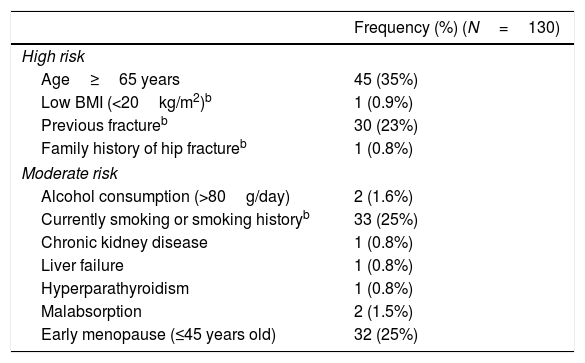
Table 1 summarizes the risk factors found in the baseline visit. Forty-nine percent of patients had one or more high-risk factors, and 47% had one or more moderate-risk factors. None of the patients had been treated with corticosteroids in the 12 months prior to starting treatment with IA. Of all fractures recorded before the beginning of AI treatment, 11 were low-energy fractures: 6 vertebral fractures, 5 wrist fractures (one patient experienced 2 wrist fractures) and 1 foot fracture.
Risk factors in study sample.a
| Frequency (%) (N=130) | |
|---|---|
| High risk | |
| Age≥65 years | 45 (35%) |
| Low BMI (<20kg/m2)b | 1 (0.9%) |
| Previous fractureb | 30 (23%) |
| Family history of hip fractureb | 1 (0.8%) |
| Moderate risk | |
| Alcohol consumption (>80g/day) | 2 (1.6%) |
| Currently smoking or smoking historyb | 33 (25%) |
| Chronic kidney disease | 1 (0.8%) |
| Liver failure | 1 (0.8%) |
| Hyperparathyroidism | 1 (0.8%) |
| Malabsorption | 2 (1.5%) |
| Early menopause (≤45 years old) | 32 (25%) |
The baseline examination of BMD was performed in a median (IR) of 3 (1–8) months after starting treatment with AI. Mean values of BMD at baseline for the overall study sample in the assessed regions were the following: 0.940 in the column (T-score −2.02), 0.875 in the hip (T-score −1.05), and 0.829 in the femoral neck (T-score −1.26). The exam revealed the presence of osteoporosis in 50 patients (39%) (the mean BMD was 0.820 in the column [T-score −3.01], 0.811 in the hip [T-score −1.59], and 0.776 in the femoral neck [T-score −1.72]). Osteopenia was identified in 71 patients (55%) (the mean BMD was 0.984 in the column [T-score −1.65], 0.894 in the hip [T-score −0.89], and 0.847 in the femoral neck [T-score −1.1]). Only 1 (1%) patient received treatment with bisphosphonates in the 12 months preceding the baseline visit.
Aromatase inhibitors treatment and cancer progressionPatients were treated with AI for a median (IR) of 60 (36–60) months. Table 2 summarizes the main characteristics of AI treatment, including the treatment regimen administered. Seven (5%) patients received hormonal treatment in the 12 months preceding the baseline visit.
At the time of starting the analysis, 15 (11%) patients had cancer relapse (local in 2 cases, and metastatic in 13 cases), and 13 (10%) had died, 9 of them because of cancer.
Fracture incidence and risk factorsThe proportion of patients experiencing fractures was greater among those with at least one high-risk factor than those without high-risk factors (15% vs. 3%). Furthermore, the time to first fracture was significantly shorter in patients with at least one high-risk factor: mean of 99 months (95% CI 88–111) vs. 102 months (95% CI 98–105) (P=0.023). On the other hand, the proportion of fractures among patients with moderate-risk factors was similar to that of patients without moderate-risk fractures (9.8% vs. 7.6%), and no significant differences were observed in the time to first fracture: mean of 106 months (95% CI 99–114) and 96 months (95% CI 90–101) for patients with and without moderate-risk factors, respectively (P=0.290). The baseline risk factors identified in patients experiencing a fracture were age over 65 years (8 patients), previous fracture (3 patients), early menopause (i.e. before 45 years of age) (3 patients), smoking history or currently smoking (2 patients), liver failure (1 patient), and hyperparathyroidism (1 patient). The comparative analysis of each risk factor did not reveal significant differences regarding fracture incidence.
Preventive intervention and bone eventsThe comprehensive assessment of bone health was performed in a median (IR) of 3 (1–8) months after initiating AI treatment. The baseline assessment was performed considering the risk factors proposed by the Spanish Society of Rheumatology in 101 patients (77.7%) and the risk factors proposed by Hadji et al. in 29 patients (22.3%). As a result of the assessment, 102 (79%) patients started treatment with bisphosphonates, administered orally in 94 (72%) patients (alendronate, risedronate or ibandronate) and intravenously in 8 (6%) patients (zoledronate). Reasons for using the intravenous route were low tolerability to an oral bisphosphonate (5 cases), osteoporosis with multiple fractures (1 case), and worsening in the BMD score during treatment with oral bisphosphonates (2 cases). Overall, 114 (88%) patients received calcium supplement and 102 (79%) vitamin D supplement.
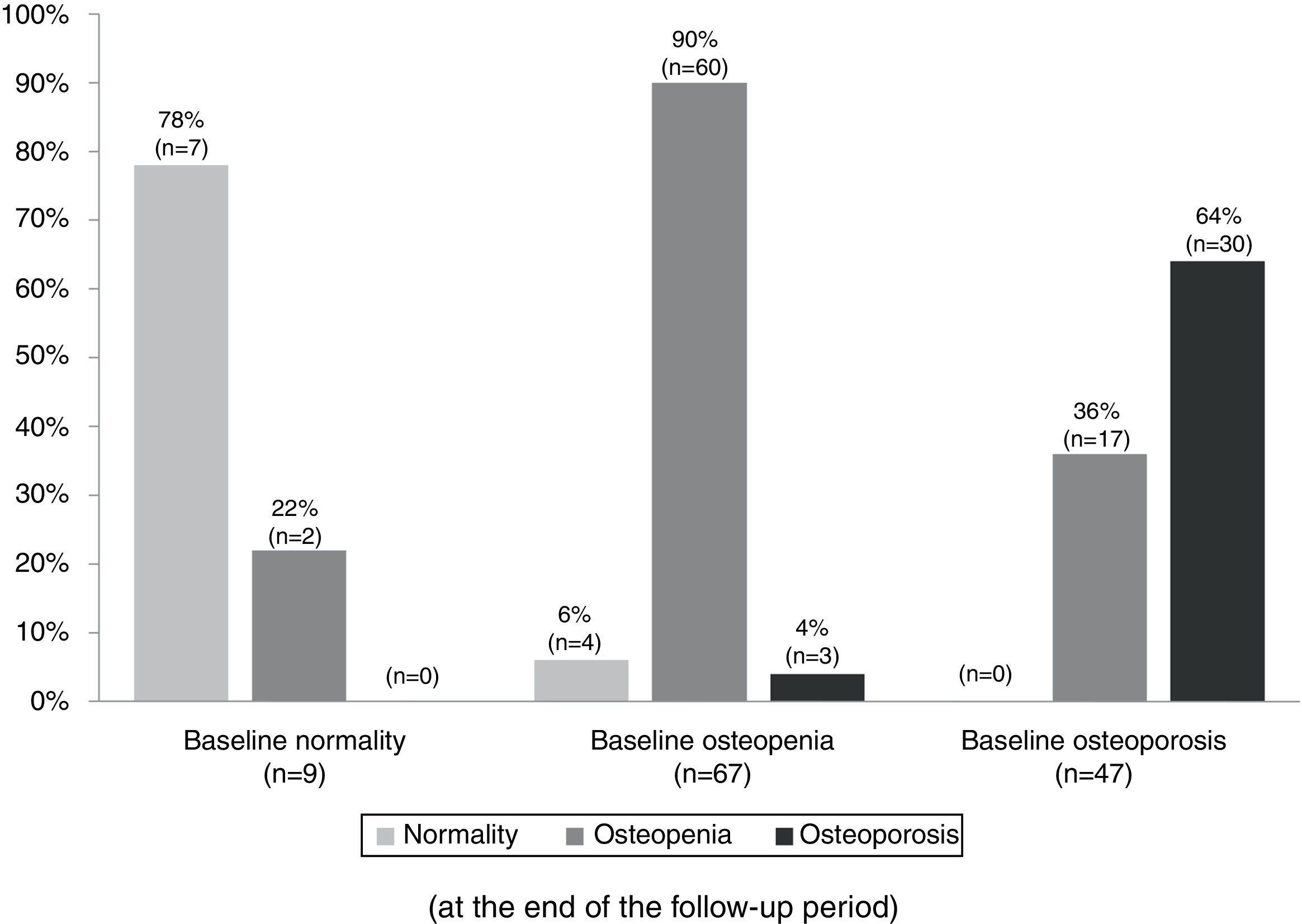
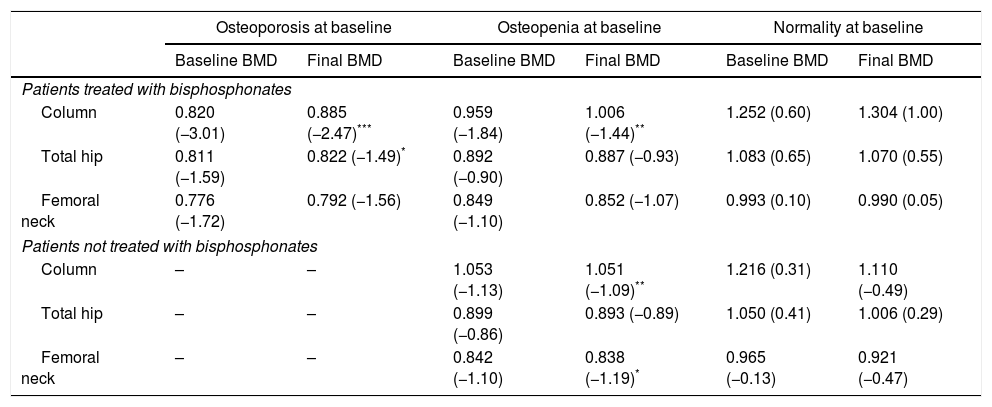
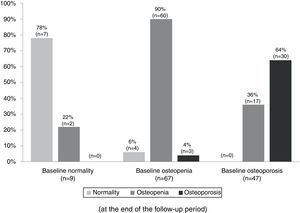
Table 3 summarizes the mean BMD values and T-scores of patients with and without bisphosphonate treatment at baseline and at the end of the follow-up period (median follow-up of 65 months; IR 50–77). Overall, 97 patients (79%) maintained their baseline BMD diagnosis (i.e. normality, osteopenia or osteoporosis), 5 (4.1%) experienced a worsening of their BMD diagnosis, and 17 (14%) an improvement. Fig. 2 summarizes the BMD diagnosis at the end of the follow-up period, grouped according to the baseline BMD diagnosis.
Bone mineral density (BMD) measurements at baseline and at the end of the follow-up period. Results are presented as mean g/cm2 (T-score).
| Osteoporosis at baseline | Osteopenia at baseline | Normality at baseline | ||||
|---|---|---|---|---|---|---|
| Baseline BMD | Final BMD | Baseline BMD | Final BMD | Baseline BMD | Final BMD | |
| Patients treated with bisphosphonates | ||||||
| Column | 0.820 (−3.01) | 0.885 (−2.47)*** | 0.959 (−1.84) | 1.006 (−1.44)** | 1.252 (0.60) | 1.304 (1.00) |
| Total hip | 0.811 (−1.59) | 0.822 (−1.49)* | 0.892 (−0.90) | 0.887 (−0.93) | 1.083 (0.65) | 1.070 (0.55) |
| Femoral neck | 0.776 (−1.72) | 0.792 (−1.56) | 0.849 (−1.10) | 0.852 (−1.07) | 0.993 (0.10) | 0.990 (0.05) |
| Patients not treated with bisphosphonates | ||||||
| Column | – | – | 1.053 (−1.13) | 1.051 (−1.09)** | 1.216 (0.31) | 1.110 (−0.49) |
| Total hip | – | – | 0.899 (−0.86) | 0.893 (−0.89) | 1.050 (0.41) | 1.006 (0.29) |
| Femoral neck | – | – | 0.842 (−1.10) | 0.838 (−1.19)* | 0.965 (−0.13) | 0.921 (−0.47) |
Changes in the bone mineral density diagnosis during 5 years of treatment with aromatase inhibitors in patients with BMD assessment at the end of the follow-up (n=123). Patients were classified into three groups according to the baseline BMD assessment: normality, osteopenia, and osteoporosis. The percentages displayed in the bar diagram correspond to patients with normality, osteopenia, and osteoporosis at the end of the follow-up period.
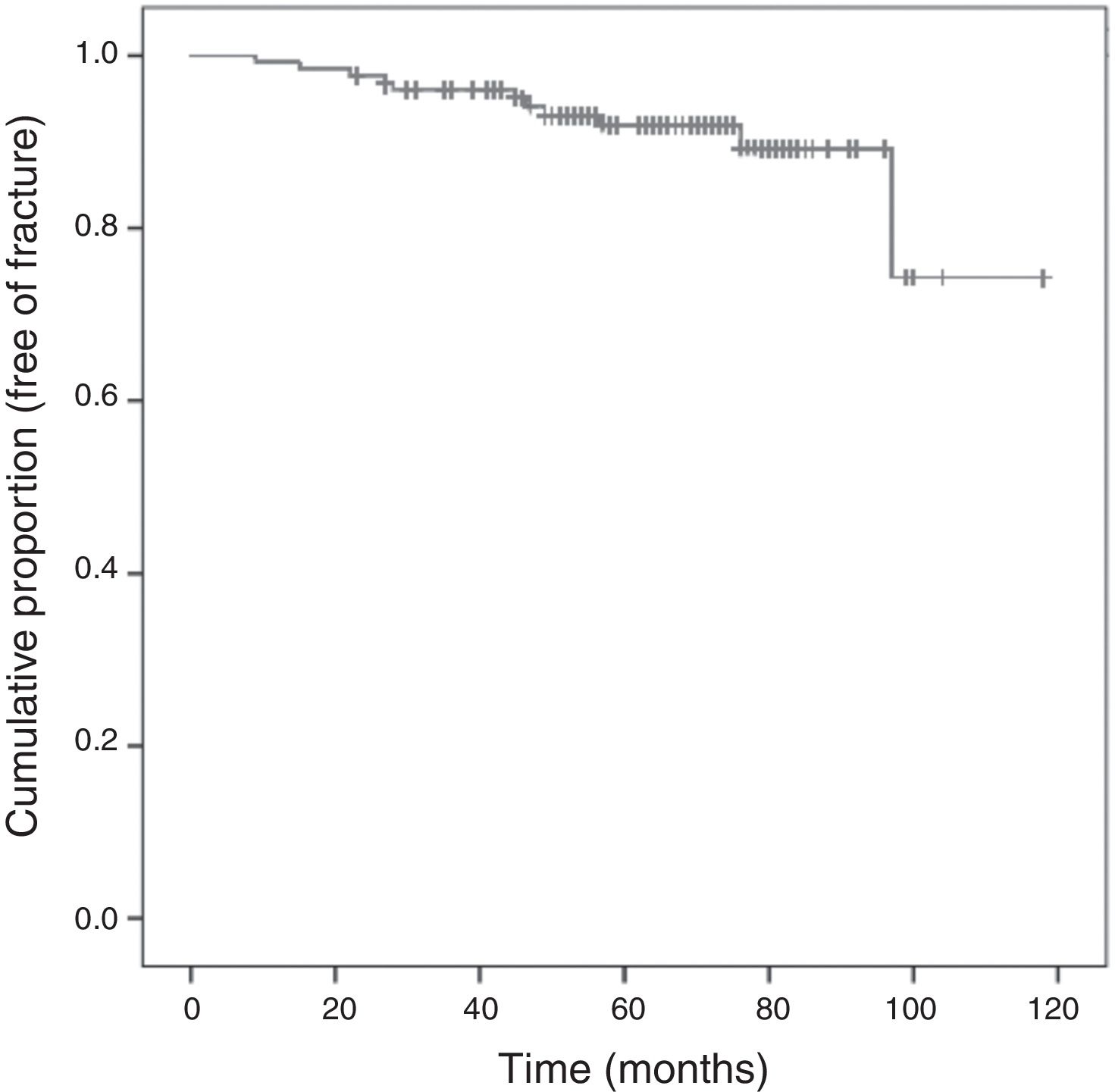
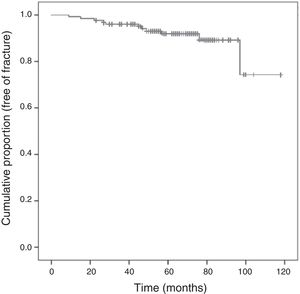
Following the screening criteria for low-energy fractures detailed in the methods section, 77 patients (59.7%) underwent plain X-ray at baseline visit, and 117 (90.0%) during the follow-up period. During that time, 11 patients (8.5%) experienced a total of 15 fractures; Fig. 3 shows the survival curve of recorded fractures. In 5 patients (45%), the fracture occurred during the first 3 years of AI treatment. In most cases, patients experienced only one fracture, with the exception of two patients who experienced 2 and 4 fractures, respectively during the follow-up. Regarding the fracture location, 9 (60%) affected the vertebrae, 4 (26%) the limbs, 1 (7%) the pelvis-sacrum, and 1 (7%) the rib. No hip fractures were recorded. Eighty percent of vertebral fractures were asymptomatic and were therefore identified by X-ray in a follow-up visit. All patients who experienced a fracture were receiving preventive treatment for bone loss: 9 were being treated with calcium, vitamin D, and bisphosphonates (administered orally and intravenously in 7 and 2 patients, respectively), one was being treated with a bisphosphonate and vitamin D, and one was being treated with calcium and vitamin D.
Regarding the fractures in patients treated with the various AIs, of 71 patients receiving anastrozole, 6 (8%) experienced a fracture; all of them had a history of previous fracture (5 vertebral, 1 in the wrist, and 1 in the sacrum), 5 had osteoporosis at baseline and 1 osteopenia. Of 36 patients treated with letrozole, 1 (3%) experienced a fracture; the patient had previous vertebral fracture and osteoporosis at baseline. Finally, of 23 patients treated with exemestane, 4 (18%) experienced a fracture; all of them had a history of previous fracture (2 vertebral, one in the wrist, and one patient had fractures in the rib, wrist, and vertebrae), 3 had osteoporosis at baseline and 1 osteopenia.
DiscussionDespite the risk of bone loss associated with AI treatment, bone health assessment in patients starting hormone treatment after breast cancer surgery is unusual in real-life practice. Our results show that breast cancer survivors who initiate treatment with AI have a high prevalence of fracture risk factors and may benefit from a specialized assessment in an osteoporosis unit at the beginning of AI treatment.
In our cohort, which included women with a mean age (62 years) similar to that found in large trials assessing the efficacy and safety of AI,8,24,25 nearly half of the patients had one or more high-risk factors for fracture, indicating that patients starting treatment with AI in real-life practice have a risk profile for bone events. In addition to age, a history of previous fracture is one of the most relevant risk factors.18,26 In the overall population, women with a previous fracture have 86% more risk of experiencing a new fracture, and some authors have suggested that the risk associated with this event could be independent of the patient's BMD.26,27 However, information about previous fracture in patients initiating treatment with AI is limited and large trials assessing the efficacy of AI treatment do not explore the presence of bone injuries actively. As a result, low-energy fractures (mostly asymptomatic, as commonly occurs in vertebral fractures) may be unnoticed, leading to an underestimation of the prevalence of previous fractures. In our retrospective cohort, 23% of patients had a history of previous fracture, mostly vertebral (4.6%) or wrist (3.0%) fractures. The prevalence of previous fracture reported in other observational studies assessing bone health in patients receiving hormone treatment is disparate. In line with our results, Servitja et al. reported a prevalence of 4.1% and 3.2% for vertebral and wrist fractures, respectively,28 while the corresponding percentages in the cohort of Bouvard et al. were 20.0% and 8.0% – even after excluding patients diagnosed with osteoporosis at the beginning of AI treatment.29 Despite these differences, the greater prevalence of vertebral and wrist fractures compared with other locations is common in all studies, and it is consistent with the epidemiology of low-energy fractures in Spain.30
The baseline assessment of the BMD revealed a remarkable number of patients (93%) with T-scores in the range of osteoporosis/osteopenia. During the 5 years of follow-up, only 2.4% of patients developed osteoporosis, a proportion remarkably lower than that reported in randomized clinical trials assessing the efficacy of AI (nearly 5%).8,13 Moreover, 14% of patients improved the BMD diagnosis, despite AI treatment.
During the follow-up, 8.5% of patients experienced at least one fracture, nearly half of them during the first three years. Interestingly, no hip fractures were reported; this finding is clinically significant, as it is among the fractures with greater morbidity and mortality risk.31–33 With the exception of Jackesz et al., who reported a particularly low fracture incidence during AI treatment (2% in 28 months of follow-up),34 most of the large randomized controlled trials reported greater incidence rates than that observed in our cohort: 7% in 58 months of follow-up (2–3 years with AI),25 9% in 60 months of follow-up,35 and 11% in 68 months of follow-up.36 It is worth mentioning that due to the lack of fracture screening in patients initiating AI treatment – both in the real clinical setting and in large clinical trials – the real fracture incidence might be even greater than reported.
In the general population, treatment recommendations for the prevention of bone events are based mainly on BMD and/or the presence of osteoporotic fractures.16 However, due to the increased risk of bone loss associated with AI treatment, various authors have stressed the need for extending the circumstances for initiating preventive treatment by also considering the baseline risk factors for fractures.10,18,19 In fact, our study patients with high-risk factors for fractures (according to the updated consensus of the Spanish Society of Rheumatology)16 experienced the first fracture significantly earlier than those without high-risk factors. As a retrospective study based on real-life practice, our intervention evolved throughout the study period by adopting the various risk factors lists proposed for making decisions on preventive treatment. At the time of study start, our baseline assessment was performed based on the T-score cutoffs proposed by the NOF19 and the risk factors described by the Spanish Society of Rheumatology.21 Later on, we used the comprehensive algorithm proposed by Hadji et al.18, which maintained the same T-score thresholds and simplified the risk factor list – albeit preserving key risk factors such as age, weight/BMI, health behavior, personal/family history, and corticosteroid use. Irrespective of the risk factors considered, 79% of patients in our cohort received preventive treatment for bone events. The analysis of fractured patients revealed that all of them had been identified as risk profiles for fracture in the baseline visit and hence experienced the fracture despite treatment with bisphosphonates, calcium and/or vitamin D. In a previous observational study describing the results of a preventive action based solely on the assessment of the BMD, 15 of 27 patients experiencing a fracture had not been identified as having a risk profile and therefore had not been treated.37
In addition to the moderate size of our cohort, the scope of our results must be appraised considering two important characteristics of the study design, both associated with the intrinsic limitations of studies based on real-life practice. First, no control group was included (i.e. patients without bone health assessment at the beginning of AI treatment). The recent approval of AI treatment at the time of study start precluded the use of a historical reference in our center. Likewise, we could not use large clinical trials as a reference for fracture incidence because the lack of screening for low-energy fractures hampered any comparison between our results and those reported in these trials. Second, the traditional lack of consensus regarding the risk factors to be included in a treatment-decision algorithm precluded a consistent assessment throughout the study period. In our osteoporosis unit, the algorithm proposed by Hadji et al. was adopted rapidly as we found it the most easy-to-apply and convenient option for establishing a preventive treatment. However, the baseline assessment of many patients was performed before the publication of the algorithm. It is noteworthy that most risk factors were consistent in both lists, including the above-mentioned key risk factors. Irrespective of the risk factors considered for the assessment, the DMO cutoffs for establishing preventive treatment where consistent throughout the time period.
Our specific assessment of bone health in patients beginning AI treatment, which included the screening for asymptomatic fractures, revealed a high prevalence of fracture risk factors, even greater than that observed in randomized trials assessing the efficacy and safety of AI. This finding supports the implementation of a systematic evaluation of bone health in patients who start treatment with AI. Furthermore, the low incidence of bone events reported during the 5 years of follow-up suggests that the assessment and treatment algorithm used may contribute to the prevention of bone events in these patients, and stresses the need for randomized controlled trials to confirm the efficacy of this intervention.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestP. Martínez, E. Galve, V. Arrazubi, M.A. Sala, S. Fernández, C.E. Pérez, J.F. Arango, and I. Torre declare that they have no conflict of interest.
The authors would like to thank BioClever, S.L., particularly Gerard Carot-Sans, PhD, for providing medical writing assistance. This study was funded by the EITB Maratoia, 2011 edition (grant number BIO11/CM/014).