To describe efficacy, safety, and patient-reported outcomes (PROs) in patients with rheumatoid arthritis (RA) with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) treated with tofacitinib or biological DMARDs (bDMARDs) in real-life conditions.
MethodsA noninterventional study was performed between March 2017 and September 2019 at 13 sites in Colombia and Peru. Outcomes measured at baseline and at the 6-month follow-up were disease activity (RAPID3 [Routine Assessment of Patients Index Data] score), functional status (HAQ-DI [Health Assessment Questionnaire] score), and quality of life (EQ-5D-3L [EuroQol Questionnaire]). The Disease Activity Score-28 (DAS28-ESR) and frequency of adverse events (AEs) were also reported. Unadjusted and adjusted differences from baseline were estimated and expressed as the least squares mean difference (LSMD).
ResultsData from 100 patients treated with tofacitinib and 70 patients with bDMARDs were collected. At baseline, the patients’ mean age was 53.53 years (SD 13.77), the mean disease duration was 6.31 years (SD 7.01). The change from baseline at month 6 was not statistically significant different in the adjusted LSMD [SD] for tofacitinib vs. bDMARDs for RAPID3 score (−2.55[.30] vs. −2.52[.26]), HAQ-DI score (−.56[.07] vs. −.50[.08]), EQ-5D-3L score (.39[.04] vs. .37[.04]) and DAS28-ESR (−2.37[.22] vs. −2.77[.20]). Patients from both groups presented similar proportions of nonserious and serious AEs. No deaths were reported.
ConclusionChanges from baseline were not statistically significantly different between tofacitinib and bDMARDs in terms of RAPID3 scores and secondary outcomes. Patients from both groups presented similar proportions of nonserious and serious AEs.
Clinical trial numberNCT03073109.
Describir la eficacia, la seguridad y los desenlaces reportados por los pacientes (PRO) en pacientes con artritis reumatoide (RA) con una respuesta inadecuada a los fármacos antirreumáticos modificadores de la enfermedad sintéticos convencionales (csFARME) tratados con tofacitinib o FARME biológico (bFARME) en condiciones de la vida real.
MétodosEstudio no intervencional realizado entre marzo de 2017 y septiembre de 2019 en 13 centros de Colombia y Perú. Los desenlaces evaluados al inicio y a los seis meses de seguimiento fueron la actividad de la enfermedad (puntaje Routine Assessment of Patients Index Data [RAPID3]), el estado funcional (puntaje Health Assessment Questionnaire [HAQ-DI]) y la calidad de vida (EuroQol Questionnaire [EQ-5D-3L]). El puntaje de actividad de la enfermedad-28 (DAS28-ESR) y la frecuencia de eventos adversos (EA). Se estimaron las diferencias no ajustadas y ajustadas con respecto a los valores basales y se expresaron como diferencia de medias por mínimos cuadrados (LMD).
ResultadosSe recolectó información de 100 pacientes tratados con tofacitinib y 70 pacientes con bFARME. Al inicio del estudio, la edad media de los pacientes era de 53,53 años (DE 13,77) y la duración media de la enfermedad de 6,31 años (DE 7,01). El cambio con respecto al valor basal en el mes 6 no fue estadísticamente significativo en la LMD ajustada (SE) para tofacitinib vs. los bFARME para RAPID3 (−2,55 [0,30] vs. −2,52 [0,26]), puntuación HAQ-DI (−0,56 [0,07] vs. −0,50 [0,08]), puntuación EQ-5D-3L (0,39 [0,04] vs. 0,37 [0,04]) y DAS28-ESR (−2,37 [0,22] vs. −2,77 [0,20]). Los pacientes de ambos grupos presentaron proporciones similares de EA no graves y graves. Ninguna muerte fue reportada.
ConclusionesLos cambios desde el inicio no fueron estadísticamente significativos entre tofacitinib y los bFARME en RAPID3 y en los desenlaces secundarios. Los pacientes de ambos grupos presentaron proporciones similares de EA no graves y graves.
Número de ensayo clínicoNCT03073109.
Rheumatoid arthritis (RA) is an autoimmune, chronic, systemic disorder that affects approximately 1% of the world's adult population.1 RA is characterized by synovial membrane swelling and causes joint swelling, stiffness and pain, which lead to cartilage and bone tissue progressive erosion and destruction at the affected joints. Between 17.85% and 40.90% of patients with RA may experience extra-articular manifestations that may involve skin, eye, respiratory, oral, cardiovascular, neurological, hematological or vascular function.2–5 Patients with RA are also likely to experience depression, sexual dysfunction, and social relationship disruption.6,7
Furthermore, RA represents an important burden for informal caregivers (spouse, relatives) who spend much time helping patients with their daily activities, personal care, social activities and financial matters.8,9 This caregiver overload contributes substantially to a loss of productivity and the total disease burden.
For patients, RA remains an incurable disease; thus, the treatment goals are to relieve disease signs and symptoms, control disease activity, improve physical function and patient quality of life and inhibit structural damage progression in the disease course.10–12
There are effective therapies for RA. Commonly, patients initiate nonsteroidal anti-inflammatory drugs (NSAIDs) or low-dose glucocorticoids and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) as soon as possible after diagnosis. Biological disease-modifying anti-rheumatic drugs (bDMARDs) and targeted synthetic disease-modifying anti-rheumatic drugs (tsDMARDs), such as tofacitinib, are drugs used for preventing or reducing the swelling caused by RA after the failure of csDMARDs.2–5
Currently, tofacitinib 5mg twice daily (BID) (approved dosage for treatment of RA) has demonstrated consistent efficacy in reducing the signs and symptoms of RA and has led to improvements in patient-reported outcomes (PROs), with manageable safety profiles across six phase III studies and one phase IV study in different stages of arthritis, either as monotherapy or in combination with csDMARDs.13–18 Tofacitinib has demonstrated a consistent safety profile in open-label long-term extension clinical studies.19
There is limited information on the use of tofacitinib in current practice in Latin America,20 including studies comparing tofacitinib with bDMARDs directly in a real-world Latin American setting. Due to the great importance of the studies that are focusing on comparisons of broad types of treatments in the management of RA patients in current clinical practice, we aimed to describe the related physical activity, disease activity, quality of life and safety in Latin American patients with RA treated with tofacitinib or bDMARDs after the failure of csDMARDs in real-life conditions.
MethodsSetting and populationA noninterventional, hybrid study (retrospective and prospective study) with two arms comparing tofacitinib to bDMARDs treatments in patients with RA after the failure of csDMARDs was performed from March 2017 through September 2019. The retrospective period corresponds to the time from when the physician prescribed the studied drug until the patient decided to participate in the study at maximum of three weeks, while the prospective period is the period from recruitment until six months of follow-up. This study was conducted in 13 sites from Colombia (10 sites) and Peru (3 sites). Patients were followed up for 6 months and were in primary care. Changes to the treatment and use of concomitant medications during the follow-up were within current practice guidelines and were decided upon by their rheumatologist under real-world conditions. The protocol was approved by the Independent Ethics Committee at each center. All the patients provided written informed consent. Clinical trial number: NCT03073109.
The patients were ≥18 years of age, had been diagnosed with moderate to severe RA for more than 6 months before enrollment, had a Disease Activity Score 28-joint based on erythrocyte sedimentation rate (DAS28-ESR) score ≥3.2, with inadequate response to the continuous use of methotrexate or combination of csDMARDs for at least 12 weeks before the study entry, with no previous bDMARDs use, and had been prescribed tofacitinib or bDMARDs in the last three weeks at doses established by the American College of Rheumatology (ACR) guidelines published in 2015 and following medical criteria. The definition of RA diagnosis was based on the criteria used in medical practice for each rheumatologist.
The exclusion criteria were patients who were not able to answer the questionnaires; patients diagnosed with autoimmune rheumatic diseases other than RA and Sjogren's syndrome; patients treated with bDMARDs as monotherapy; patients who had participated in other studies; pregnant or breastfeeding women; and patients with any current malignancy or history of malignancy with the exception of adequately treated or excised nonmetastatic basal cell or squamous cell cancer of the skin or cervical carcinoma in situ, lymphoproliferative disorders, a history of lymphoma, leukemia, or signs and symptoms suggestive of current lymphatic disease.
Sampling strategySample size was estimated based on the primary objective to compare the disease activity between both groups as measured by the Routine Assessment of Patient Index Data 3 (RAPID3). Taking into account the results obtained in prior studies with bDMARDs, a difference of 2 points in the RAPID3, with a standard deviation (SD) of 6 in both arms, a confidence level of 95% and a statistical power of 80%, approximately 142 patients were required in each arm. With a correction factor of 15% considering the proportion of patients who could drop out during the study, the minimum number of patients required was approximately 160 per arm. Sampling was conducted at the main sites using tofacitinib in the participating countries.
Convenience sampling was conducted due to the limited access to patients with tofacitinib. For patients using a biological DMARD, data was collected randomly with replacement; for this purpose, at the beginning of the week patients from each site that had started treatment with biological DMARD the previous week was listed consecutively following the order of visit and was selected through random numbers generated by computer. The candidate patients were contacted by phone and those who do not want to participate was replaced by the next patient until completing the required number of patients.
Outcome measuresAll the outcomes defined in the study were measured through PROs at month 0, it was the time when the patient signed the consent inform where the treatment was already prescribed, and at month 6 through questionnaires completed by the patients. The scales used had been previously validated in the Spanish language and registered the information reported in the medical records. The primary assessed outcome was disease activity, which was measured through the RAPID3. The other involved outcomes were functional status, which was measured through the adapted Health Assessment Questionnaire – Disability Index (adapted HAQ-DI) and quality of life, which was measured with the EuroQoL 5-Dimension 3-Level (EQ-5D-3L) Latin-American language. The only outcome measure that was extracted from the medical records was the Disease Activity Score 28-joint count assessment-erythrocyte sedimentation rate (DAS28-ESR) because it is routinely used in clinical practice.
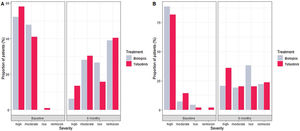
High severity disease activity using DAS28-ESR was defined as score higher that 5.2, 5.1–3.2 as moderate severity, 3.1–2.6 as low severity, and lower than 2.6 as in remission. With RAPID3, patients who scores between 10 and 4.3 was defined high severity, 2.3 and 4.0 as moderate, 2.0 and 1.3 as low severity, and 0 and 1.0 near remission.
Adverse event (AE) frequency and severity occurring due to the prescription of therapies under evaluation were assessed after 6 months of follow-up. AE frequency and severity were measured from the information on the medical record or were spontaneously reported by the patients during the medical visit.
Other data related to the patient's demographic and clinical characteristics, treatment details such as previous (glucocorticoid, methotrexate or leflunomide) and concomitant treatments, and barriers in the health care system were abstracted from the medical records to describe the population and other possible potential confounders.
Health insurance corresponded to the type of insurance in which the patient has access to the treatment. It can be through public or private insurance, complementary which is additional insurance to the previously mentioned, or bought by the patient. Access barrier was defined as any constraints caused by administrative issues with the health care insurance or supplier reported by the patient during the follow up. Interruption was considered if the patient loses one day or more with treatment. The time to supply was measured only for the first prescription dosage delivery from the Health Maintenance Organization (HMO) or supplier to the patient, and it was expressed as the number of days required for the delivery of treatment from the time of prescription.
Statistical analysisMatched analysis was planned through propensity score matching; however, the achieved number of recruited patients did not allow propensity score matching. Descriptive statistics were produced for all variables. These included estimates of the mean, standard deviation (SD), 95% confidence intervals of the mean, median, interquartile ranges and frequency distributions for continuous-scale variables and frequency distributions for categorical-scale variables.
DAS28-ESR was used to measure disease activity estimated by the rheumatologist. Several PROs were used to measure functional status (adapted HAQ-DI), quality of life (EQ-5D-3L) and disease activity (RAPID3) were analyzed by directly estimating the difference in means using least square means (LSM) between patients treated with tofacitinib and patients treated with bDMARDs and between periods. A bivariate and multivariable analysis was performed to identify the association between the baseline variables and the disease activity. Linear regression was conducted for the multivariable analysis of all PROs. The adjusted full model was composed by all potential confounding variables such as demographic and clinical characteristics, concomitant treatment, previous treatment and access variables (e.g. age, gender, country of origin, previous treatments, neutrophils, access barriers, insurance, baseline clinical data such as DAS28). The reduced model was developed from the results of multivariable analysis selecting the variables with p value less than 0.05. The detail results of multivariable analysis for mean changes of PROs comparing treatments were reported in the Supplementary material Tables S1–S4. The comparison of score for each group of treatments at the baseline and month 6 was conducted with a t paired in bivariate analysis and mixed-effect regression for multivariable analysis. The statistical criterion to select the final model was looking for the minor variance comparing with simple model. Multiple imputation was used to manage the missing data from the different variables using the package MICE in R software (version 4.0.5).
ResultsCharacteristics of the participants and study completionDuring the study period, 170 patients were enrolled in the study, including 100 patients treated with tofacitinib and 70 treated with bDMARDs (etanercept (39.7%), rituximab (16.2%), tocilizumab (16.2%), infliximab (13.2%), golimumab (8.8%), abatacept (2.9%), adalimumab (1.1%), and certolizumab (1.1%)). Eighty-six patients were from Colombia, and 83 patients were from Peru. At the 6-month follow-up, 92.9% (158) of enrolled patients completed the study, including 90 in the tofacitinib group and 68 in the bDMARDs group. Nine patients withdrew due to a loss to follow-up (tofacitinib (n=8) and bDMARDs (n=1)) for administrative reasons, however it was not possible to stablish if patients continued with the treatment; 2 patients treated with tofacitinib discontinued for other reasons. Only 1 patient from the bDMARDs group discontinued the study due to AEs. Although the mean time to supply at the start of the treatment was similar in both groups, more dispersion was observed in patients who received tofacitinib.
The mean age was 53.53 years (SD 13.77); 88% were women, and 97% were living in urban areas. The mean disease duration since diagnosis was 6.31 (SD 7.02) years. Methotrexate (58.82%), leflunomide (19.41%), or any chloroquine (11.76%) were the most frequent csDMARDs previously used. Corticosteroids were used previously in 82.94% of patients.
When comparing baseline data, the main differences were found in insurance characteristics and previous treatments. Patients had mainly private health insurance (n=91), followed by public health insurance (n=53). More patients with tofacitinib had coverage through private health insurance (67%), while the majority of the bDMARDs group had coverage through public health insurance (59%). The group that received tofacitinib reported more access barriers than patients with bDMARDs (29% vs 14%). Another observed difference in both studied groups was in the proportions of patients with concomitant treatment with leflunomide, previous use of methotrexate, and previous use of prednisolone. Although the mean time to supply to start the treatment was similar in both groups, more dispersion was reported in patients who received tofacitinib (Table 1).
Demographic and clinical characteristics of patients by groups.
| bDMARDs group | Tofacitinib group | p-Value | |
|---|---|---|---|
| Number of subjects | 70 | 100 | |
| Age mean (SD) | 51 (13) | 55 (14) | 0.072 |
| Female – no. of patients | 65 (93%) | 85 (85%) | 0.11 |
| Country – no. of patients | |||
| Peru | 45 (64%) | 39 (39%) | 0.002 |
| Colombia | 25 (36%) | 61 (61%) | |
| Area – no. of patients | |||
| Rural | 1 (1.4%) | 5(5%) | 0.41 |
| Urban | 69 (99%) | 95 (95%) | |
| Access information | |||
| Health insurance – no. of patients | |||
| Complementary | 1 (1.4%) | 2 (2%) | <0.001 |
| Patient | 1 (1.4%) | 10 (10%) | |
| Private | 24 (34%) | 67 (67%) | |
| Public | 41 (59%) | 12 (12%) | |
| No report | 3 (4.3%) | 9 (9%) | |
| Access barriers – no. of patients | 10 (14%) | 29 (29%) | 0.024 |
| No report | 3 (4.3%) | 9 (9%) | |
| Time to supply (days) mean (SD) | 24 (31) | 22 (45) | 0.0017 |
| Disease duration (year) mean (SD) | 6 (6.8) | 6.5 (7.2) | 0.81 |
| Time previous treatment (months) mean (SD) | 34 (37) | 26 (31) | 0.091 |
| Concomitant therapy – no. of patients (%) | |||
| Leflunomide – no. of patients | 20 (29%) | 15 (15%) | 0.05 |
| Methotrexate – no. of patients | 30 (56%) | 52 (52%) | 0.75 |
| Aminoquinolines – no. of patients | 7 (10%) | 16 (16%) | 0.37 |
| Corticosteroids – no. of patients | 60 (86%) | 77 (77%) | 0.22 |
| Clinical characteristics | |||
| Lymphocytes/mm3mean (SD) | 2,200 (865) | 2,600 (1,800) | 0.71 |
| Neutrophils/mm3mean (SD) | 4,400 (2,500) | 4,600 (2,200) | 0.58 |
| Swollen joins mean (SD) | 6.9 (4.2) | 8.7 (7.2) | 0.18 |
| Tender joints mean (SD) | 10 (4.4) | 11 (7.3) | 0.57 |
| Comorbidities | 1.4% (1) | 7 (7%) | 0.18 |
| DAS28-ESR mean (SD) | 5.9 (4.5) | 5.2 (1) | 0.28 |
| Previous treatment – no. of patients (%) | |||
| Deflazacort – no. of patients | 9 (13%) | 22 (22%) | 0.19 |
| Leflunomide – no. of patients | 11 (16%) | 13 (13%) | 0.78 |
| Methotrexate | 18 (26%) | 48 (48%) | 0.0055 |
| Prednisolone – no. of patients | 40 (57%) | 37 (37%) | 0.015 |
| Folic acid – no. of patients | 0 (0%) | 1 (1%) | 1 |
| Chloroquine – no. of patients | 4 (5.7%) | 8 (8%) | 0.79 |
| Hydroxychloroquine – no. of patients | 2 (2.9%) | 2 (3%) | 1 |
| Sulfasalazine | 2 (2.9%) | 0 (0%) | 0.33 |
| Methylprednisolone | 1 (1.4%) | 1 (1%) | 1 |
bDMARDs: biological disease-modifying anti-rheumatic drugs; DAS28-ESR: Disease Activity Score 28-joint count assessment-erythrocyte sedimentation rate; SD: standard deviation.
The patients had severe disease activity according to the DAS28-ESR reported at baseline, 5.9 (SD 4.5) for bDMARDs and 5.2 (SD 1) for tofacitinib. Seven percent of patients prescribed tofacitinib had any comorbidity, whereas this value was 1.4% for the bDMARDs group. Lymphocyte counts, neutrophil counts, and the numbers of swollen joints and tender joints were similar between the study groups.
Disease activityThe disease activity was evaluated with DAS28-ESR. DAS28-ESR data were available for all patients at baseline; however, at the second visit, DAS28-ESR data were reported in only 86 patients treated with tofacitinib and 61 treated with bDMARDs.
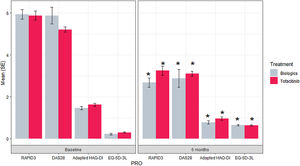
The reduction of DAS28-ESR for both groups was statistically significant comparing the score at baseline and month 6 (p value<0.001) (Fig. 1). During the 6 months, the difference of score in DAS28-ESR in both visits were similar between treatments both bivariable and multivariate analyses (p value 0.099 and 0.728, respectively) (Supplementary material Table 5S). For low disease activity, 11% more bDMARDs patients than tofacitinib patients reported low disease activity (Fig. 2). Comparing the score of DAS28-ESR between baseline and month 6 visit, a reduction of the activity was found in both treatment groups with consistency of the results with bivariate and multivariable analysis (Supplementary material Table 6S).
Results of the PROs at baseline and 6 months for each treatment. *p value <0.001 for the difference between 6-month outcome value and baseline outcome value. bDMARDs: biological disease-modifying anti-rheumatic drugs; DAS28-ESR: Disease Activity Score 28-joint count assessment-erythrocyte sedimentation rate; EQ-5D-3L: EuroQoL 5-Dimension 3-Level; HAQ-DI: Health Assessment Questionnaire – Disability Index; PRO: patient-reported outcomes; RAPID3: Routine Assessment of Patient Index Data 3; SE: standard error.
An exploratory analysis, the difference between treatments in the change in the baseline and month 6 was statistically no significant (p value 0.695).
PRO resultsThe measurement of outcomes (RAPID3, adapted HAQ-DI, EQ5D) was conducted at baseline in 100 patients in the tofacitinib group and in 70 patients in the bDMARDs group. Ninety and sixty-eighty patients in the tofacitinib and bDMARDs groups, respectively, completed the study and had RAPID3, adapted HAQ-DI and EQ5D scores recorded at the last visit.
Reduction of RAPID3 scores from baseline to month 6 was observed in both studied treatments with statistical difference between visits (p value>0.001) (Fig. 1 and Table 2). At the end of follow-up, a similar proportion of patients treated with tofacitinib and bDMARDs achieved remission, while 18% more patients from the bDMARDs group than the tofacitinib group achieved low disease activity (Fig. 2). The RAPID score obtained at month 6 compared to baseline for both groups (bDMARD and tofacitinib) were minor with difference statistically significant being steady results in the bivariate and multivariable analysis (Supplementary material Table 7S).
PRO mean changes from baseline to 6 months for both treatments.
| bDMARDs | Tofacitinib | |
|---|---|---|
| Baseline | ||
| RAPID3 | 5.95 (5.34–6.56) | 7.12 (6.35–7.89) |
| Adapted HAQ-DI | 1.50 (1.31–1.70) | 1.64 (1.39–1.89) |
| EQ-5D-3L | 0.23 (0.16–0.30) | 0.19 (0.09–0.29) |
| DAS28-ESR | 5.21 (4.99–5.44) | 5.59 (5.15–6.04) |
| 6 months | ||
| RAPID3 | 2.46 (1.84–3.08) | 3.73 (2.97–4.50) |
| Adapted HAQ-DI | 0.77 (0.57–0.96) | 0.93 (0.68–1.18) |
| EQ-5D-3L | 0.67 (0.60–0.74) | 0.54 (0.45–0.64) |
| DAS28-ESR | 2.61 (2.37–2.84) | 3.02 (2.54–3.49) |
| Changes from baseline to 6 month* | ||
| RAPID3 | −3.49 (−4.07–(−2.92)) | −3.38 (−4.02–(−2.74)) |
| Adapted HAQ-DI | −0.74 (−0.91–(−0.57)) | −0.71 (−0.92–(−0.50)) |
| EQ-5D-3L | 0.44 (0.34–0.54) | 0.35 (0.25–0.45) |
| DAS28-ESR | −2.61 (−2.92–(−2.30)) | −2.54 (− 2.92–(−2.24)) |
Values are the mean (interval confidence). bDMARDs: biological disease-modifying anti-rheumatic drugs; DAS28-ESR: Disease Activity Score 28-joint count assessment-erythrocyte sedimentation rate; HAQ-DI: Health Assessment Questionnaire – Disability Index; EQ-5D-3L: EuroQoL 5-Dimension 3-Level; PRO: patient-reported outcomes; RAPID3: Routine Assessment of Patient Index Data 3.
The reduction in RAPID3 score during the follow-up between the bDMARDs and tofacitinib groups was not statistically significant different (p value 0.154). The multivariable analysis composed by significant variables has the same tendency remained.
Pain and health status are other dimensions included in the RAPID3. Patients who received tofacitinib reported a mean reduction in pain of 3.94±0.89, while bDMARDs patients reported a mean reduction of 3.28±0.77. In the health status dimension, the tofacitinib group presented an improvement of 3.79±0.47, whereas the bDMARDs group presented an improvement of 2.78±0.47. Differences in both groups for these outcomes were not found (p value 0.577 and 0.104).
Regarding other secondary PROs, patients who received tofacitinib or bDMARDs reported an improvement in their functional status and quality of life (Table 2). There were not statistically significant difference between groups in the mean change during 6 months (p value: 0.256 and 0.986, respectively).
The results of the bivariate analysis, multivariable analysis with all variables, and reduced model comparing the score for all PROs by treatment and by visits were consistent (Supplementary material Tables 7S–12S).
SafetyTwenty patients treated with tofacitinib and sixteen treated with bDMARDs reported any AEs. The most frequent events (over 2%) in patients treated with bDMARDs were diarrhea, pharyngitis, falls, headache, urinary tract infection, and nasopharyngitis, while those for tofacitinib group were headache, influenza, and pharyngotonsillitis. Herpes simplex and herpes zoster were reported in 2 patients treated with tofacitinib (Table 3). Cardiovascular, malignancies or thromboembolism AEs were not reported. Two serious AEs were reported by one patient treated with tofacitinib (appendicitis and peritonitis that were reported as not related to the treatment).
Adverse events reported by groups.
| Tofacitinib group | bDMARDs group | |||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Patients evaluable for adverse events | 100 | 70 | ||
| Number of subjects with serious adverse events | 1 | (1.00) | 0 | (0.00) |
| Number of subjects with non-serious adverse events | 20 | (20.00) | 16 | (22.86) |
| Number of non-serious adverse events | 30 | (30.00) | 20 | (28.57) |
| Number (%) of subjects with one (1) adverse event | 12 | (60.00) | 12 | (75.14) |
| Number (%) of subjects with two (2) adverse events: | 6 | (30.00) | 4 | (5.71) |
| Number of subjects with three (3) adverse events | 2 | (10.00) | 0 | (0.00) |
| Non serious adverse events according to System Organ Classes (SOC) | ||||
| Blood and lymphatic system disorders | 2 | (2.00) | 1 | (1.43) |
| Bicytopenia | 1 | (1.00) | 0 | (0.00) |
| Neutropenia | 0 | (0.00) | 1 | (1.43) |
| Purpura | 1 | (1.00) | 0 | (0.00) |
| Endocrine disorders | 1 | (1.00) | 0 | (0.00) |
| Oligomenorrhea | 1 | (1.00) | 0 | (0.00) |
| Eye disorders | 2 | (2.00) | 0 | (0.00) |
| Photophobia | 1 | (1.00) | 0 | (0.00) |
| Vision blurred | 1 | (1.00) | 0 | (0.00) |
| Gastrointestinal disorders | 1 | (1.00) | 4 | (5.71) |
| Abdominal pain | 0 | (0.00) | 1 | (1.43) |
| Diarrhea | 1 | (1.00) | 3 | (4.29) |
| General disorders and administration site conditions | 1 | (1.00) | 0 | (0.00) |
| Malaise | 1 | (1.00) | 0 | (0.00) |
| Infections and infestations | 2 | (2.00) | 0 | (0.00) |
| Herpes simplex | 1 | (1.00) | 0 | (0.00) |
| Herpes zoster | 1 | (1.00) | 0 | (0.00) |
| Injury, poisoning and procedural complications | 0 | (0.00) | 2 | (2.86) |
| Fall | 0 | (0.00) | 2 | (2.86) |
| Musculoskeletal and connective tissue disorders | 3 | (3.00) | 2 | (2.86) |
| Arthralgia | 1 | (1.00) | 0 | (0.00) |
| Coccydynia | 0 | (0.00) | 1 | (1.43) |
| Lumbar vertebral fracture | 1 | (1.00) | 0 | (0.00) |
| Musculoskeletal pain | 0 | (0.00) | 1 | (1.43) |
| Myalgia | 1 | (1.00) | 0 | (0.00) |
| Nervous system disorders | 4 | (4.00) | 2 | (2.86) |
| Headache | 3 | (3.00) | 2 | (2.86) |
| Spinal pain | 1 | (1.00) | 0 | (0.00) |
| Renal and urinary disorders | 3 | (3.00) | 2 | (2.86) |
| Cystitis | 1 | (1.00) | 0 | (0.00) |
| Pollakiuria | 1 | (1.00) | 0 | (0.00) |
| Urinary tract infection | 1 | (1.00) | 2 | (2.86) |
| Reproductive system and breast disorders | 1 | (1.00) | 0 | (0.00) |
| Vulvovaginitis | 1 | (1.00) | 0 | (0.00) |
| Respiratory, thoracic and mediastinal disorders | 8 | (8.00) | 7 | (10.0) |
| Cough | 1 | (1.00) | 0 | (0.00) |
| Influenza | 3 | (3.00) | 1 | (1.43) |
| Nasopharyngitis | 0 | (0.00) | 2 | (2.86) |
| Pharyngitis | 1 | (1.00) | 3 | (4.29) |
| Pharyngitis bacterial | 1 | (1.00) | 0 | (0.00) |
| Pharyngotonsillitis | 2 | (2.00) | 0 | (0.00) |
| Rhinorrhea | 0 | (0.00) | 1 | (1.43) |
| Skin and subcutaneous tissue disorders | 2 | (2.00) | 0 | (0.00) |
| Alopecia | 1 | (1.00) | 0 | (0.00) |
| Ecchymosis | 1 | (1.00) | 0 | (0.00) |
This is the first study that evaluated PROs in RA patients from two Latin American countries and provided information related to the effectiveness of tofacitinib or biologic DMARDs. The results suggest similar baseline changes at month 6 in the measured PROs for tofacitinib or bDMARDs, each given in combination with csDMARDs, in RA patients with moderate to severe diseases who were nonresponders to csDMARDs. Both treatments achieved comparable changes in the score for the primary outcome, RAPID3 score. Likewise, the magnitude of the response in the other PROs and outcome measures were similar for both treatment groups, including disease activity, functional status, and quality of life. However, these results should be interpreted with caution given the study design and sample size. The rates of AE were balanced for both tofacitinib and bDMARDs.
Difference in the number of patients treated with bDMARDs or tofacitinib in Colombia and Peru was observed mainly by reimbursement system available in each country which could influence in the decision to use one or another medication. Fewer patients with bDMARDs were presented due to the number expected patients being overestimated and the type of sampling, in which from 3 patients with bDMARDs one was selected, was used looking for more representability of patients under this type of treatment and avoiding any selection bias. Additionally, the probability of being recruited in the study was independent to the treatment given that the invitation to participate happens after the patients were prescribed but more of them have not received the treatment by the insurance company. The average of received treatment after the prescription was 24 and 22 days for tofacitinib and bDMARDs, respectively. Based on the information of recruitment, only two patients in bDMARDs decided not to participate while all the patients with tofacitinib accepted. Therefore, the risk of not participate in the study by adverse event inefficient related to the treatment, or type treatment was low.
The results are consistent with those observed in a clinical trial that compared tofacitinib monotherapy versus tofacitinib or adalimumab combined with methotrexate in patients with rheumatoid arthritis with inadequate response to methotrexate. Tofacitinib has not been approved to be used as monotherapy in the country participants during the study. The HAQ-DI score and pain as PROs were assessed. The results of changes from baseline at 6 months showed comparable results for tofacitinib and adalimumab in combination with methotrexate for both self-report questionnaires.21
Although the RAPID3 is not commonly used in clinical trials, it has been widely studied. One study found an association between this PRO and quality of life, explaining 81% of the variability in the RAPID3.22 This finding is consistent with the results of this study, where the changes from baseline were similar between the RAPID3 and the EQ5D-3L.
Studies have reported a correlation between the RAPID3 and the DAS28-ESR of 0.62 and 0.71, which is considered moderate.23,24 In contrast to the DAS28-ESR, the RAPID3 includes pain which is important for the patient, as well as quality of life and the presence of new erosion via imaging.25 However, the DAS28-ESR remains the main established composite measure of disease activity to measure disease activity in clinical trials. The results in this study showed similar changes from baseline to month 6 for RAPID3 scores and DAS28-ESR in both studied groups, but in the classification of the diseases, changes in disease activity fluctuated, with a higher percentage of patients in remission or with low disease activity according to the DAS28-ESR.
Despite our study not showing a difference between tofacitinib and biologics and there were no cases of MACE, malignancies or thrombosis reported, a recently completed Phase 3b/4 randomized, open-label safety study found that the non-inferiority criterion was not met comparing the tofacitinib doses combined (5mg BID and 10mg BID) vs TNFi for the co-primary endpoints of adjudicated MACE and malignancies excluding NMSC.26 Also, previously reported data from an ad hoc safety analysis of the same study showed an increased risk of venous thromboembolic events for tofacitinib relative to TNFi.27
Tofacitinib is associated with an increased risk of herpes zoster compared with bDMARDs.27 This study reported one case of simple herpes zoster and another of herpes simplex in patients treated with tofacitinib, with an incidence similar to that in an extended study of 9.5 years of use of tofacitinib, which reported an incidence of 2.7 per 100 patients per year.28
The study has some limitations. The target sample size was not met, affecting the power of the study and limiting the conclusions of the study. Patients selected for enrollment in the study represented a “convenience sample”, ensuring that the records were obtained from physicians willing to be involved in the study. Thus, the results of the study may not be applicable to the general RA population or to physicians treating RA in the countries included, and the characteristics of the patients who agreed to participate in the study could be different from those of the population of the study. There was heterogeneity among patients, which was controlled by multivariable analysis; however, this was limited to observable covariables. There may be other variables which were not measured that potentially could affect the association between treatment and the clinical outcomes, known limitations of the observational studies. The estimation of EQ-5D score was calculated with the health states of Chilean population due to not available of other reported in countries included in the study.29 Eight patients with tofacitinib and one from bDMARDs were lost to follow-up for administrative causes, however, it was not possible to know if they continued with the treatment remaining the potential risk of bias that this lost were associated to the treatment. Finally, the report of adverse events and their severity can vary between the centers depending on the interpretation and accuracy of assessments the rheumatologist.
The results of this study can be generalized to other Latin American countries with health care systems and access barriers to the treatment similar to those of the countries of this study. Additionally, the results can be applied only for patients with RA with a failure of csDMARDs or with treatment in combination with any csDMARDs. In Peru and Colombia tofacitinib and bDMARDs are reimbursed by the government, in the first one as part of the national formulary to treatment of moderate to severe RA in patients who failed a methotrexate-based therapy and in Colombia only tofacitinib was not included in the national formulary at the time this study was conducted.
The study has also strengths. There were specific criteria in the selection of sites ensuring the quality of data in the medical records. Additionally, despite the limitations of studies in real-world settings, this study used validated composite measures of disease activity and PROs without any influence from physicians due to the design of outcome measures and PROs, as per current clinical practice. Although the sample size achieved was lower than planned, most of the patients came from several sites in Colombia and Peru that were more representative of the different management of RA. Last, there was not high heterogeneity between study groups in the measured variables, increasing the validity of the results.
In conclusion, in patients diagnosed with RA and inadequate response to csDMARDs, tofacitinib and bDMARDs combined with csDMARDs demonstrated similar clinical results in outcome measures and PROs, as well as frequencies of AEs. Further studies are necessary to confirm these results.
Ethical approvalThe protocol and that informed consent has been obtained from the subjects were approved by the Independent Ethics Committee at site: Centro Integral de Reumatología del Caribe Circaribe, SERVIMED, Centro de Investigaciones en Reumatología y Especialidades Médicas SAS, Reumalab, Fundación Valle de Lili, Clínica Jockey Salud, Clínica San Judas Tadeo, Clínicos IPS, Centro Medico CEEN, Fundación Instituto de Reumatología Fernando Chalem, Clínica de Occidente, Artmedica and IDEARG. The study was conducted in accordance with the Declaration of Helsinki and the local regulation of the participant countries.
Authors’ contributionsAll authors contributed to the study conception and design. Material preparation was performed by JMR, MG, DPL and TL. The data collection was conducted by the different site participants. The analysis was executed by JMR and MG, and reviewed by all the authors. The first draft of the manuscript was written by JMR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
FundingThis work was funded by Pfizer Inc.
Competing interestsReyes JM, Gutierrez MV, Ponce de Leon D, Lukic T, and Amador L are employees of Pfizer. Del Castillo D and Izquierdo J have received speakers fees from several pharmaceutical companies. The other authors have no competing interests.
We thank all the following Research Centers for allowing us to conduct the study from 2017 to 2019: Centro Integral de Reumatología del Caribe Circaribe, SERVIMED, Centro de Investigaciones en Reumatología y Especialidades Médicas SAS, Reumalab, Fundación Valle de Lili, Clínica Jockey Salud, Clínica San Judas Tadeo, Clínicos IPS, Centro Medico CEEN, Fundación Instituto de Reumatología Fernando Chalem, Clínica de Occidente, Artmedica and IDEARG and all the investigators who contributed their knowledge, expertise and the integrity of the work as a whole. Likewise, we thank all the patients that accepted to participate in the study.