Inflammatory rheumatic diseases usually affect women of childbearing age treated with biologic drugs. However, there is a lack of literature on the efficacy and toxicity of biologic disease-modifying drugs during pregnancy. The aim of this study was to determine the presence of pregnant patients treated with bDMARDs in a real-world dataset and to examine the impact of pregnancy and lactation on the evolution of rheumatic disease in a registry of Spanish patients.
MethodThis was a multicentre prospective study with a real-world setting. Information was obtained from BIOBADASER registry. Patients included are women who got pregnant until November 2020 from 19 rheumatology units. We conducted proportions, means, and standard deviations (SD) to describe the study population and the use of treatments. T-test and Chi-square test were applied to assess differences between groups.
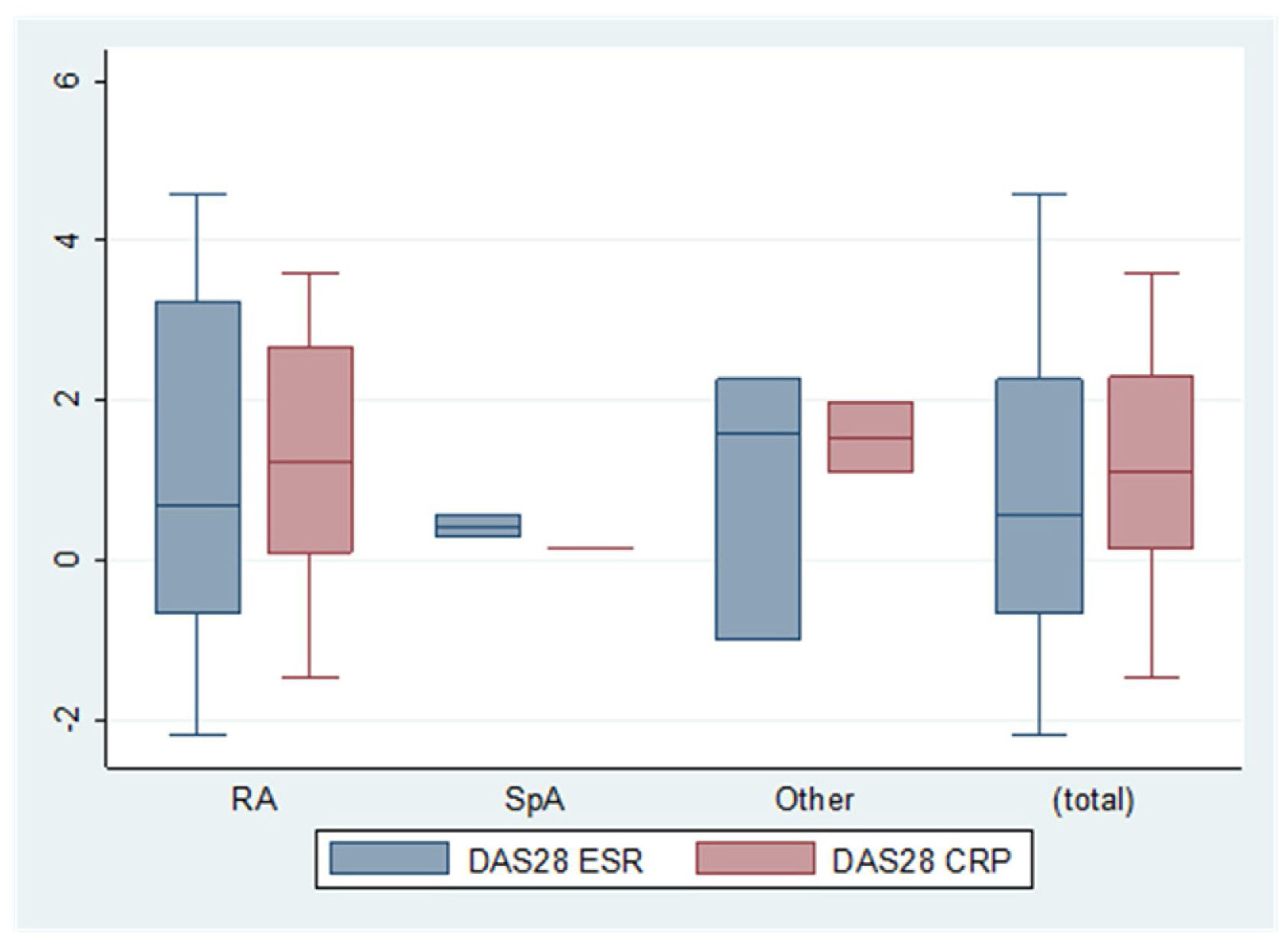
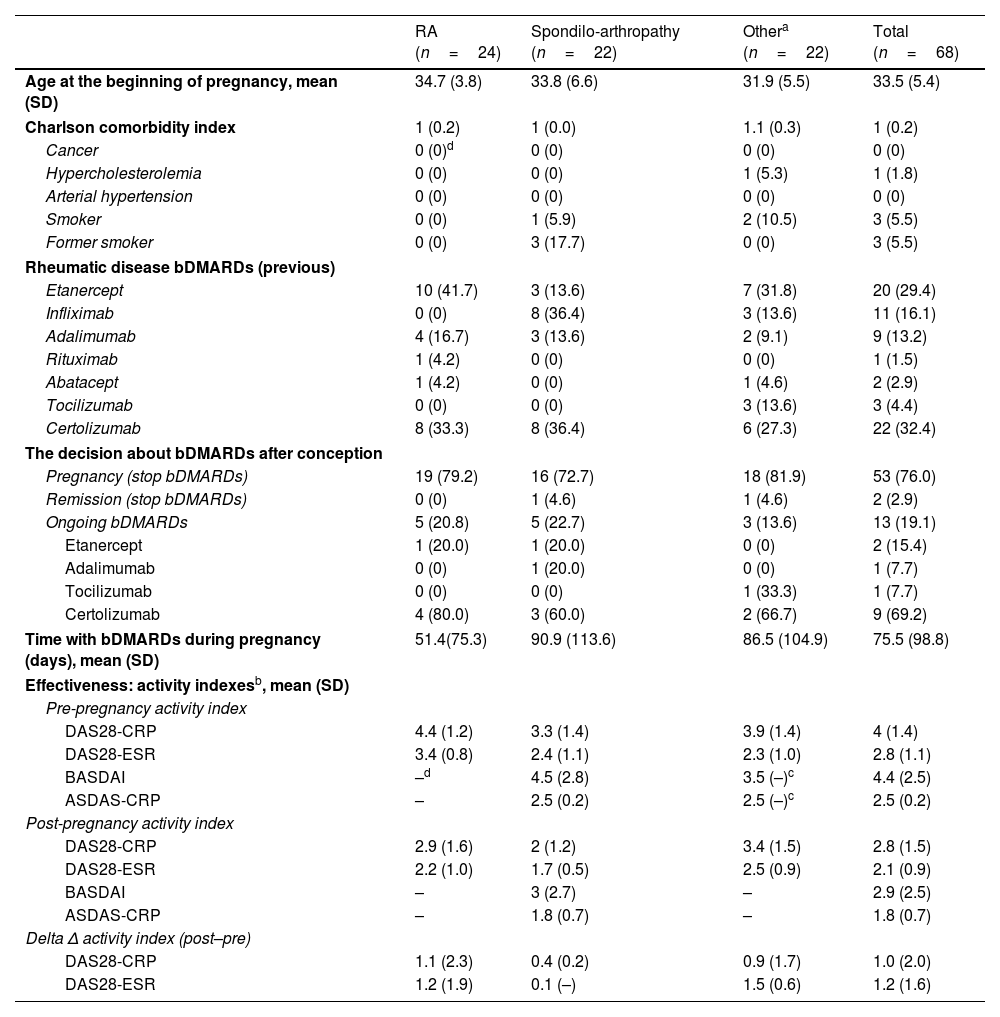
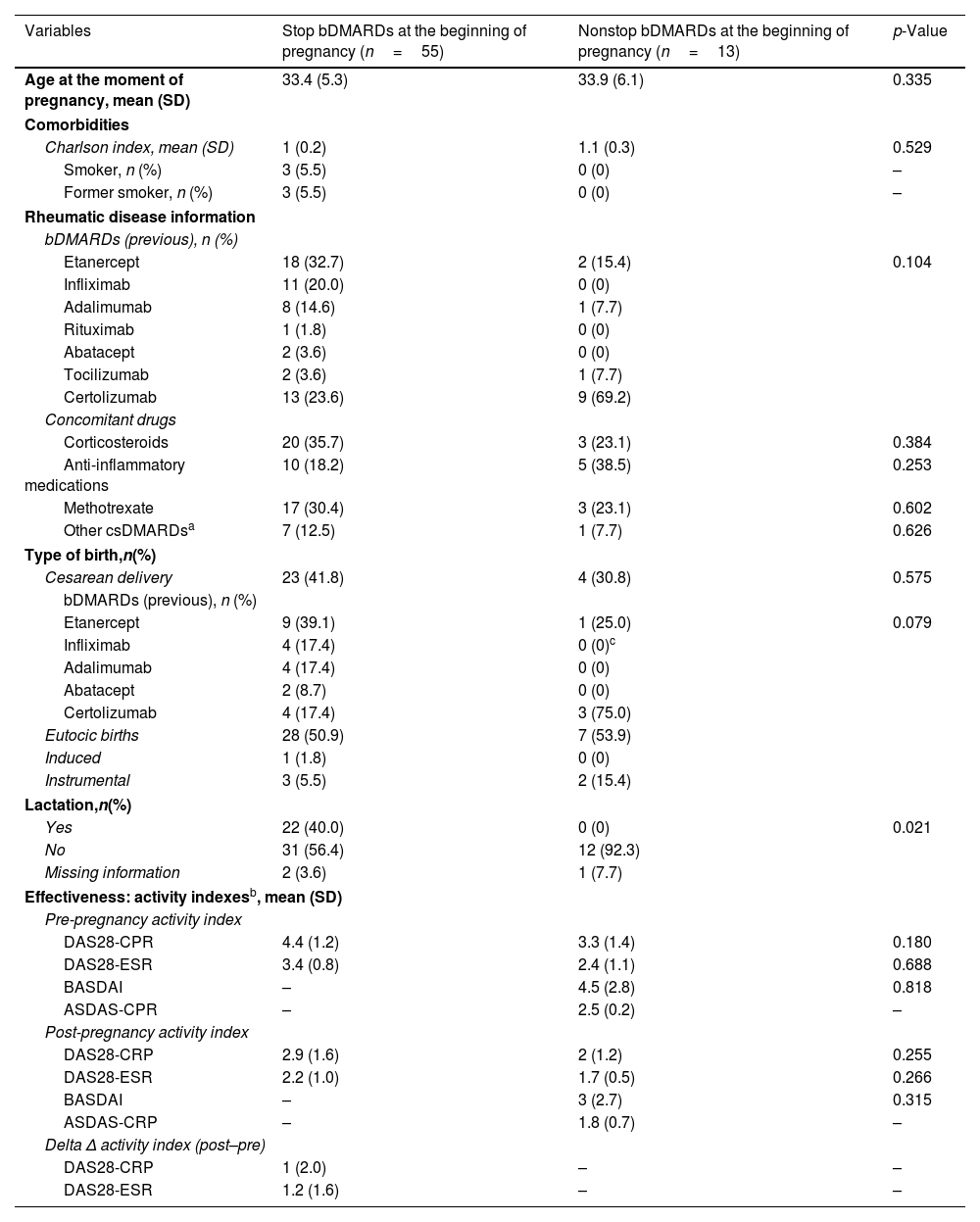
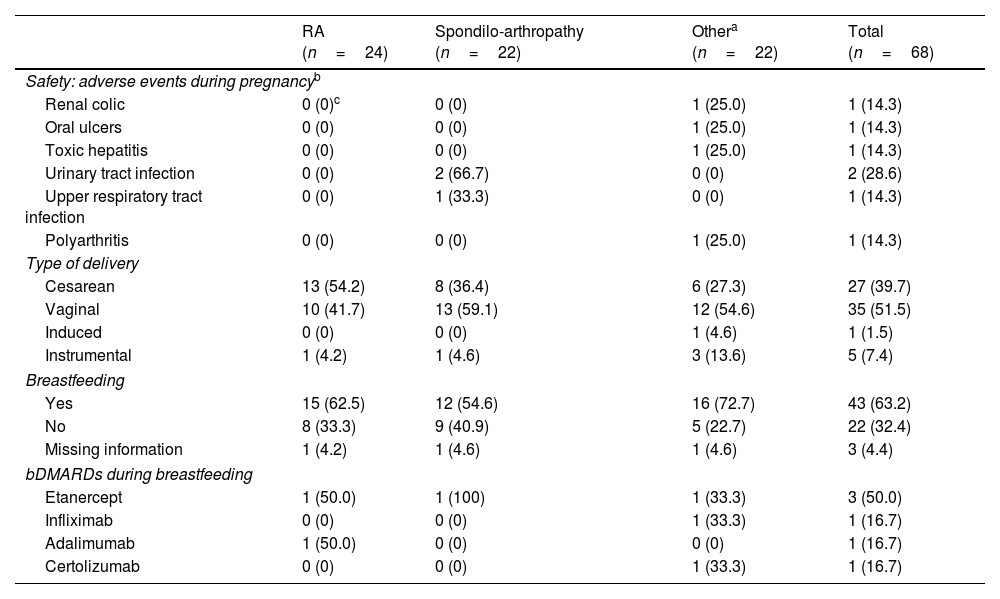
ResultNinety cases of pregnancy were registered (n=68 full-term pregnancies; n=22 spontaneous miscarriages). Most of the cases discontinued bDMARDs during pregnancy (78.9%) but 13 cases continued treatment during pregnancy, mainly using certolizumab pegol. These cases were obtaining better management of rheumatic disease, although the differences were not statistically significant [DAS28-CRP, 2.9 (SD: 1.6) vs. 2.0 (1.2), p=.255; DAS28-ESR, 2.2 (1.0) vs. 1.7 (.5), p=.266]. No serious adverse events were reported during pregnancy and lactation.
ConclusionBeing pregnant is still an uncommon condition in patients with rheumatic diseases and using bDMARDs. Our results show that rheumatic disease tended to progress better during pregnancy in patients who continued to take bDMARDs.
Las enfermedades reumáticas inflamatorias afectan normalmente a mujeres en edad fértil tratadas con fármacos biológicos. Sin embargo, escasea la literatura sobre la eficacia y la toxicidad de los fármacos modificadores de la enfermedad (FAME) biológicos durante el embarazo. El objetivo de este estudio fue determinar la presencia de pacientes embarazadas tratadas con FAME biológicos en un conjunto de datos del mundo real y examinar el impacto del embarazo y la lactancia en la evolución de la enfermedad reumática en un registro de pacientes españoles.
MétodoEstudio prospectivo multicéntrico en un entorno del mundo real. La información se obtuvo del registro BIOBADASER. Los pacientes fueron mujeres embarazadas hasta el mes de noviembre del 2020, de 19 unidades de Rreumatología. Obtuvimos proporciones, medias y desviaciones estándar (DE) para describir la población de estudio y el uso de tratamientos. Se realizaron las pruebas t y χ2 para evaluar las diferencias entre grupos.
ResultadoSe registraron 90 casos de embarazo (n=68 embarazos a término; n=22 abortos espontáneos). La mayoría de los casos suspendieron el tratamiento con FAME biológicos durante el embarazo (78,9%), pero 13 casos prosiguieron el tratamiento durante el embarazo, utilizando principalmente certolizumab pegol. Dichos casos obtuvieron un mejor manejo de la enfermedad reumática, aunque las diferencias no fueron estadísticamente significativas (DAS28-CRP, 2,9 [DE 1,6] vs. 2 [1,2], p=0,255; DAS28-ESR, 2,2 [1] vs. 1,7 [0,5], p=0,266). No se reportaron episodios adversos graves durante el embarazo y la lactancia.
ConclusiónLa situación de embarazo sigue siendo infrecuente en las pacientes con enfermedades reumáticas que utilizan FAME biológicos. Nuestros resultados reflejan que la enfermedad reumática tendió a progresar mejor durante el embarazo en las mujeres tratadas con FAME biológicos.