Peripheral and follicular helper T lymphocytes (Tph and Tfh, respectively) have an important role in B cell immune responses and the pathogenesis of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Although several studies on the number of Tph and Tfh cells in these conditions have been published, different phenotypes have been employed for their analysis. In this study, we assessed the levels and function of Tph and Tfh cells in blood samples from patients with RA and SLE by using an extended immunophenotype.
Materials and methodsIn a cross-sectional pilot study, blood samples from twenty-seven patients with RA and fifteen with SLE, and twenty-six healthy controls were studied. The levels of Tph (CD4+PD-1+CXCR5−CD38+CD69+ICOS+) and Tfh (CD4+PD-1+CXCR5+CD38+CD69+ICOS+) cells were analyzed by flow cytometry. In addition, the function of Tph/Tfh cells was estimated by measuring the synthesis of IL-21 by these lymphocytes as well as the number of circulating plasmablasts (CD19+CD27+CD20−CD38hi).
ResultsIncreased percentages of Tph and Tfh lymphocytes were detected in patients with RA and SLE. Furthermore, the synthesis of IL-21 tended to be higher in both conditions, and higher levels of plasmablasts were detected in these patients, compared to controls. In patients with SLE, the number of Tph cells was associated with disease activity and with the levels of circulating plasmablasts, whereas in patients with RA a significant correlation between Tph cells and evolution time was observed.
Discussion and conclusionsOur data of Tph and Tfh lymphocytes, based in the analysis of an extended phenotype of these cells, provides further evidence on their involvement in the pathogenesis of RA and SLE.
Los linfocitos T de ayuda foliculares y de ayuda periféricos (Tfh y Tph) tienen un papel importante en la respuesta inmune humoral y la patogenia de la artritis reumatoide (AR) y el lupus eritematoso generalizado (LEG). Aunque se han publicado estudios sobre el número de células Tfh y Tph en sangre venosa periférica de pacientes con AR y LES, esto se ha realizado utilizando diferentes inmunofenotipos. En este estudio se analizó el número y la función de estos linfocitos aplicando un inmunofenotipo extendido.
Material y métodosEn un estudio de casos y controles se incluyeron a 27 pacientes con AR, 15 con LES y 26 controles sanos. Los niveles de células Tph (CD4+PD-1+CXCR5−CD38+CD69+ICOS+) y Tfh (CD4+PD-1+CXCR5+CD38+CD69+ICOS+) se analizaron por citometría de flujo. Además, la función de las células Tph/Tfh se estimó mediante el análisis de la producción de IL-21 y los niveles de plasmablastos (CD19+CD27+CD20−CD38hi) circulantes.
ResultadosSe encontraron niveles incrementados de células Tfh y Tph en pacientes con AR y LES. Además, los niveles de IL-21 tendieron a ser más elevados en estos pacientes y el número de plasmablastos fue más alto en los mismos. Por otra parte, se encontró en LES una correlación significativa entre el número de células Tph y la actividad de la enfermedad y el número de plasmablastos, mientras que en AR se observó una asociación significativa entre niveles de células Tph y tiempo de evolución de la enfermedad.
Discusión y conclusiónConsideramos que nuestros resultados, basados en un análisis de fenotipo extendido de células Tfh y Tph, apoyan adicionalmente la participación de estas células de ayuda en la patogenia de la AR y el LES.
Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are chronic autoimmune diseases that result from the interaction between polygenic risk factors and environmental influences, which causes loss of immune tolerance to self-antigens, leading to the synthesis of autoantibodies.1,2 These patients show abnormal levels and dysfunction of different immune cells, including CD4+CD25highFoxp3+ T regulatory (Treg) cells, Th17/Th22 helper lymphocytes, NK cells as well as CD69+ Treg and Tr1 regulatory cells.3–5 Thus, SLE and RA, are characterized by the presence of different autoantibodies, rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA) in RA, and anti-nuclear antibodies (among many others) in SLE.1,2
Follicular T helper cells (Tfh) were identified as a unique CD4+ T cell subset found in germinal centers of secondary lymphoid organs (SLO), mainly composed by B lymphocytes.6 The phenotype of Tfh lymphocytes is characterized by the expression of different membrane molecules, mainly CD4, CXCR5 and PD-1,7 which allowed to detect their presence in the peripheral blood. These helper cells are characterized by their ability of synthetize IL-21, a key cytokine in the differentiation of B cells into either, plasmablast or memory B lymphocytes.8 Thus, Tfh cells provides help to B cells, promoting the synthesis of antibodies. Accordingly, different studies have supported the involvement of this lymphocyte subset in the pathogenesis of autoimmune diseases characterized by presence of autoantibodies, including SLE, RA and autoimmune thyroid disease.8,9
Peripheral helper T (Tph) lymphocytes were initially detected in the inflamed synovial tissue of patients with RA,10 This lymphocyte subset is closely related to Tfh cells, sharing phenotypic markers (e.g., PD-1) as well as the capability to synthesize IL-21, and to provide help to B lymphocytes.11 However, unlike Tfh cells, Tph lymphocytes express the chemokine receptors CCR2 and CCR5, but not CXCR5, which allows their recruitment to inflamed tissues.12 Thus, the phenotype that allow the detection of these cells (and its discrimination from Tfh lymphocytes) corresponds to CD4+PD-1+CXCR5−, which can be easily detected by flow cytometry.12,13 However, in different reports distinct phenotypes have been employed to analyze Tph cells. In this regard, in the case of RA and SLE, the quantitative analysis of Tph cells has been based on the following immunophenotypes: CD4+PD-1highCXCR5−,14 CD4+PD-1+ICOS+,15 CD4+PD-1+CXCR5−,16 CD3+CD4+CD45RA−PD-1highCXCR5−,17 CD4+CXCR5−CXCR3+PD-1high,18 and CD4+TCRβ+CD45RA−CXCR5low.19 Furthermore, additional molecules are expressed by both, Tph and Tfh cells, including the activation markers CD38 and CD69, and the costimulatory receptor ICOS, which could be important for the functions of these cells.12,13,20 Accordingly, we and others12,13,21 consider that the expression of these molecules may indicate that Tph and Tfh lymphocytes are active and functional. According to this, the aim of this study was to carry out a reassessment on the possible involvement of Tph/Tfh cells in two inflammatory autoimmune conditions. Thus, we have performed a simultaneous and detailed analysis of the blood levels of Tph and Tfh cells in patients with RA and SLE, in order to corroborate previous reports and to provide further evidence on the involvement of these lymphocyte subsets in the pathogenesis of autoimmune diseases. Therefore, in this study, we have hypothesized that the analysis of an extended immunophenotype of Tph (CD4+PD-1+CXCR5−CD38+CD69+ICOS+) and Tfh (CD4+PD-1+CXCR5+CD38+CD69+ICOS+) cells, and other data (IL-21 synthesis and levels of circulating plasmablasts), could provide additional significant information regarding the levels and function of these cells in RA and SLE. We consider that our results further support the role of Tph cells in the pathogenesis of these conditions.
Materials and methodsIndividuals and samplesBlood samples (10.0mL) were obtained from twenty-seven patients with RA (22 females and 5 males) and a mean age of 50.2±11.3 years. In addition, according to DAS28 index at the time of the study, twelve patients were arbitrarily classified as having low activity (DAS28 ≤4) and fifteen as high activity (DAS28 >4). Twenty patients were receiving prednisone (2.5–7.5mg/day) and/or disease-modifying antirheumatic drugs (methotrexate 7.5–20.0mg/week and sulfasalazine 1.0–3.0mg/day). Five patients were untreated at the time of the study. Fifteen patients with SLE were also studied, fourteen patients were females and one male, with a mean of age 37.2±11.0, and according to the MEX-SLEDAI score, 60% of patients had a moderate to severe active disease, and 40% were in remission. Thirteen patients were receiving prednisone (2.5–7.5mg/day) and/or immunosuppressive drugs (methotrexate 10.0–15mg/week and/or azathioprine 50mg/day). Two patients were untreated at the time of the study. No patients under therapy with biologicals agents were included in the study, and no evidence of renal failure was detected in any of them. All patients were examined by a rheumatologist, and the diagnosis was established based on the criteria of American College of Rheumatology. Additionally, twenty-six healthy participants (15 females and 11 males) with an average age of 35.7±14.6, were included in the study. Main clinical and demographic data of patients included in the study are shown in Table 1, and a written informed consent was obtained from all of them. This study was approved by the Hospital Bioethical Committee (HCIMP 38-19).
Clinical and demographic data of patients with rheumatoid arthritis and systemic lupus erythematosus included in the study.
| Patients | ||
|---|---|---|
| RA | SLE | |
| n (female/male) | 27 (22/5) | 15 (14/1) |
| Age (years) | 50.2±11.3 | 37.2±11.0 |
| Time of evolution (years) | 7.93±8.17 | 5.6±4.51 |
| DAS28 >4.0 (%) | 55.6 | – |
| SLEDAI >4.0 (%) | – | 60 |
| Therapy* | ||
| Prednisone (dose range) | 20/27 (2.5–7.5mg/day) | 8/15 (2.5–10.0mg/day) |
| Methotrexate (dose range) | 20/27 (7.5–20.0mg/week) | 10/15 (12.5–15.0mg/week) |
| Sulfasalazine (dose range) | 5/27 (1.0–3.0 g/day) | – |
| Azathioprine (dose range) | – | 3/15 (50.0mg/day) |
| Biological agents | 0/27 | 0/15 |
| Untreated patients (%) | 5/27 (18%) | 2/15 (13%) |
| Rheumatoid factor (U/mL)** | 126.0 (55.0–208.4) | – |
| ACCP (U/mL)*** | 47.05 (1.7–137.5) | – |
| Anti-nuclear antibodies (>1:80) | – | 15/15 (100%) |
DAS28: Disease Activity Score 28; MEX-SLEDAI: Mexican Systemic Lupus Erythematosus Disease Activity Index; RF: rheumatoid factor; ACCP: anticyclic citrullinated peptide antibodies. Data corresponds to the arithmetic mean±SD or median and interquartile range.
Blood samples were obtained and peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation (Ficoll-Hypaque, Sigma Chemical Co., St. Louis, MO). Cells were maintained in RPMI 1640 (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), penicillin (50IU/mL), and streptomycin (50μg/mL) (Sigma). Cell viability was assessed by trypan blue staining, and it was always greater than 95%.
Flow cytometry analysisTo determine the frequency of Tph and Tfh lymphocytes, PBMCs were labeled with the following monoclonal antibodies (mAbs): anti-CD4-PerCP (BD Biosciences), anti-PD-1-Pacific Blue (BioLegend), anti-CXCR5-APC-Cy7 (BioLegend), anti-CD38-PE (eBiosciences), anti-CD69-APC (BioLegend), anti-ICOS(CD278)-FITC (Invitrogen), and anti-IL-21-APC (BioLegend). Plasmablasts were analyzed by using the following mAbs: anti-CD19-PerCP (BD Biosciences), anti-CD27-FITC (eBiosciences), anti-CD20 APC-Cy7 (BD Biosciences) and anti-CD38-PE (eBiosciences) (BD Biosciences). Then, cells were washed and resuspend for their analysis in a FACS Canto II flow cytometer with the FACS Diva Software (BD Biosciences). Tfh cells were defined as CD4+PD-1+CXCR5+CD38+CD69+ICOS+ and Tph cells corresponded to CD4+PD-1+CXCR5−CD38+CD69+ICOS+, whereas CD19+CD27+CD20−CD38hi cells were considered as plasmablasts.
Intracellular cytokine stainingTo assess the synthesis of IL-21 by Tph and Tfh cells, 1.5–2×106 PBMC were cultured in flat bottom 24-well plates for 4h, in the presence or absence of 750ng/mL of ionomycin and 50ng/mL of PMA (Sigma). Brefeldin A (10μg/mL) was added in the last 2h of cell culture. Cells were harvested, labeled for CD4, PD-1, CXCR5, CD38, and ICOS, fixed with 1% p-formaldehyde, and permeabilized with 0.05% saponin. Finally, cells were stained with an anti-IL-21 mAb (eBiosciences) tagged with APC and analyzed by flow cytometry.
Statistical analysisData are expressed as the arithmetic mean and SD for data with normal distribution, while median and interquartile range (Q1–Q3) for data with a non-Gaussian distribution. Comparisons between two groups were done with the Mann–Whitney U test, while comparisons among three groups were analyzed with the Kruskal–Wallis sum rank test, and post-hoc analysis, if necessary. Association between two quantitative variables was analyzed with the Spearman or Pearson correlation tests, as required. Data were analyzed by using the GraphPad Prism software v5.0 (San Diego, CA) and p values <0.05 were considered as significant.
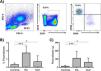
ResultsIncreased levels of circulating Tph cells in RA and SLE patientsLevels of Tph and Tfh cells in PBMC from patients with RA and SLE were analyzed by multi-parametric flow cytometry, according to the strategy shown in Fig. 1. Thus, Tfh cells were defined as CD4+PD-1+CXCR5+CD38+CD69+ICOS+, whereas CD4+PD-1+CXCR5−CD38+CD69+ICOS+ corresponded to Tph cells. We detected increased levels (both, percentages and absolute numbers) of Tph cells in patients with RA (p<0.001, compared to healthy controls, in both cases, Fig. 2A and B). Similar results were observed in the case of patients with SLE (Fig. 2A and B). Accordingly, no significant differences were detected when the levels of Tph cells of patients with RA and SLE were compared (p>0.05, in both cases, Fig. 2A and B). In the case of Tfh cells, we also detected enhanced levels (percentages and absolute numbers) of these lymphocytes in the peripheral blood from patients with RA or SLE compared to healthy controls (p<0.01 in all cases, Fig. 2C and D). Likewise, these cell levels were similar in the two groups of patients studied (p>0.05 in both cases, Fig. 2C and D).
Flow cytometry strategy for the analysis of Tph and Tfh cells. PBMC were stained with the indicated mAb and analyzed by multi-parametric flow cytometry. According to this analysis, Tfh lymphocytes were defined as CD4+PD-1+CXCR5+CD38+CD69+ICOS+, whereas Tph cells corresponded to CD4+PD-1+CXCR5−CD38+CD69+ICOS+. Data correspond to a sample from a representative healthy control.
Levels of Tfh and Tph cells in blood samples from patients with RA, SLE and healthy controls. PBMC were labeled and analyzed by flow cytometry, as stated in “Materials and methods” section. (A and B) Percent and absolute number of Tph cells. (C and D) Percent and absolute number of Tfh cells. Data correspond to the median and Q1–Q3 interquartile range. **p<0.005; ***p<0.001.
When the levels of Tfh and Tph cells were compared by using the extended (CD4+PD-1+CXCR5+CD38+CD69+ICOS+ and CD4+PD-1+CXCR5−CD38+CD69+ICOS+, respectively) and short (CD4+PD-1+CXCR5+ and CD4+PD-1+CXCR5−, respectively) phenotypes, we observed that higher levels of significance were detected in the former analysis, as it is shown in Table 2. These data strongly suggest that the analysis of Tph/Tfh cells through their extended immunophenotype by multi-parametric flow cytometry improves the detection of these lymphocyte subsets.
Comparison of the levels of statistical significance in the flow cytometry analysis of Tfh and Tph cells by using the short and extended phenotypes.
| ANOVA | Controls vs RA | Controls vs SLE | RA vs SLE | |
|---|---|---|---|---|
| Short phenotype Tfh cells(CD4+PD-1+CXCR5+) | <0.05>0.01 | <0.05>0.01 | <0.05>0.01 | n.s. |
| Extended phenotype Tfh cells(CD4+PD-1+CXCR5+ CD38+CD69+ICOS+) | <0.01>0.005 | <0.001 | <0.001 | n.s. |
| Short phenotype Tph cells(CD4+PD-1+CXCR5−) | <0.01>0.005 | <0.01>0.005 | <0.01>0.005 | n.s. |
| Extended phenotype Tph cells(CD4+PD-1+CXCR5−CD38+CD69+ICOS+) | <0.001 | <0.001 | <0.001 | n.s. |
As shown in Fig. 3A, the percentage of IL-21+ Tph cells tended to be higher in patients with RA and SLE compared to controls; however, these apparent differences did not reach statistical significance (p=0.06, Kruskal–Wallis test). A similar trend was observed in the case of Tfh lymphocytes (p=0.06, Kruskal–Wallis test, Fig. 3B).
Analysis of the synthesis of IL-21 by Tph and Tfh cells in blood samples from patients with RA and SLE and healthy controls. PBMC were stimulated with PMA and ionomycin, labeled with the mAbs to identify Tph/Tfh cells (as stated in “Materials and methods” section), permeabilized and incubated with an anti-IL-21 mAb, and analyzed by flow cytometry. (A) Percentages of Tph lymphocytes expressing IL-21. (B) Percentages of Tfh cells expressing IL-21. Data correspond to median and Q1–Q3 interquartile range.
As shown in Fig. 4A, plasmablasts, defined as CD19+CD27+CD20−CD38hi, were analyzed by flow cytometry. In these analyses, we found a higher frequency and absolute numbers of plasmablasts in blood samples from patients with RA compared to healthy controls (p<0.001, in both cases, Fig. 4B and C). Similar results were observed in the case of the patients with SLE (p<0.05, in both cases, Fig. 4B and C). Accordingly, no significant differences were detected in the levels of these cells between RA and SLE (p>0.05 in both cases, Fig. 4B and C).
Quantitative analysis of circulating plasmablasts in patients with RA and SLE. (A) Flow cytometry strategy for the analysis of circulating plasmablast. PBMC were stained with the indicated mAbs and analyzed by flow cytometry, as stated in “Materials and methods” section. Cells with the phenotype CD19+CD27+CD20−CD38hi were considered as plasmablasts. (B and C) Percent and absolute number of plasmablasts in samples from patients with RA and SLE and healthy controls. Data correspond to the median and Q1–Q3 interquartile range. *p<0.05; ***p<0.001.
As shown in Fig. 5A, a significant positive association between Tph and Tfh levels (Fig. 5A), and between Tfh cells and rheumatoid factor titers (Fig. 5B) were detected in patients with RA. Moreover, a significant but negative association between Tph or Tfh levels and evolution time of the disease was observed in this condition (Fig. 5C and D, respectively). In the case of patients with SLE, a significant positive relationship between Tph numbers and the percent of circulating plasmablasts was detected (Fig. 5E), as well as a negative association between the percent of Tfh cells and evolution time of the disease (Fig. 5F). Interestingly, additional significant positive associations were detected between the levels of Tph or Tfh cells and disease activity (Fig. 5G and H).
Although this study was not designed to perform a prospective analysis, it is of interest that when the levels of Tph cells were additionally correlated with the disease activity (SLEDAI score) approximately six months after the quantification of Tph cells in SLE patients, a significant association was detected (r=0.66, p<0.01, n=11). Likewise, in the case of patients with RA, a similar analysis also showed a significant correlation between the Tph levels and the DAS28 score detected six months later (r=0.48, p<0.05, n=19). In contrast, no significant associations were detected between disease activity and Tfh levels in this type of analysis, in both SLE and RA (data not shown).
DiscussionBoth, Tfh and Tph cells exert a relevant role in the generation of the humoral immune response, through their helper effect on B lymphocytes and plasma cell differentiation.6–10 Accordingly, several years ago it was hypothesized that these cell subsets may be involved in the pathogenesis of autoimmune disease mediated by antibodies. Thus, Tfh and Tph cells have been previously analyzed in the peripheral blood and tissues of patients with different autoimmune conditions, including RA and SLE.7–10 However, different immunophenotypes have been employed in these analyses, including, in the case of Tfh cells and in most studies, the expression of CD4 and PD-1 and the absence of the chemokine receptor CXCR5 (CD4+PD-1+CXCR5− cells).11 As expected, additional cell markers of Tfh and Tph cells have been described, including the costimulatory molecule ICOS (CD278) and the cell activation markers CD38 and CD69.11,19,20 Therefore, in this study we decided to carry out an upgraded analysis of the levels of Tfh and Tph cells in patients with RA and SLE, by using an extended immunophenotype, which could reflect an activation and functional status of these helper lymphocytes, CD4+PD-1+CXCR5−CD38+CD69+ICOS+ for Tph, and CD4+PD-1+CXCR5+CD38+CD69+ICOS+ for Tfh cells.9
As expected, many studies have detected increased levels of Tph or Tfh cells in peripheral blood or their presence in different inflamed tissues from patients with autoimmune diseases.8,13,17–19,22–24 In agreement with those studies, we have observed increased numbers of both Tfh and Tph cells in the peripheral blood from patients with RA and SLE. In this regard, we consider that our study provides interesting additional information regarding Tfh and Tph cells in these conditions given that we have performed a simultaneous analysis of these two cell subsets, including their percentages and absolute numbers. Thus, our data indicate that in the peripheral blood compartment there is not a predominance of neither of the two cell subsets analyzed in the case of RA or SLE. In this regard, it has been reported that Tph lymphocytes predominate over Tfh cells in the organized lymphoid cell infiltrate of the synovial tissue in patients with RA.25 In contrast, in the inflamed tissue from patients with IgG4-related disease Tfh cells are clearly more abundant than Tph lymphocytes.26
The possible relevance of our data on the enhanced numbers of Tph and Tfh cells in the peripheral blood from patients with RA and SLE are supported by their significant (although not very strong) associations with the enhanced levels of circulating plasmablasts. In addition, it is of interest the significant (also not very strong) associations that were detected between the levels of Tph cells and the disease activity observed six months later, in both, patients with RA and SLE. Thus, we consider that it is feasible that an increased number and an enhanced activity (i.e., IL-21 synthesis) of Tph/Tfh cells could be causally associated with a raised differentiation of B lymphocytes into plasma cells and with disease outcome.
The possible functions of the different molecules expressed by Tph and Tfh cells, which were included in our extended phenotype analysis, remain to be fully disclosed. In this regard, it has been widely described that CD38 behaves as an activation molecule and that exerts different functions in the many cell subsets in which it is expressed.27 Moreover, CD69 also behaves as an activation molecule in most immune cells and it seems to have a relevant role in the regulation of the immune system.28 Finally, ICOS (CD278) is also expressed upon activation by T cells, and the intracellular signal pathways induced after their interaction with its ligand (ICOS-L, CD275) promotes cell proliferation and survival, among others.29 Obviously, the possible overall effect of the combined presence of these molecules on Tph/Tfh cells is an interesting point to be studied. However, it is very feasible that the expression of these three activation markers indicate that these cells are fully functional, at least regarding their helper effect on B cell function and differentiation. Therefore, we consider that our data further support the role of Tph/Tfh lymphocytes in the complex pathogenesis of both, RA and SLE. Accordingly, we have detected a significant positive association between the levels of Tph cells and the serum titers of rheumatoid factor (in the case of patients with RA) as well as a significant correlation with disease activity (SLEDAI score) in the patients with SLE. Furthermore, significant association were observed in SLE between the percentage of Tph cells and the number of circulating plasmablasts, and disease activity. However, we also detected significant associations between the proportion of Tfh lymphocytes and the evolution time of the disease, in both, RA and SLE, a finding that deserves additional investigation.
In summary, we consider that our data on the simultaneous and detailed analysis of the levels and function of Tph/Tfh lymphocytes, by using an extended immunophenotype of these cells, and accompanied by other assays (detection of synthesis of IL-21 and levels of circulating plasmablasts), provides significant additional evidence on their involvement in the pathogenesis of RA and SLE. However, it seems very convenient that these results be confirmed in a larger number of patients, mainly in the case of SLE. Likewise, the apparent association between Tph blood levels and disease activity/outcome (as well as its absence in the case of Tfh cells) should be further analyzed in a prospective study, in both, patients with RA and with SLE.
FundingThis study was financially supported by an institutional fund of the Universidad Autónoma de San Luis Potosí, San Luis Potosí, SLP, Mexico.
Authors’ contributionsSG-R, investigation; VN-M, investigation; MP-A, investigation; GB-L, resources; PP-D, methodology; LE-E, methodology; GH-M, methodology; RG-A; conceptualization, writing, reviewing and editing.
Conflict of interestsThe authors declare that they have no conflict of interest.