International criteria for the diagnosis of antiphospholipid syndrome (APS) include the detection of antiphospholipid antibodies: anticardiolipin and anti-β2-glycoprotein i (anti-β2GPI) of immunoglobulin (Ig) G and IgM isotopes and lupus anticoagulant (LA), and its detection in patients with a history of thrombosis or pregnancy complications is considered to be essential in the management of APS.1–3
During the 1950s, it became evident that patients with systemic lupus erythematosus (SLE) had a circulating anticoagulant factor, and the concept of LA was coined to designate a heterogenic group of coagulation inhibitors that affect prothrombin activation by the prothrombinase enzyme complex.4 Lupus anticoagulant is currently described as an Ig like IgG/IgM that inhibits phospholipid (PL)-dependent coagulation reactions in vitro. However, is LA always associated with SLE? Does it inhibit coagulation? Is it an antibody? First, the majority of the patients who are positive for LA do not have SLE. In the absence of concomitant thrombocytopenia or a deficiency of coagulation factors or inhibitors of coagulation factors, with certain exceptions, LA is related to processes of hypercoagulability and arterial and venous thrombosis, but is not, per se, a risk factor for bleeding or hemorrhage. With the currently available scientific evidence, it can be said the LA is constituted by a group of antibodies that have yet to be characterized.5,6
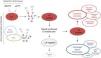
Lupus anticoagulant is detected using functional assays that demonstrate a PL-dependent prolongation of the clotting time, due to the in vitro interference of antibodies with PL-dependent function, as with certain essential cofactors in the coagulation cascade (Fig. 1) that result in the prolongation of the activated partial thromboplastin time. The International Society on Thrombosis and Haemostasis (ISTH),7 a society that has unsuccessfully attempted to change the name, established the following criteria to confirm the presence of LA:
- 1.
Phospholipid-dependent prolonged coagulation tests.
- 2.
Demonstration of the coagulation inhibitor utilizing mixed plasma.
- 3.
Demonstration of the PL dependence of the inhibitor.
- 4.
Ruling out other coagulation disorders, particularly due to deficiency of coagulation factors.
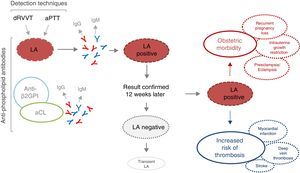
Detection, interpretation and possible clinical consequences of a positive result in lupus anticoagulant testing. aCL, anti-cardiolipin; anti-β2GPI, anti-β2-glycoprotein i; aPTT, activated partial thromboplastin time; dRVVT, dilute Russell's viper venom time; Ig, immunoglobulin; LA, lupus anticoagulant; PL, phospholipid.
Lupus anticoagulant can be detected in patients with SLE, and with other autoimmune diseases, infections like human immunodeficiency virus, hepatitis and malaria, neoplastic disease or those taking certain drugs (procainamide and chlorpromazine).6,8 A prevalence of 5% has been reported in the general adult population and up to 9.5% in women of reproductive age. Although the pathogenic mechanism has not been defined, the presence of LA has been related to stroke, transitory ischemic attack, acquired thrombophilia and obstetric events, like early and/or recurrent pregnancy loss.7 Although it is certain that, in general, antiphospholipid antibodies have been associated with the clinical manifestations of APS, this association seems to be more evident with LA both in thrombosis and in the morbidity related to pregnancy.8,9
Studies dealing with the relationship between the processes of coagulation and inflammation would establish the clinical relevance of the detection of LA alone, as well as the emergent association of LA to C-reactive protein and mortality. The therapeutic management of asymptomatic carriers of LA could require prophylactic treatment given the presence of cardiovascular risk factors or autoimmune disease.5,8
In view of the potential thrombotic risk in patients who are positive for LA, it is essential to develop an accurate method for performing its assessment in terms of the diagnosis and follow-up of these patients and the decision on the anticoagulant therapy they should receive. Unfortunately, since we lack a technique to serve as a reference for the detection of LA, laboratories utilize heterogeneous and non-quantitative assays, impeding the characterization of positive results in terms of low or elevated titers.10,11 This requires that the validation of the results be performed only by expert staff. In turn, it impedes their being standardized, the establishment of a consensus and the automation of this determination.6,11 A study published by Devreese et al. points out the need to analyze other additional procoagulant markers, like P-selectin (a marker of platelet activation) and coagulation factor vii in patients with weak LA, in order to optimize its clinical utility.12,13 It is evident that both overrating and underrating LA would expose our patients to long-term anticoagulation or to an increase in the risk of recurrent thrombosis, respectively.13 Likewise, it should be possible to report LA quantitatively to enable the identification of low titers or those near the reference value. There is an unquestionable need to perform prospective studies, that examine the relevance of these laboratory tests. This, together with possible new prognostic laboratory parameters, would help in the stratification of the patients in accordance with the risk groups and in making therapeutic decisions.
The authors wish to thank Dr. Nora Butta (Laboratory for Research on Coagulopathies and Hemostatic Disorders, Hematology Unit, Hospital Universitario La Paz-IDIPaz, Madrid, Spain) for her critical reading and the corrections she made in this manuscript.
Please cite this article as: Valor L, Hernández-Flórez D, Martínez-Barrio J, López Longo FJ. Una reflexión sobre el anticoagulante lúpico: cómo lo definimos, determinamos e interpretamos. Reumatol Clin. 2018;14:120–122.