To review the clinical evidence on abatacept and to formulate recommendations in order to clear up points related to its use in rheumatology.
MethodAn expert panel of rheumatologists objectively summarized the evidence on the mechanism of action, practicalities, effectiveness and safety of abatacept, and formulated recommendations following a literature review. The level of evidence and degree of recommendation was established.
ResultsThe document presents 21 statements focused on evidence or recommendations on abatacept (14 evidence summaries and 9 recommendations). The level of evidence was 2b or higher according to the Oxford Centre for Evidence-Based Medicine scale on 14 occasions. The degree of the recommendation was A in two recommendations, C in one, and D in the rest. It was considered important to make recommendations on aspects with lower levels of evidence.
ConclusionsThis is a practical document to supplement the summary of product characteristics.
Revisar la evidencia clínica sobre abatacept y emitir recomendaciones con objeto de aclarar su uso en Reumatología.
MétodoUn panel de expertos reumatólogos resumió de forma objetiva las pruebas existentes sobre el mecanismo de acción, modo de uso, eficacia y seguridad de abatacept y emitió recomendaciones de uso en situaciones concretas, previa revisión de la bibliografía. Se estableció el nivel de evidencia de las pruebas y el grado de apoyo de dichos datos a las recomendaciones emitidas.
ResultadosEl documento presenta 21 enunciados resumen de la evidencia encontrada o recomendaciones sobre abatacept (14 enunciados y 9 recomendaciones). El nivel de evidencia es superior a 2b según la escala de Oxford del Centro de Medicina Basada en la Evidencia en 14 ocasiones. El grado de apoyo de las recomendaciones es A en 2 recomendaciones, C en una y D en el resto. Se consideró importante realizar recomendaciones precisamente en los aspectos con menor grado de evidencia.
ConclusionesSe trata de un documento práctico como complemento a la información en ficha técnica.
Since the advent of biological agents, clinicians have accumulated a large amount of relevant information quickly. This has made it difficult to disentangle the differences between the various biological agents: 5 TNF inhibitors, anakinra, rituximab, abatacept and tocilizumab. This has led to a series of simplistic conclusions such as that “all biological agents are equal” or that “everyone would have a similar safety and tolerability profile’. The absence of direct comparative studies has also helped, although there are meta-analysis based clinical trials that have provided some light on these differences.1–4
Abatacept is a parenterally administered drug characterized by a different mechanism of action than other biological agents. It is a fusion protein consisting of the extracellular domain of CTLA-4 expressed on the T cell and a modified Fc fragment of human immunoglobulin IgG1. This protein modulates costimulation when the antigen presenting cell interacts with the T lymphocyte.
The indications for abatacept are restricted to rheumatoid arthritis (RA) and polyarticular juvenile idiopathic arthritis (JIA). It is currently approved for patients with inadequate response to previous treatment with one or more disease modifying drugs (DMARDs) or TNF inhibitor (at least one in the case of JIA).5
The objective of this document is to provide the clinician with a review of the evidence of this biological agent, focusing especially on the most relevant aspects of its efficacy in RA and existing data to date on safety. The document presents 21 statements focused on evidence or recommendations, whose mission is to try to resolve any questions that might arise with its use.
MethodsThe head of the project (EMM) selected 7 rheumatologists; all were experts in RA. All have extensive experience in the use of biologic therapies, as well as being authors of scientific articles on the subject. Besides these features, the panelists were chosen based on their geographical distribution, which tried to be as close as possible due to their small number. There were two meetings, the first on May 20, 2011 and a second one on November 14, 2011. At the first meeting, issues were agreed upon, as well as the scope and format the document should have, and tasks were distributed. All experts conducted a non-systematic review of the scientific literature and were responsible for drafting the document. In addition, 3 reviewers (LL, LR and LC) from the previous group were incorporated into the project from the beginning, conducted systematic reviews of questions that the panelists considered as disputable and that were not included in the Cochrane meta-search for abatacept.6 During the last meeting, consensus statements were drafted summarizing late evidence and its clinical applicability. The level of evidence (LE) and degree of recommendation (DR) were established by one reviewer (LC), based on the Oxford Centre for Evidence-Based Medicine7 scale. When the DR could not be used because of lack of a recommendation, it was labeled “not applicable” (NA).
ResultsA tabular summary of the evidence and recommendations is shown in Table 1 together with the appropriate level of evidence and degree of recommendation. Below is a description of the various aspects of abatacept.
Evidence and Use Recommendations for Abatacept in Rheumatoid Arthritis With Level of Evidence, Degree of Recommendation, Agreement and Applicability.
| Evidence or Recommendation | LE | DR | |
| 1 | Abatacept is administered as a short term intravenous perfusion and has a low incidence of infusion related reactions | 1b | NA |
| 2 | Abatacept is effective in the reduction of signs and symptoms of rheumatoid arthritis in patients with failure to DMARD or TNF inhibitors | 1b | NA |
| 3 | Efficacy of abatacept is maintained in the long term | 2b | NA |
| 4 | Abatacept is effective in recent onset RA with no prior DMARD treatment and poor prognosis factors although this indication has not been approved | 1b | NA |
| 5 | Abatacept reduces progression of joint structural damage in a maintained manner over time | 2b | NA |
| 6 | Abatacept has high rates of compliance in long term studies | 2b | NA |
| 7 | Effectiveness data of abatacept in clinical practice in registrie are consistent with clinical trials | 2b | NA |
| 8 | Before administering abatacept and during its monitoring, general recommendations proposed for biologic therapy must be followed | 5 | D |
| 9 | The safety profile of abatacept and its tolerability are satisfactory | 1b | NA |
| 10 | In clinical trials, rates of infection with abatacept are similar to those of patients treated with MTX and numerically inferior to other biologics | 1b | NA |
| 11 | The rate of tuberculosis in patients treated with abatacept seems inferior to that of TNF inhibitors. However, in patients to be treated with abatacept, latent tuberculosis must be looked for according to the SER guidelines | 2b; 5 | NA; D |
| 12 | Safety of abatacept is unknown in patients with hepatitis B or C. Its use must be guided by a strict control of liver function and viral load | 5; 5 | NA; D |
| 13 | The risk of appearance of tumors in patients treated with abatacept is not superior to what is expected in RA patients | 2b | NA |
| 14 | The use of abatacept in patients with stage IV heart failure must be approached with caution | 5 | D |
| 15 | In patients with COPD, abatacept must be administered only after a rigorous analysis of the risk benefit balance | 1b | A |
| 16 | No cases of interstitial lung disease (ILD) have been detected in patients treated with abatacept. In cases with preexisting disease its safety profile is unknown. | 4; 5 | NA |
| 17 | In patients with demyelinating disease, the use of abatacept is not contraindicated; in case of use, close neurological follow up is merited | 2b | D |
| 18 | IV Abatacept is poorly immunogenic | 4 | NA |
| 19 | In patients treated with abatacept the antibody response to inactivated germ vaccines or those with cellular components is inferior to the general population; live attenuated germ vaccines should be avoided | 4 | C |
| 20 | Abatacept may be administered without performing a washout period after the use of anti-TNF, but should not be administered with another biologic agent | 1b | A |
| 21 | Abatacept should be stopped at least 3 months before the onset of pregnancy | 5 | D |
A: applicability; DA: degree of agreement; LE: Level of evidence; DR: degree of recommendation; NA: not applicable (as is not a recommendation).
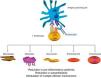
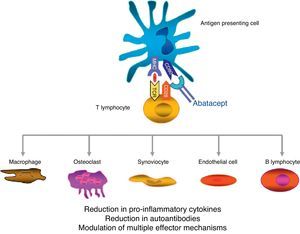
Abatacept is the first therapeutic agent approved for treatment of RA that acts by selectively blocking the activation of T cells by disrupting coestimulatory8 signals. The T cell plays a key role in the pathogenesis of RA.9,10 In a simplified form, T cell activation requires 2 signals by key antigen presenting cells.11 The first is that of antigen presented in the context of the major histocompatibility complex system, recognized by the T cell receptor. The second signal is provided by costimulatory molecules such as CD80 and CD86, binding to CD28 on the T cell. This binding to CD28 leads to T cell proliferation and production of cytokines.8,12 CTLA-4 is expressed physiologically on the surface of activated T cells and its basic task is facilitating CD28 binding to CD80/CD86, and thus suppressing T cell activation.12,13 Abatacept is a fusion protein consisting of the extracellular domain of human CTLA-4 linked to an Fc fragment of human IgG1 that selectively inhibits the second signal, blocking the binding of CD80/CD86 to CD28 (Fig. 1).
Several in vitro and in vivo models have shown that selective abatacept induced blockade of costimulation is accompanied by a regulation of the function of CD4+T cells, a reduction of proinflammatory cytokines, autoantibodies and metalloproteinases and a increased function of regulatory T cells.14–19 Although data on the mechanism of action in RA patients are limited, it is known that abatacept reduces the inflammatory component of rheumatoid synovium and the expression of genes involved in bone destruction.18 In addition, CTLA-4 has an antiresorptive effect, binding directly to the precursors of osteoclasts and inhibiting their differentiation.12,20
The Fc fragment of abatacept is designed with several mutations that inactivate it. Thus, abatacept does not bind to low affinity receptors CD16 and CD32 and does so suboptimally to high affinity receptor CD64.21 Because of this, abatacept is not accompanied by antibody-dependent cellular or complement cytotoxicity.
Particular Mode of Administration and Evidence/Recommendation 1. Abatacept is Administered as an Intravenous Infusion of Short Duration and Has a Low Incidence of Infusion Reactions (LE: 1b, DR: NO)Abatacept is administered as a short intravenous infusion at a dose of approximately 10mg/kg. After the first infusion, abatacept is administered at 2 and 4 weeks and then every 4 weeks.5 Details on dosage, route of administration, and precautions to take before and during the administration are shown in Table 2. Intravenous administration of abatacept has the characteristic that is administered in 30min and rarely requires the use of premedication since infusion reactions are infrequent.6,22,23 In general, the dose and administration intervals of abatacept are often kept constant over time.24
Administration of Abatacept: Dosage, Form of Administration and Precautions.
| • Composition: Each vial contains 250mg of abatacept |
| • Form of administration: Intravenous perfusion. Each vial must be reconstituted with 10ml water for injectable preparations. Afterwards, the reconstituted solution must be diluted in 100ml sodium chloride 9mg/ml (0.9%) solution, before perfusion |
| • Duration of perfusion: 30min |
| • Premedication before perfusion: Not needed |
| • Dosage: The dose of abatacept is approximately 10mg/kg. Abatacept is administered on weeks 0, 2 and 4, and then every 4 weeks |
| Patient Weight | Dose | Number of Vials |
| <60kg | 500mg | 2 |
| ≥60 to ≤100kg | 750mg | 3 |
| >100kg | 1.000mg | 4 |
| • Usefulness period: Closed vial: 3 years. After reconstitution and dilution: 24h between 2°C and 8°C |
| • Precaution in special populations: No dose adjustment is needed in the elderly. No information exists fo patients with kidney and/or liver failure |
Although abatacept is expected to be approved for subcutaneous administration within the next few months,25,26 at present in Europe it is only available for intravenous administration. Subcutaneous administration offers patients the convenience of being home administered. In any case, the patient's preferences with respect to the route of administration must be explored. It is reported that 50% of patients prefer the intravenous to subcutaneous administration due to the calming effect of the presence of medical personnel and security of hospital treatment, the distaste for self-administration and frequency of administration, generally more spaced intervals than with subcutaneous.27
There is a substudy of the AGREE trial in which patients who achieved remission at 2 years (ESR DAS28<2.6) were randomized in a double-blind fashion to receive abatacept at doses of 10mg/kg or 5mg/kg. One year after treatment, the proportion of patients with reactivation of the disease was similar in both groups.5
EffectivenessThe efficacy of abatacept has been demonstrated in both short and long-term clinical trials (Table 3) and long-term extension studies (Table 4), and its effectiveness can be seen in routine clinical practice conditions through registries.
Description of Abatacept Clinical Trials.
| Study | Design | t | Population Included | Intervention | Comparator | Measured Outcomes |
| Phase IIb Kremer (2003, 2005)28,29 | DB | 6m | 339 RA with insufficient response to MTX | ABA 2mg/kg+MTX (n=105)ABA 10mg/kg+MTX (n=115) | PBO+MTX (n=119) | ACR20 at 6 months – ACR50 and 70 – SF-36 QL – Safety |
| AIM Kremer (2006)30 | DB | 1 year | 652 AR with inadequate response to MTX | ABA 10mg/kg+MTX | PBO+MTX | ACR20 at 6 months and 1 year – ACR50 and 70 – HAQ-DI – X ray progression (Genant-modified Sharp) – DAS28 at 6 and 12 months – SF-36 QL |
| ATTAIN Genovese (2005)32 | DB | 6m | 393 RA with insufficient response to anti TNF | ABA 10mg/kg+MTX (n=258) | PBO+MTX (n=133) | ACR 20, 50, 70DAS28HAQ |
| ASSURE Weinblatt (2006)63 | DB | 1 year | 1441 RA with insufficient response to DMARD, biologic or not.Patients with chronic stable disease were included (HF, COPD, DM and asthma) | ABA 10mg/kg (n=959)a | PBO (n=482)a | Safety measuring infusion site reactions, adverse events and autoimmunity.HAQGlobal evaluation of disease and pain as referred by the patient |
| ATTEST Schiff (2008)31 | DB | 6m | 431 RA with insufficient response to MTX | ABA 10mg/kg/4s+MTX (n=156) | IFX 3mg/kg+MTX (n=165)Placebo+MTX (n=110) | DAS28-ESREULAR response criteriaHAQ |
| AGREE Westhovens (2009)34 | DB | 1 year | 509 RA with no prior MTX treatment | ABA 10mg/kg/4 week+MTX (n=256) | PBO+MTX (n=253) | X ray progression (Genant score)DAS28-CRPACR 50, 70 and 90HAQ |
| ARRIVE Schiff (2009)33 | O | 6m | 1.046 RA with insufficient response to anti TNF | ABA 10mg/kg/4s no washout period (n=517) | ABA 10mg/kg/4s with washout (n=449) | Safety – DAS28-CRP |
O: open; ABA: abatacept; RA: rheumatoid arthritis; DB: double blind; RCT: randomized clinical trial; DMARD: disease modifying antirheumatic drug; m: months; MTX: methotrexate; PBO: placebo.
Abatacept Efficacy Results in Long Term Extension Studies. Proportion of Patients That Achieved the Outcome at Study End With Respect to Patients Reaching the End of Study.
| Study | No Prior MTX | DMARD Failure | Anti-TNF Failure | ||
| AGREE | IIB | AIM | ATTEST | ATTAIN | |
| Extension time | 1 year | 7 years | 5 years | 1 year | 4.5 years |
| Initial N/final na | 459/433 | 219/114 | 539/390 | 372/344 | 317/79 |
| Retention rate in the last year | 94.3 | 52 | 70.4 | 90.9 | 47.9b |
| ACR 20/50/70 | |||||
| RCT | –/57/32c | 77/53/29 | 82/54/32 | 67/40/25 | 50/20/10 |
| LTE | –/60/50c | 84/68/51 | 84/61/40 | 87/61/41 | ND |
| Low activity | |||||
| RCT | 54.3 | 48.2 | 44.1 | 23 | 27.7 |
| LTE | 60 | 69.7 | 54.7 | 45 | 40 |
| Remission | |||||
| RCT | 46.1 | 25.3 | 25.4 | 20 | 15 |
| LTE | 55.2 | 48.2 | 33.7 | 26 | 25.7 |
| HAQ response | |||||
| RCT | 71.9 | 49.6 | 63.7 | 61.5 | 47.3 |
| LTE | 81.5 | Maintained | 74.2 | Maintained | Maintained |
RCT, clinical trial; LTE: long term extension; DMARD: disease modifying antirheumatic drug; MTX, methotrexate.
Meta-analysis of Maxwell et al. shows a clear efficacy of abatacept vs placebo.6 A phase IIb clinical trial in patients with established RA and inadequate response to methotrexate (MTX) showed that abatacept plus MTX was more effective than MTX monotherapy, with ACR20 response rates at 6 months of 60% with abatacept and 35% with placebo.28 This significant clinical improvement persisted at 12 months of treatment, with remission rates DAS28-CRP (<2.6) higher in the abatacept-treated patients than in those receiving placebo (35% vs 10%).29 The AIM30 study, a randomized phase III multicenter, double blind trial, compared abatacept (+MTX) vs placebo (+MTX) in 652 patients with an inadequate response to MTX. The ACR20 response rate at 6 months, the primary endpoint, was significantly higher in the abatacept-treated than in the placebo group (68% vs 40%). These differences were even slightly increased after one year of treatment (73% vs 40%). ACR50 and ACR70 responses were also significantly higher in the abatacept-treated group than in the placebo group at both 6 and 12 months. Significant differences between groups were observed after 30 days of treatment. There was also a significant improvement in HAQ-measured disability assessed in the active treatment group vs placebo at one year.
ATTEST31 study, a double-blind trial, separately analyzes the effectiveness of abatacept and infliximab vs placebo in patients with RA and inadequate response to MTX. At 6 months of treatment, both biological agents were superior to placebo. There is a greater speed of action of infliximab in the first 3 months and slower abatacept ACR20 responses at one year. The study was not designed to specifically evaluate the comparative efficacy of the 2 biological agents.
The ATTAIN32 study is a randomized, double-blind, randomized, placebo-controlled trial evaluating the efficacy of abatacept vs placebo in patients with failure to TNF inhibitors (infliximab or etanercept). 393 patients with active RA were included after a washout period of TNF inhibitors and were randomized 2:1 to treatment with abatacept (+MTX and/or FAME) or placebo (+MTX and/or FAME). At 6 months of treatment, ACR20 response rates, the primary endpoint, were significantly higher in the abatacept-treated group than in the placebo group (50% vs 20%), as were ACR50 (20% vs 4%) and ACR70 responses (10% vs 2%). There was also a significant improvement in disability (HAQ improvement>0.3) in the abatacept group compared to placebo (47% vs 23%).
The ARRIVE33 study not only is an open trial of abatacept in 1046 RA patients who had failed to TNF inhibitors, whose primary objective was to evaluate the safety of abatacept administered with or without washout of TNF inhibitors, but also analyzes their effectiveness, demonstrating a significant improvement of clinical activity of RA (reduction of DAS28-PCR greater than 1.2) in more than 50% of patients at 6 months of follow up.
Evidence/Recommendation 4. Abatacept Is Effective in Recent Onset DMARD-Naive RA With Poor Prognostic Factors, Although it Is not Approved for This Indication (LE: 1b, DR: NO)In the AGREE study, abatacept with MTX also demonstrated more effectiveness than MTX monotherapy in patients with recent onset RA who had not previously received MTX and had poor prognostic factors, such as positive autoantibodies or x-ray34 erosions. The rate of remission (DAS28-CRP) at 12 months was significantly higher in patients treated with abatacept+MTX than in those assigned to placebo+MTX: 41% vs 23%. Abatacept, however, has no indication at present for the treatment of patients who have not received prior MTX or DMARD.
Evidence/Recommendation 5. Abatacept Slows the Progression of Structural Joint Damage and This Effect Is Sustained Over Time (LE: 2b, DR: NO)Before presenting the results of studies, it is important to note that in the assessment of structural damage with abatacept, the Genant-modified Sharp van der Heijde was used instead, which is usually the one used in the trials of inhibitors TNF, and the Genant index ranks damage lower (score of 0–290) than the van der Heijde (0–448), although both correlate.35
In the AIM30 study the difference in the rate of erosion and total Genant score after a year of treatment compared to baseline was significantly lower with abatacept+MTX than with placebo+MTX (erosion rate: 0.63 with abatacept compared to 1.14 in placebo, P=.029, total Genant index: 1.21 vs 2.32, P=.012),30 which means a reduction of radiological progression of 45% for erosions and 48% in the total index. This reduction is lower than that obtained with TNF inhibitors, which is approximated to 90%.36 The difference is probably not due to the use of the Genant index instead of the van der Heijde, but with abatacept improvement is slower.
These results are confirmed in the AGREE study performed in RA patients without prior MTX treatment, who showed a 39% reduction in the progression of total damage and 44% for erosions.34 After a year, 61% of patients treated with abatacept+MTX had not progressed radiographically compared to 53% among patients who received MTX alone, representing an estimated difference of 8% between groups.34 No details of radiological progression in patients with a previous TNF inhibitor were given, nor is it known whether there is significant radiological progression in patients with no clinical response to abatacept.
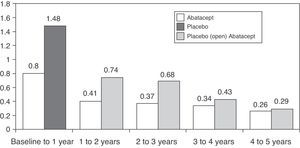
Regarding the long-term progression of the 547 patients who completed the AIM study, 539 (83%) were treated with abatacept during the open period. Mean radiographic progression was significantly reduced in the 2 groups of patients, but more so in those initially treated with abatacept: 1.07 and 0.46 vs 2.40 and 0.75 in the 1st and 2nd years, respectively.37 The rate of radiological progression was reduced even more during the 5 year open follow-up period however, until the 5th year, the rate of progression in the placebo group initially did not match those initially treated with abatacept (Fig. 2).38 The AGREE study data at 2 years confirmed these results. The progression of structural damage in the 2nd year was 57% lower than in the first year in patients initially treated with abatacept. At 2 years in patients who were treated with abatacept+MTX there was less radiographic progression than those who had been treated with MTX alone (0.84 vs 1.75 in the total score, P<.001).39 A recently study published analyzing all patients together, irrespective of initial treatment, showed that the total radiographic progression was significantly reduced in the 3rd year of treatment compared to the 2nd year (P=.022).40
Mean change in the Genant radiological score followed for 5 years in the AIM study. X-axis reflects absolute change of the Genant score with respect to the study time periods. The first year includes the blind abatacept or placebo period and afterwards patients on placebo received open label treatment with abatacept.91
Both phase IIb trials and AIM,40–43 as well as the ATTEST and AGREE38,39 trials have long-term extension studies(Table 4). It is important to understand that there is an intention to treat analysis unless the results of the patients completing the study are not discussed.
In the Phase IIb study of 220 patients treated with abatacept during the double-blind period, 152 entered the long-term extension phase, in which they received abatacept 10mg/kg every 28 days.41,42 Of these, 92 and 85 completed the 5th and 7th years of follow up with retention rates of 60% and 52%, respectively. Of the 73 patients who withdrew, 19 did so due to lack of effect, although this being a rare cause of withdrawal from the 2nd year of the extension study onward, with only 9 patients leaving the study during the last 6 years. The response obtained in the double-blind phase were low activity and improved physical function, as measured by mHAQ, maintained up to 7 years in patients who completed the study.39,41,42
Of the 385 patients initially treated with abatacept who completed the double-blind phase on AIM, 378 entered the extension phase, all receiving 10 abatacept mg/kg every 28 days and ending 266 after 5 years of follow-up, representing a retention rate of 70%.43 20% of the 112 patients who withdrew did so for lack of efficacy. The responses obtained in the remaining patients were maintained until the 5th year. A high proportion of patients achieved remission or low disease activity at the end of double-blind period, maintained throughout the extension period.44 At the end of double-blind study, 116 patients (31%) had a normal HAQ score. Of these, 74% remained normal throughout the length of the study.44,45
Both the ATTEST38 as well as the AGREE39 retention rate after one year was over 90%. The ACR20, 50 and 70 and the HAQ confirmed that efficacy was maintained over 2 years in both studies. After the double-blind, 6-month ATTAIN trial, 317 patients entered the extension phase,46 of which 79 (25%) and 73 (23%) reached the 5th year. During the extension period, 165 patients left the study, 69 of them (22%) due to lack of efficacy.45,46
Finally, in terms of quality of life, both extension studies of the AIM and ATTAIN trials have shown sustained improvement in various aspects of sleep quality using the MOS-Sleep and Sleep Problem Index, and in physical and mental subscales of the SF36, a fatigue scale, the number of days with limited activity and “participation in activities” (APAQ).47–49
Evidence/Recommendation 7. The Effectiveness Data of Abatacept in Clinical Practice in the Registries Are Consistent With Those in Clinical Trials (LE: 2b, DR NA)The data on effectiveness of abatacept in clinical practice derived from registries have appeared mainly in the last 3 years, with most coming from conference proceedings (see supplementary material). In general we can say that the data correspond to what is already known through clinical trials, although such comparisons must always take into account the biases that involve treatment in clinical practice, where patients are very different from those usually in clinical trials.
A retrospective observational study analyzed the results of the administration of abatacept in 100 patients of whom 97% had previously received a TNF inhibitor, showing that at 6 months it was 80% in those treated with abatacept and 44% and 34% with low activity and those that achieved remission, respectively.50 The French ORA51,52 registry reported data on effectiveness in 133 patients treated with abatacept, of which only 9.5% had not received biological drugs previously, showing that at 6 months, 66% achieved a EULAR response (good or moderate). The DANBIO53 Danish registry presented data on 150 patients treated with abatacept. As in the previous registry, only a small proportion, 5%, received prior TNF inhibitors. The DAS28CRP at baseline and at 24 and 48 weeks was 5.3, 3.4 and 3.3, respectively. The American CORRONA54 registry analyzed patients treated with abatacept and TNF blockers, which had not previously received other biological drugs. After adjusting, the analysis at 2 years of treatment showed that the probability of discontinuation due to a loss of effectiveness was similar in patients receiving abatacept or anti-TNF (RR 0.88, 95% CI 0.51–1.52). There were no differences in clinical response assessed by the patient in what concerned the overall situation and pain.54 Monitoring of patients treated with abatacept in this registry has also shown a gradual improvement in fatigue and sleep disorders and a tendency to reduce the average working day loss.55 A Canadian registry also showed that a significant percentage of patients treated with abatacept achieved a clinically significant improvement in functional capacity assessed by the HAQ (≥0.3), which occurred after about 2 months of treatment and was not influenced by the number TNF inhibitors the patients had previously received.56 Finally, recent data from the French registry of patients treated with abatacept showed a superior response in patients who are RF/anti-CCP positive.57 However, in a previous study with a different analysis, Buch et al.58 found no differences between the two populations.
Comparison of Abatacept With Other BiologicalsThere are no randomized studies that allow a direct comparison of abatacept with other biologicals. Only the ATTEST31 study, in which abatacept and infliximab were compared individually with placebo, is able to extract some comparative data.
In the systematic review we performed (available in supplementary material) and analyzed, 7 studies included comparative efficacy or safety of abatacept with other biologics in RA. Among these, the ATTEST study showed similar efficacy between abatacept and infliximab (3mg/kg), with a favorable safety profile for abatacept.31 Systematic reviews and metaanalyses were also included2,3,59,60 as well as a literature review.4 From these reviews it can be concluded that abatacept is not very different from other biological regarding effectiveness. In terms of safety, the meta-analysis of abatacept 20114 by Singh, which also include infliximab, adalimumab, etanercept, anakinra, rituximab and tocilizumab, made indirect comparisons in cases where a statistical model that allowed for these. The result is that both abatacept and anakinra were significantly associated with fewer serious adverse effects compared with the others and abatacept was also associated with significantly fewer serious infections compared to infliximab and tocilizumab.
As for cost-effectiveness, Lopez-Olivo et al.61 show in a paper presented at the ACR conference of 2010 that 80% of studies funded by the pharmaceutical industry come to the conclusion that the intervention is cost effective, as opposed to 54% of studies funded by other agencies.
Safety and Risk Management of AbataceptEvidence/Recommendation 8. Before and During Administration of Abatacept, the General Monitoring Recommendations Proposed for Biological Therapies Should Be Followed (LE: 5; DR: D)The recommendations of the SER for risk management in biologic therapy62 should be followed in all patients in whom treatment with abatacept is being considered and should include a clinical history and physical examination, in which data on relevant comorbidities, repeat infections and active infection (including TB) should be followed. A routine virus serology in addition to liver function tests, chest X-rays and Mantoux (+booster) testing must be routinely performed according to known recommendations.62 Before treatment onset, vaccination of patients against influenza and pneumococcus should be considered and other vaccines evaluated according to the situation of each patient. Avoid vaccination with attenuated or live germs. In patients of reproductive age pregnancy while abatacept is administered should be discouraged. During treatment with abatacept should be monitored for infections, cancer, or worsening of respiratory function in patients with chronic obstructive pulmonary disease (COPD) (for a detailed review on COPD, see below, following recommendations accordingly). In case of major elective surgery, abatacept treatment should be discontinued during the perioperatory period.62 Comorbidities are common in patients with RA and may be conditioned by treatment with biologics, such as abatacept.
Recommendation/Evidence 9. The Safety Profile and Tolerability of ABA Is Satisfactory (LE: 1b, DR: NO)In controlled clinical trials of abatacept with placebo (AIM,30 ATTAIN,32 ASSURE63 and ATTEST31), adverse reactions with abatacept were reported at rates similar to placebo-treated patients, between 55% and 80%. The adverse reactions most commonly reported (≥5%) were headache and nausea.5 The Cochrane meta-analysis of Maxwell and Singh shows that total adverse events were significantly higher in the abatacept group compared with the placebo group, but the relative risk was low (RR 1.05, 95% CI: 1.01–1.08).6
The rate in clinical trials of with drug administration associated reactions is about 7.7 (3.8–13.8) per 100 patient years, which is lower than that of other intravenous biological drugs.5 The acute events related to infusion occur with a frequency of 0.1%–1% of patients included cardiopulmonary symptoms such as hypotension, increased or decreased blood pressure, dyspnea, nausea, flushing, urticaria, cough, hypersensitivity, pruritus, rash, and wheezing. Most of these reactions were mild to moderate.5 In a clinical trial with abatacept 2 patients discontinued the drug due to serious adverse reactions that occurred in the first hour of infusion.30 Overall, the proportion of patients who discontinued treatment was slightly higher in patients treated with abatacept than with placebo (5% vs 2.2%).30 In another trial, in which a significant number of patients were treated concomitantly with MTX, the adverse reactions related to infusion in the first hour were more frequent in the abatacept group, although these were only mild to moderate.32 Only one patient developed anaphylaxis of 2688 patients treated with abatacept during 4764 patient-years.64
Recommendation/Evidence 10. Clinical Infection Rates With Abatacept Are Similar to Those of Patients Treated With MTX and Numerically Lower Than Those of Other Biological Therapies (LE: 1b, DR: NO)In clinical trials of abatacept, infection rates vary between 3.2% and 89.4%, and severe infections between 0.01% and 7.8% (or 1.8–5 episodes per 100 patient-years).4 These figures reflect the heterogeneity of the populations included in studies. In a meta-analysis of clinical trials of biologics, the risk of serious infections with abatacept was numerically lower than the rest, and significantly lower in comparison with infliximab, tocilizumab and certolizumab.4 Overall, it appears that the risk of serious infection is lower with abatacept than with other biologics. That assessment is further supported by work showing that the incidence rates of overall infections and pneumonia requiring hospitalization were similar in patients in clinical trials with abatacept than in cohorts treated with DMARD.65 In the meta-analysis by Singh et al., the odds ratio of serious infections with abatacept vs placebo is 0.57 (95% CI 0.30–1.08).4 As with other biologics, pneumonia is one of the most common serious infections in patients with RA treated with abatacept. They appear in varying rates between 0.36 and 1.43 per 100 patient-years in relation to the patient profile (see review in supplementary document). In any case, they are higher in patients who previously failed treatment with TNF inhibitors, which probably reflects rates in patients with more severity.66–68 The rates of opportunistic infections per 100 patient-years are between 0.01 and 0.36 and vary by prior therapy with abatacept.
Evidence/Recommendation 11. The Rate of Tuberculosis in Patients Treated With Abatacept Appears Inferior to That of TNF Inhibitors (LE: 2b, DR: NA). However, Patients Who Are to Be Treated With Abatacept Should Undergo a Search for Latent Tuberculosis in Accordance With the Recommendations of the SER (LE: 5; DR: D)In a study conducted in mice infected with M tuberculosis, evoking a model of chronic tuberculosis, abatacept or a murine anti-TNF was administered, with the primary endpoint being survival. While all mice treated with abatacept survived, those receiving anti-TNF died due to an exacerbation of tuberculosis.69
In all clinical trials with a total of 4149 patients and a rate of 12132 patients per year, we found 8 cases of tuberculosis, reflecting an incidence rate of 0.07 [0.03–0.13] per 100 patient-years.69 These cases were: infection with cervical lymphadenitis due to tuberculosis, 3 cases of pulmonary tuberculosis, a case of thoracic Pott's disease, a case of submandibular lymphadenitis, 1 case of latent tuberculosis, a case of suspected tuberculosis with placebo and 1 case of “suspected” tuberculosis. This represents a rate lower than that shown in patients with RA treated with TNF inhibitors. However, the number of patients treated with abatacept to date and the time they have undergone treatment after implementation of guidelines for the prevention of TB reactivation, prevents stronger conclusions.
Evidence/Recommendation 12. The Safety of Abatacept in Patients With Hepatitis B or C (LE: 5; DR: NA). Abatacept Use Should Be Guided by Close Monitoring of Liver Function and Viral Load Tests (LE: 5; DR: D)The safety of abatacept in patients chronically infected with hepatitis viruses B and C has not been studied in depth, since these patients were excluded from clinical trials, and there are no consensus guidelines for use in these situations. There are only a few references to case reports in which abatacept has been used in patients with no active infection by hepatitis B virus, which is usually associated to prophylaxis. There are also isolated cases of RA associated with hepatitis C and treated with abatacept without any significant impairment of liver function.70–72 Given the lack of specific recommendations for treatment with abatacept in patients chronically infected with hepatitis viruses B and C, it is not recommended for use unless no other options are available and always with close monitoring of liver function and viral loads.
Evidence/Recommendation 13. The Risk of Tumors in Patients Treated With Abatacept Is not Higher Than Expected in Patients With RA (LE: 2b, DR: NO)Clinical trials with abatacept excluded patients with a history of cancer in the previous 5 years and excluded those with mammograms suggestive of breast cancer. On the other hand it is known that patients with RA have a higher risk of developing lymphoma and lung cancer.73 Simon et al.74 performed a comparative analysis of all patients treated with abatacept during clinical development (4134 patients from 7 clinical trials) vs patients with RA from 5 cohorts (RA 41529, with a median follow up of 1, 8, and 9.3 years).74 In the population that was treated with abatacept, after excluding skin cancers other than melanoma, there was a total of 51 tumors: 7 breast, 2 colorectal, 13 lung and 5 lymphomas, whereas the control population showed 522 cancers, 275 of the breast, 470 colorectal and 184 lung lymphomas. The number of cancers in patients treated with abatacept was within the expected range in concordance with RA cohorts. The incidence rate of lung cancer with abatacept (0.15/100 person-years) is similar to that of the various RA cohorts (0.09–0.26) and for lymphoma occurred at a rate of 0.06/100 vs 0.06–0.11 person-years. Therefore, the data suggest that the risk of these tumors with abatacept is different from that expected in a population with RA.
Evidence/Recommendation 14. The Use of Abatacept in Patients With Class IV Heart Failure Must Be Cautious (NE: 5; GR: D)Clinical trials excluded patients with NYHA class IV heart disease. The remaining patients without heart disease or below class IV had no evidence of worsening or the appearance of “de novo” heart failure.5
Evidence/Recommendation 15. In Patients With Chronic Obstructive Pulmonary Disease, Abatacept Should Be Given Only After a Thorough Analysis of Risk–benefit Balance (LE: 1b, DR: A)In the ASSURE63 study, patients with chronic obstructive pulmonary disease (COPD) treated with abatacept developed more respiratory adverse reactions than those treated with placebo: 43% vs 24%. Similarly, serious adverse events of respiratory origin were more frequent in patients treated with abatacept than in those receiving placebo: 10% vs 0%. Adverse reactions noted were relapses of COPD, bronchitis and pneumonia. Therefore in clinical practice, abatacept should be indicated only after a thorough review of risk/benefit.
Evidence/Recommendation 16. There Have Been no Reports of Interstitial Lung Disease in Patients Treated With Abatacept (LE: 4; DR: NA). Safety is Unknown in Cases of Pre-existing Impairment (LE: 5; DR: NO)Two systematic reviews of the literature aimed at assessing the occurrence of interstitial lung disease (ILD) following administration of different biological drugs found no published cases related to abatacept.75,76 On the other hand, it is unknown what effect abatacept would have in patients with prior interstitial lung disease. Despite all this, and given previous experience with biological therapies, administration of abatacept should be performed with very close monitoring of respiratory symptoms and lung function of patients.
Evidence/Recommendation 17. In Patients With Demyelinating Diseases Abatacept Is Contraindicated and, if Used, Requires Close Neurological Monitoring (LE: 2b, DR: D)So far a few cases of demyelinating disease, one in RA77 and another in juvenil78 chronic arthritis, have been reported in all clinical trials with abatacept and there is no clear increase over expected rates of demyelinating disease. Furthermore, treatment with abatacept is safe and could be effective in multiple79 sclerosis. However, we must await the results of longer-term pharmacovigilance to clarify this.
Evidence/Recommendation 18. Abatacept Administered Intravenously Is a Poorly Immunogenic Drug (LE: 4; DR: NO)In 3985 patients with RA treated with abatacept for up to 8 years, 4.8% developed antibodies against abatacept during treatment.5 In patients who were evaluated after discontinuing abatacept for antibodies (>42 days after the last dose) 5.5% were positive.5 The presence of antibodies was not associated with adverse reactions, including those related to the administration, or with changes in efficacy or serum abatacept concentrations. The rates of ANA and anti-DNA in clinical trials were not different from those of the placebo5 treated group.
In clinical trials with abatacept have been cases of psoriasis 0.57 per 100 (95% CI 0.44–0.72), Sjögren's syndrome rate 0.19 (95% CI 0.12–0.29) and vasculitis reported rate 0.18 (95% CI 0.11–0.28).80 No cases of SLE have been reported.
Evidence/Recommendation 19. In Patients Treated With Abatacept, Antibody Responses to Inactivated Vaccines or Germ Cell Components Is Lower Than the General Population; Live Attenuated Vaccines Should Be Avoided (LE: 4; DR: C)The information on the efficacy and safety of different vaccines with abatacept therapy is limited. No data exist on their potential risk on vaccination with live attenuated organisms, such as oral polio, MMR, varicella and yellow fever. There are some facts about inactivated germs vaccines or cellular components. In a double-blind trial in healthy volunteers, there was a response to vaccination with tetanus and pneumococcal vaccines which was slightly lower after administration of a dose of abatacept in controls.81 A substudy of ARRIVE33 evaluated the efficacy of vaccination with 7 strains of Streptococcus pneumoniae in 21 patients with RA. The response was positive at least for one strain in 81% of cases, and 48% to 3 strains or more.82 The ARRIVE study also analyzed the response to influenza vaccination (strains H1N1, H3N2 and influenza B) in 20 patients with RA, 50% responded to at least 2 of these 3 strains.83 Moreover, in an observational study in Brazil, the rate of seroconversion in a group of 11 patients with abatacept, compared to that of 33 patients with RA treated with MTX and 33 healthy controls, was significantly inferior.84 Therefore, vaccination with live attenuated organisms is considered contraindicated until 3 months after cessation of treatment with abatacept, or at least 2 weeks before the start or its reintroductio.5 Inactivated vaccines or germ cell components can be administered at any time, although its efficacy may be somewhat diminished. For this reason, if possible, it also recommended that administration occurs 3 months after discontinuation.
Evidence/Recommendation 20. Abatacept May Be Given Without Making a Washout Period After the Use of Anti-TNF Drugs, But Not Administered in Combination With Other Biological Agent (LE: 1b, DR: A)Sequential administration of abatacept after failure of a TNF inhibitor is not associated with increased adverse event rates including serious infections, after 6 months of drug administration. This was demonstrated in the ARRIVE trial where prior to administration of abatacept a washout period to TNF antagonists was performed for at least 2 months in 449 patients and was not done in 597; there was no difference in rates of serious infections between groups.33 However, whenever you perform the transition from treatment with a TNF inhibitor to abatacept, patients should be monitored closely for signs of infection.
As is the case with other biologics, combination therapy of abatacept and a TNF inhibitor is associated with a significant increase in infections in general and specifically severe63 infections,85 which are a contraindication for this type of administration. There is no evidence on the safety and efficacy of abatacept in combination with anakinra or rituximab. As for the combination with other drugs, abatacept can be combined safely with MTX to the fact that the population pharmacokinetic analysis did not detect any effect of these on the clearance of abatacept, or found any significant safety issues with the use of abatacept in combination with DMARDs during development.5 No dose adjustment is necessary when used in combination with other DMARDs, glucocorticoids, salicylates, NSAIDs or analgesics.
Evidence/Recommendation 21. Abatacept Should Be Discontinued at Least 3 Months Before the Start of Pregnancy (LE: 5; DR: D)Abatacept crosses the placental barrier, although preclinical studies showed no adverse effects at doses up to 29 embryos-times the human dose of 10mg/kg.5 Data on women who became pregnant accidentally treated with abatacept are very limited. Of 8 women in the 5 pivotal studies, 7 were receiving concomitant MTX and another leflunomide.5 In 3 cases there were spontaneous abortions in the first quarter (2 of them previously had abortions) and 2 pregnancy were terminated voluntarily. In the AGREE study, 2 accidental pregnancies ended in spontaneous and induced abortion.34 Therefore, pregnancy in women treated with abatacept should be avoided until 3 months after the suspension.
Other interesting aspects related to abatacept.
Lipid ProfileIn a paper presented as a Congress summary there was an increase in mean HDL cholesterol at 24 weeks of starting treatment with abatacept in 17 patients: 1.7–2.1mmol/l (P=.008) and a decrease in the atherogenic index from 3.0 to 2.7 (P=.03), without changes in total cholesterol, triglycerides or LDL86,87 cholesterol. The increase in lipids with no significant increase in the atherogenic index seems a common occurrence to infliximab, etanercept, adalimumab, abatacept, rituximab and tocilizumab.88
DiscussionThe document presents 21 summary recommendations gathered from the evidence on abatacept (14 statements and 9 recommendations). A 2b level of evidence is 14 times higher on the Oxford Centre for Evidence-Based Medicine scale. However, it was considered important to precisely point out those aspects with lesser degrees of evidence, which is why, despite a good level of evidence in the statements without recommendation, the degree of support of the recommendations is A in 2 recommendations, C and D in the rest. Recommendations were not extracted from many statements of evidence so the clinician uses them for their own decision making process; for example, the fact that abatacept has demonstrated efficacy in certain situations does not mean one would specifically recommend abatacept. We think that for certain recommendations it is important to consider the overall therapeutic options, individual patient characteristics and approved therapeutic indications and sequences.
Evidence 1 corresponds to the administration of abatacept in a short period of time (30min) and the low incidence of infusion reactions. The level of evidence is high (1b). The administration in this time frame is a great advantage because it aleviates the burden of care and contributes to a more rational use of hospital resources. In addition, the small number of infusion reactions is an added advantage22,23 which has been detailed in evidence number 8. It should also be remembered that abatacept has also been developed for subcutaneous use,25,26 and its use in this way is approved in the U.S.
Abatacept clinical development [evidence 2 (LE: 1b) and 3 (LE: 2b)] has been carried out through several clinical studies, including most notably AIM30 study, in patients with inadequate response to MTX, and the ATTAIN32 study, in patients with previous failure to anti-TNF in which the control arm was additionally treated with placebo, MTX or other DMARDs. In both trials, abatacept was significantly superior in efficacy with respect to the control arm, which corroborates the effectiveness of the drug. In the ATTEST31 study, abatacept and infliximab (at a fixed dose of 3mg/kg) combined with MTX were compared with placebo (+MTX). The objective of this study was to assess the magnitude of the response of each of the two biological agents compared with placebo (+MTX). At 6 months, the study showed that both were superior to placebo with a similar response. Failure to increase the dose of infliximab to 5mg/kg in non-responders patients, a common clinical, could be considered one of the limitations of the study, but the trial design looked only to administer infliximab at an approved dose, and abatacept treatment after one year had an overall safety profile similar to infliximab.31 Another interesting study conducted in patients with early RA and a poor prognosis34 showed a significantly higher remission rate in patients treated with abatacept and MTX compared to those treated only with MTX [evidence 4 (LE: 2b)]. This study highlights the theoretical utility the use of this biological agent in early stages of disease would have. However, remember that abatacept treatment is not indicated in these phases of the disease.
Abatacept in combination with MTX has been shown to significantly reduce structural damage in the medium and long terms [evidence 5 (LE: 2b)].30,38,39,41 The overall reduction is estimated at about 48% per year of treatment and a reduction in damage increases gradually in the long term38 studies,.39 It has been speculated that abatacept had a lesser impact than anti-TNF on radiological damage, because with the latter an overall 90% reduction is estimated.36 It is very possible that the impact on structural damage has little or no clinical manifestation or radiological progression because in either case it is very small.
Extension studies after several years of treatment with abatacept have shown high retention rates [evidence 6 (LE: 2b)]. Thus, in the AIM study, after 5 years of treatment, 70% of patients were receiving abatacept.43 In other studies with shorter extension periods (one year) retention 90% rates were reached,38,39 while in the ATTAIN study, in which, as an inclusion criterion patients had to have failed TNF inhibitors and therefore it was constituted by patients with poor therapeutic response, the retention rate was 25% after the 5th year of treatment.46 However, one must be cautious when interpreting retention rates in clinical trials as there are many external factors, including access to biological agents that can influence in one direction or the other.
Most of the data on effectiveness in clinical practice [evidence 7 (LE: 2b)] are from meeting abstracts.50,52–56 Reading the results confirms the effectiveness of the drug, especially in a population with severe RA, since in most cases abatacept was used in patients who had failed anti-TNF.
On the other hand abatacept is well tolerated and rarely presents infusion reactions, these being generally mild (see reviews of the evidence 1 and 8). Also in a recent meta-analysis that included all biological agents sold, a total of 163 trials and 46 extensions,4 abatacept was the biological agent, together with anakinra, which had the lowest number of serious adverse events, although this was not statistically significant compared to the control arm [evidence 9 (LE: 1b)]. The same meta-analysis also showed that when the active drug was compared with the control arm, abatacept was the biological agent associated with fewer serious infections, significantly lower when compared with certolizumab pegol, infliximab and tocilizumab [evidence 10 (LE: 1b)].
With regard to tuberculosis [evidence 11 (LE: 2b)], so far it has only been described in 8 cases in the extension phase of the abatacept clinical development program.80 When conducted with abatacept clinical research already knew the likelihood that reactivation of latent tuberculosis after administration of an anti-TNF. This meant that in clinical studies with this drug, a scrupulous screening is made for the existence of this infection. To what extent these measures have been able to determine a decline in tuberculosis is not known. However, as in every patient treated with biological agents, the Spanish Society of Rheumatology recommends that screening for tuberculosis is the same as when administering a TNF antagonist, which this panel supports [second part of evidence 11 (LE: 5)].
No conclusive data exist on the safety of abatacept in patients infected with hepatitis B or C virus [evidence 12 (LE: 5)] or in patients with an advanced degree of heart failure [recommendation 14 (LE: 5)]. In these cases the level of evidence is 5 and it has to be the rheumatologist who decides management with abatacept based on the risk/benefit. With regard to an increased risk of cancer [evidence 13 (LE: 2b)], abatacept, like all other biological agents, has not shown an increase of new tumors74 in those receiving this agent; however, no data exist on safety of abatacept in patients with a history of a tumor.
Patients with COPD treated with abatacept are at increased risk of lower respiratory tract infections compared to patients treated with placebo [evidence 15 (LE: 1b)],63,89 therefore, in all patients with this condition, risk/benefit must be carefully evaluated when abatacept is administered.
One of the problems facing the clinician when prescribing a biological agent is the risk of aggravation of previous interstitial pneumonitis or even the risk that the drug could be an inducing agent for such a pathology [evidence 16 (LE: 4 and 5)]. To date there are some cases of interstitial pneumonitis described in relation to anti-TNF, even with rituximab75 and tocilizumab.76,90 However, neither we nor other authors have conducted an extensive literature review of similar cases related to abatacept.75,76 For this reason, abatacept could be a candidate as a therapeutic alternative in patients with prior interstitial pneumonitis. However, any patient in this situation should be subject to close monitoring of respiratory function.
Abatacept is not formally contraindicated in patients with demyelinating disease [evidence 17 (LE: 2b)] and in fact clinical trials have been carried out in very early stages in patients with multiple sclerosis.79 In addition, abatacept intravenously administered is poorly immunogenic [evidence 18 (LE: 4)] and after 8 years of treatment, antibodies only appeared in 4.8% of cases.5 It is not associated to a higher incidence of ANA or anti-DNA antibodies in the control population.5
One of the most common problems in daily practice concerns the vaccination of patients about to receive a biological [evidence 19 (LE: 4)]. With Abatacept, as with other biologic therapies, there are no studies with live attenuated virus vaccines. On the other hand, several studies have shown a slightly decreased response to tetanus toxoid, pneumococcal vaccine and flu vaccines.81–83 Therefore vaccination is contraindicated with live attenuated viruses and pneumococcal vaccination should be done preferably before administration of abatacept. Regarding the flu vaccine, as it is a seasonal administration, it must be performed when appropriate, assuming that its effectiveness may be lower.
Another very common clinical situation is the patient who needs a change in biological therapy. In the event that this situation (replacing an anti-TNF for abatacept) arises [evidence 20 (LE: 1b)], the ARRIVE33 study showed that abatacept can be administered safely without a washout period in patients who have suspended an anti-TNF.
Finally with respect to pregnancy [evidence 21 (LE: 5)] a recommendation is only made based on prudence and common sense. The evidence on this point, as expected, is zero. There are only some clinical cases described in which pregnancy occurred accidentally and did not provide any light on the possible teratogenic effect of the drug.
This panel of experts has attempted to develop a practical document with a summary of the evidence discussed regarding abatacept and have added recommendations on issues that, even after thorough review, show no conclusive evidence. We believe that it could be a good addition to the available information sheet, in order to guide rheumatologists in the use of abatacept.
FinancingThis document has been funded through an unrestricted grant made to the Rheumatology Service, Hospital Universitario La Paz (AREPAZ) by Bristol Myers Squibb (Spain). The choice of the experts was the sole responsibility of the project coordinator (EMM). No employee of the company participated in scientific meetings or in its development until the project was completed.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Martín Mola E, et al. El uso de abatacept en artritis reumatoide: revisión de la evidencia y recomendaciones. Reumatol Clin. 2013;9:5–17.